Open Access Article
Open Access ArticlePeptide nanotubes self-assembled from leucine-rich alpha helical surfactant-like peptides†
Valeria
Castelletto
 a,
Jani
Seitsonen
b,
Janne
Ruokolainen
b,
Cristian
Piras
a,
Rainer
Cramer
a,
Charlotte J. C.
Edwards-Gayle
a,
Jani
Seitsonen
b,
Janne
Ruokolainen
b,
Cristian
Piras
a,
Rainer
Cramer
a,
Charlotte J. C.
Edwards-Gayle
 c and
Ian W.
Hamley
c and
Ian W.
Hamley
 *a
*a
aDepartment of Chemistry, University of Reading, Whiteknights, Reading RG6 6AD, UK. E-mail: I.W.Hamley@reading.ac.uk
bNanomicroscopy Center, Aalto University, Puumiehenkuja 2, FIN-02150 Espoo, Finland
cDiamond Light Source, Harwell Science and Innovation Campus, Chilton, Didcot, Oxfordshire OX11 0DE, UK
First published on 2nd September 2020
Abstract
The designed arginine-rich surfactant-like peptide R3L12 (arginine3–leucine12) is shown to form a remarkable diversity of self-assembled nanostructures in aqueous solution, depending on pH, including nanotubes, mesh-like tubular networks in three-dimensions and square planar arrays in two-dimensions. These structures are built from α-helical antiparallel coiled–coil peptide dimers arranged perpendicular to the nanotube axis, in a “cross-α” nanotube structure. The aggregation behavior is rationalized based on the effects of dimensionality, and the balance of hydrophobic and electrostatic interactions. The nanotube and nanomesh structures display arginine at high density on their surfaces, which may be valuable for future applications.
Peptide nanotubes are biomolecular self-assemblies with remarkable structural and functional properties.1–14 Several classes of peptide nanotube have been reported including those based on helically wrapped β-sheets,15–20 laminates of cyclic peptide dimers forming antiparallel β-sheet stacks,10,21,22 and stacks of alternating D,L-cyclic peptides stabilized by hydrogen bonded antiparallel β-sheets.1,7,23 In addition, the parallel packing of coiled coil peptide arrays can lead to tubular structures.24–26 Here, we report on a distinct class of peptide nanotube based on a designed surfactant-like peptide (SLP).27 We have recently reported on the self-assembly of a number of SLPs with hydrophobic alanine repeats and various charged ‘headgroups’. These form β-sheet nanofibrils,28,29 and in some cases nanotubes.19,20,30
Here, we explore the self-assembly of SLPs containing leucine repeats instead of alanine sequences, since leucine has a strong α-helix propensity.31 The peptide studied is R3L12 (and is capped at both termini, ESI,† Scheme S1). In dilute aqueous solution, nanotube structures comprising α-helical peptide dimers stacked perpendicular to the walls were observed, which have a thickness that corresponds to the length of the dimer comprising antiparallel α-helical peptides. We also unexpectedly observed continuous nanotubular channel network structures that are accessible via pH variation. These structures present arginine residues at the surfaces of the nanotube and tubular network structures, providing a highly cationic surface for future applications. In addition, an ordered square lattice array was observed in thin films under certain conditions of pH. We rationalize the formation of this diverse range of previously unreported peptide nanostructures on the basis of the balance between hydrophobic and electrostatic effects.
We studied pH-dependent self-assembly of R3L12 at pH 4 (native), or lower, pH values being tuned by addition of 10 mM HCl (pH 2) or 100 mM HCl (pH 1). Circular dichroism (CD) spectra shown in Fig. 1, measured for 0.04 wt% R3L12, confirm the formation of α-helical coiled coil structures for all solution conditions studied (the CD spectra for 0.07 wt% solutions shown in ESI,† Fig. S1 also confirm α-helix structure).32 For 0.04 wt% R3L12, the α-helical content fα (and [θ]222/[θ]208 ratio, a measure of coiled coil aggregation33,34) are, respectively, 78% (0.85), 42% (0.86) and 17% (1.25) at pH 4, 2 and 1 (inset Fig. 1; the expressions used to calculate these quantities are provided in the ESI†). Decreasing the pH reduces fα significantly, while [θ]222/[θ]208 increases.
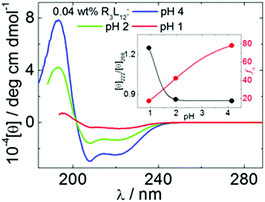 | ||
| Fig. 1 CD spectra measured for 0.04 wt% R3L12 at pH 4 (native), 2 and 1. The inset shows fα and [θ]222/[θ]208 calculated from the CD curves. | ||
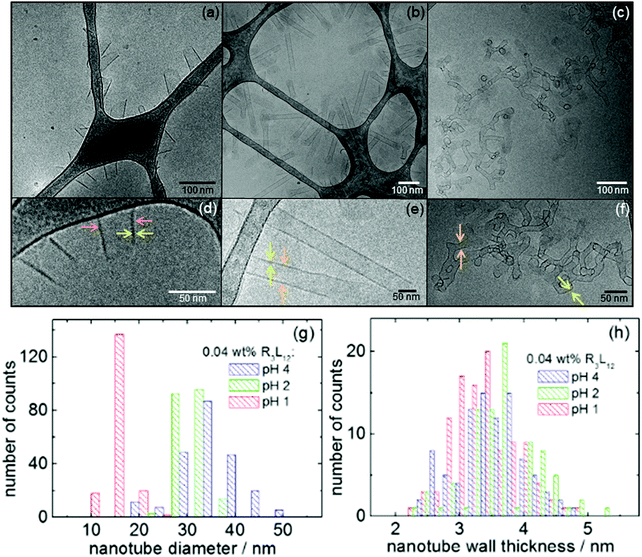
Having established the conformation of SLP R3L12, we then used the powerful combination of cryo-TEM, TEM (TEM: transmission electron microscopy) and SAXS (small-angle X-ray scattering) to probe self-assembly behavior. Fig. 2 displays representative cryo-TEM images obtained for 0.04 wt% solutions of R3L12 at pH 4, 2 and 1. These images (and others shown in ESI,† Fig. S2–S4) show the presence of short nanotubes at pH 4 (Fig. 2a and d). Decreasing the pH from 4 to 2 the nanotubes become better defined, and their population and length both increase (Fig. 2b, e and Fig. S3, ESI†). Decreasing the pH even further to pH 1 leads to the formation of a continuous tubular network aggregates (Fig. 2c, f and Fig. S4, ESI†).
The shape of the nanotubes at pH 2 is very well defined, several cryo-TEM images clearly show the nanotube cross section (Fig. S3, ESI†). The continuous tubular network assemblies at pH 1 seem to mainly comprise three-fold connecting nodes, such as those highlighted in Fig. S4 (ESI†). To determine the nanotube diameter and wall thickness, histograms were created (Fig. 2g and h) based on a series of cryo-TEM images for samples at each pH value. These results will be discussed below together with the parameters extracted from the fitting of the SAXS data.
Fig. 3 shows synchrotron SAXS data for 0.04 wt% solutions of R3L12. Samples at pH 4 and pH 2 present strong oscillations arising from nanotube wall interference features in the form factor, with a period set by the nanotube diameter. This features is absent from the data for the pH 1 sample, which as shown by cryo-TEM (Fig. 2) forms a tubular network structure instead of narrow dispersity diameter nanotubes. As described in detail in the ESI,† high quality form factor fits (shown as lines in Fig. 3) of the SAXS data were performed which provide the nanotube diameter, as well as wall thickness. The SAXS fitting parameters are listed in Table S1 (ESI†). Table S2 (ESI†) compares the parameters measured from cryo-TEM images with those calculated from the fitting of the SAXS curves. The results are in good agreement and these results indicate that, from TEM, the thickness of the nanotube wall, t, is 3.4 ± 0.5 nm, 3.7 ± 0.6 nm and 3.4 ± 0.5 nm pH 4, 2 and 1 respectively. From SAXS, the wall thickness is 3.3 ± 0.1 nm, 3.0 ± 0.1 nm and 3.0 ± 0.1 nm at pH 4, 2 and 1 respectively. The diameter of the nanotube from cryo-TEM data is 32.5 ± 6.3 nm, 30.6 ± 2.8 nm and 17.4 ± 2.2 nm at pH 4, 2 and 1 respectively, and from SAXS it is 36.6 ± 3.4 nm, 33.0 ± 3.0 nm and 18.0 ± 4.0 nm respectively. These results indicate that the nanotube thickness is approximately 3 nm within the experimental error. The cryo-TEM and SAXS results also show that the nanotube diameter dramatically decreases at pH 1, i.e., in the continuous tubular network structure (Fig. 2c, f and Fig. S4, ESI†). The nanotube wall thickness corresponds closely to the length of R3L12 estimated using average residue spacings,35l = (1.5n) + (3.4p) = 28.2 Å (∼3 nm) where n = 12 (number of L-residues in the α-helix) and p = 3 (number of R-residues).
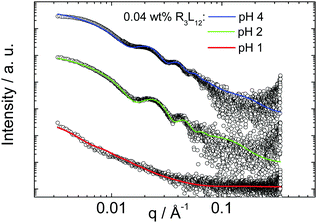 | ||
| Fig. 3 SAXS spectra measured for 0.04 wt% R3L12 at pH 4 (native), 2 and 1. The full lines indicate the fitting to the SAXS curves according to the models explained in the text and in the ESI.† The SAXS data has been arbitrarily shifted for clarity. | ||
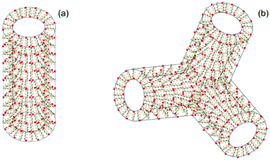
We thus propose that the nanotube walls comprise opposed dimers of α-helices oriented perpendicular to the main axis of the nanotube, as shown in Fig. 4. The antiparallel configuration is also reasonable as it will minimize electrostatic repulsion between arginine residues (peptide charge +3 under all pH conditions studied). The nanotubes and nanotubular network structures formed are coated with arginine on inner and outer surfaces. Nanotube formation results from the balance of hydrophobic and hydrogen bonding of the leucine residues that form antiparallel coiled coil dimers and electrostatic repulsion of arginine residues. The formation of the tubular network structure at very low pH 1 is ascribed to the unfavorable electrostatic penalty associated with having uncapped tubes exposed to large H+ ion concentrations, leading to closure into tubular networks.
Remarkably, distinct structures were observed in dried films of the pH 1 sample. TEM images (representative examples shown in Fig. S5, ESI†) consistently showed a population of sheet structures containing prominent lattices with square arrays 3 nm × 3 nm in size. This size corresponds approximately to the estimated length of an antiparallel α-helical dimer,35 considering the closely packed leucine residues, but allowing for splaying of the longer terminal arginine residues. We propose that this structure is a rotator-type phase, in which electrostatic repulsion of arginine residues is minimized by 90° rotations of helical dimers (Fig. S5c, ESI†), analogous, for example, to certain 2D spin lattice nanomagnet systems.36 We propose that this structure is favored in dried films due to confinement which leads to α-helices parallel to the interface, whereas in bulk the helical dimers are able to orient perpendicular to the nanotube surfaces.
Additional studies were performed on solutions containing a higher concentration, 0.07 wt%, of R3L12. Fig. S6–S8 (ESI†) display cryo-TEM and fitted SAXS form factor data. Nanotube parameters obtained from the fitting to the SAXS curves and measures of the cryo-TEM images are listed in Tables S1 and S2 (ESI†). Cryo-TEM images at pH 4 show unwrapped nanotubes (Fig. S6, ESI†), coexisting with a population of short folded nanotubes (inset Fig. S6a, ESI†). Increasing the pH to 2 gives well defined long nanotubes (Fig. S7a–c, ESI†), and a tubular structure network is formed at pH 1 (Fig. S8, ESI†). These results are similar to those at the lower concentration 0.04 wt%, with the exception of the unwrapped nanotubes observed at pH 4. This points to the subtle interplay of electrostatic and hydrophobic interactions, modulated by peptide concentration. Nanotube dimensions were obtained from cryo-TEM and SAXS (Fig. S7d, e, S8c, d and Tables S1 and S2, ESI†) and are generally very similar to those measured for 0.04 wt% R3L12 samples, as discussed above. Analysis of CD spectra (Fig. 1 and ESI,† Fig. S1) indicates that there is a slight increase in α-helical content and a stronger tendency to form coiled coil helices at 0.07 wt% compared to 0.04 wt% peptide. The stability of R3L12 in very acidic solution (pH 1) was investigated using state-of-the-art liquid AP-MALDI MS. The spectra shown in ESI,† Fig. S9 show that no peptide degradation was detectable for the solutions at pH 4 or 1.
In summary, designed SLP R3L12 exhibits remarkable pH-dependent self-assembly of α-helical peptide structures, including nanotubes and tubular network structures with molecular thickness arginine-coated walls based on opposed dimeric coiled coils. Our results contrast with observations for the related peptide K3L12 which forms bilayer discs, fibrils or vesicles depending on pH, resulting from self-assembly of α-helical peptides.37 It is also distinct from the behavior of longer “block” polypeptides such as R60L20 which self-assembles to form vesicles.38 The self-assembly of SLP R3L12 can also be contrasted with conventional coiled coil peptides with heptad repeats which can be engineered to form extended coiled–coil assemblies, such as fibrils and nanotubes,24,39–42 since these generally contain arrays of helices parallel to the long axis, although radial arrangements have been reported,43 in particular in the recently discovered cross-α fibril structure.44 Peptide R3L12 forms distinctive cross-α nanotube structures. As well as remarkable self-assembly properties, R3L12 may have interesting bioactivities and functionalities (for example potential cell-penetrating, antimicrobial or biocatalytic activities) arising from the high-density arginine-coating of the nanotubes and tube networks.
The work of VC was supported by EPSRC Platform grant EP/L020599/1 “Nanostructured Polymeric Materials for Healthcare” to IWH. We are grateful Diamond for beamtime on B21 (ref. SM22925-1). We acknowledge access to the Chemical Analysis Facility Laboratory (Univ. of Reading).
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. R. Ghadiri, J. R. Granja and L. K. Buehler, Nature, 1994, 369, 301–304 CrossRef CAS.
- D. T. Bong, T. D. Clark, J. R. Granja and M. R. Ghadiri, Angew. Chem., Int. Ed., 2001, 40, 988–1011 CrossRef CAS.
- M. Reches and E. Gazit, Science, 2003, 300, 625–627 CrossRef CAS.
- X. Gao and H. Matsui, Adv. Mater., 2005, 17, 2037–2050 CrossRef CAS.
- T. Shimizu, M. Masuda and H. Minamikawa, Chem. Rev., 2005, 105, 1401–1443 CrossRef CAS.
- S. Scanlon and A. Aggeli, Nano Today, 2008, 3, 22–30 CrossRef CAS.
- R. J. Brea, C. Reiriz and J. R. Granja, Chem. Soc. Rev., 2010, 39, 1448–1456 RSC.
- X. H. Yan, P. L. Zhu and J. B. Li, Chem. Soc. Rev., 2010, 39, 1877–1890 RSC.
- G. Rosenman, P. Beker, I. Koren, M. Yevnin, B. Bank-Srour, E. Mishina and S. Semin, J. Pept. Sci., 2011, 17, 75–87 CrossRef CAS.
- C. Valéry, F. Artzner and M. Paternostre, Soft Matter, 2011, 7, 9583–9594 RSC.
- K. L. Morris, S. Zibaee, L. Chen, M. Goedert, P. Sikorski and L. C. Serpell, Angew. Chem., Int. Ed., 2013, 52, 2279–2283 CrossRef CAS.
- I. W. Hamley, Angew. Chem., Int. Ed., 2014, 53, 6866–6881 CrossRef CAS.
- L. Adler-Abramovich and E. Gazit, Chem. Soc. Rev., 2014, 43, 6881–6893 RSC.
- P. Zelenovskiy, I. Kornev, S. Vasilev and A. Kholkin, Phys. Chem. Chem. Phys., 2016, 18, 29681–29685 RSC.
- K. Lu, J. Jacob, P. Thiyagarajan, V. P. Conticello and D. G. Lynn, J. Am. Chem. Soc., 2003, 125, 6391–6393 CrossRef CAS.
- M. J. Krysmann, V. Castelletto, J. M. E. McKendrick, I. W. Hamley, C. Stain, P. J. F. Harris and S. M. King, Langmuir, 2008, 24, 8158–8162 CrossRef CAS.
- A. K. Mehta, K. Lu, W. S. Childers, S. Liang, J. Dong, J. P. Snyder, S. V. Pingali, P. Thiyagarajan and D. G. Lynn, J. Am. Chem. Soc., 2008, 130, 9829–9835 CrossRef CAS.
- I. W. Hamley, A. Dehsorkhi, V. Castelletto, S. Furzeland, D. Atkins, J. Seitsonen and J. Ruokolainen, Soft Matter, 2013, 9, 9290–9293 RSC.
- I. W. Hamley, A. Dehsorkhi and V. Castelletto, Chem. Commun., 2013, 49, 1850–1852 RSC.
- J. Madine, V. Castelletto, I. W. Hamley and D. A. Middleton, Angew. Chem., Int. Ed., 2013, 52, 10537–10540 CrossRef.
- C. Valery, M. Paternostre, B. Robert, T. Gulik-Krzywicki, T. Narayanan, J. C. Dedieu, G. Keller, M. L. Torres, R. Cherif-Cheikh, P. Calvo and F. Artzner, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 10258–10262 CrossRef CAS.
- C. Tarabout, S. Roux, F. Gobeaux, N. Fay, E. Pouget, C. Meriadec, M. Ligeti, D. Thomas, M. Ijsselstijn, F. Besselievre, D. A. Buisson, J. M. Verbavatz, M. Petitjean, C. Valery, L. Perrin, B. Rousseau, F. Artzner, M. Paternostre and J. C. Cintrat, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 7679–7684 CrossRef CAS.
- J. D. Hartgerink, J. R. Granja, R. A. Milligan and M. R. Ghadiri, J. Am. Chem. Soc., 1996, 118, 43–50 CrossRef CAS.
- C. F. Xu, R. Liu, A. K. Mehta, R. C. Guerrero-Ferreira, E. R. Wright, S. Dunin-Horkawicz, K. Morris, L. C. Serpell, X. B. Zuo, J. S. Wall and V. P. Conticello, J. Am. Chem. Soc., 2013, 135, 15565–15578 CrossRef CAS.
- N. C. Burgess, T. H. Sharp, F. Thomas, C. W. Wood, A. R. Thomson, N. R. Zaccai, R. L. Brady, L. C. Serpell and D. N. Woolfson, J. Am. Chem. Soc., 2015, 137, 10554–10562 CrossRef CAS.
- M. Nambiar, M. Nepal and J. Chmielewski, ACS Biomater. Sci. Eng., 2019, 5, 5082–5087 CrossRef CAS.
- S. Santoso, W. Hwang, H. Hartman and S. Zhang, Nano Lett., 2002, 2, 687–691 CrossRef CAS.
- V. Castelletto, R. H. Barnes, K. A. Karatzas, C. J. C. Edwards-Gayle, F. Greco, I. W. Hamley, R. Rambo, J. Seitsonen and J. Ruokolainen, Biomacromolecules, 2018, 19, 2782–7294 CrossRef CAS.
- V. Castelletto, C. J. C. Edwards-Gayle, I. W. Hamley, G. Barrett, J. Seitsonen and J. Ruokolainen, ACS Appl. Mater. Interfaces, 2019, 11, 9893–9903 CrossRef CAS.
- J. Madine, H. A. Davies, C. Shaw, I. W. Hamley and D. A. Middleton, Chem. Commun., 2012, 48, 2976–2978 RSC.
- P. Y. Chou and G. D. Fasman, Biochemistry, 1974, 13, 222–245 CrossRef CAS.
- S. M. Kelly, T. J. Jess and N. C. Price, Biochim. Biophys. Acta, 2005, 1751, 119–139 CrossRef CAS.
- J. Y. Su, R. S. Hodges and C. M. Kay, Biochemistry, 1994, 33, 15501–15510 CrossRef CAS.
- G. Vandermeulen, C. Tziatzios and H. Klok, Macromolecules, 2003, 36, 4107–4114 CrossRef CAS.
- T. E. Creighton, Proteins. Structures and Molecular Properties, W. H. Freeman, New York, 1993 Search PubMed.
- J. M. Porro, S. A. Morley, D. A. Venero, R. Macedo, M. C. Rosamond, E. H. Linfield, R. L. Stamps, C. H. Marrows and S. Langridge, Sci. Rep., 2019, 9, 11 CrossRef.
- H. C. Fry, G. d. Q. Silveira, H. M. Cohn and B. Lee, Langmuir, 2019, 35, 8961–8967 CrossRef CAS.
- E. P. Holowka, V. Z. Sun, D. T. Kamei and T. J. Deming, Nat. Mater., 2007, 6, 52–57 CrossRef CAS.
- M. J. Pandya, G. M. Spooner, M. Sunde, J. R. Thorpe, A. Rodger and D. N. Woolfson, Biochemistry, 2000, 39, 8728–8734 CrossRef CAS.
- D. N. Woolfson, Adv. Protein Chem., 2005, 70, 79–112 CrossRef CAS.
- Y. Y. Wu, P. K. Norberg, E. A. Reap, K. L. Congdon, C. N. Fries, S. H. Kelly, J. H. Sampson, V. P. Conticello and J. H. Collier, ACS Biomater. Sci. Eng., 2017, 3, 3128–3132 CrossRef CAS.
- Y. Tian, F. B. Polzer, H. V. Zhang, K. L. Kiick, J. G. Saven and D. J. Pochan, Biomacromolecules, 2018, 19, 4286–4298 CrossRef CAS.
- E. H. Egelman, C. Xu, F. DiMaio, E. Magnotti, C. Modlin, X. Yu, E. Wright, D. Baker and V. P. Conticello, Structure, 2015, 23, 280–289 CrossRef CAS.
- E. Tayeb-Fligelman, O. Tabachnikov, A. Moshe, O. Goldshmidt-Tran, M. R. Sawaya, N. Coquelle, J. P. Colletier and M. Landau, Science, 2017, 355, 831–833 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures including materials, sample preparation, experimental data obtained from several techniques (liquid MALDI-AP MS, CD, SAXS, Cryo-TEM and TEM) and tables listing the parameters extracted from the fitting to the SAXS data and the analysis of cryo-TEM images are included. See DOI: 10.1039/d0cc04299d |
| This journal is © The Royal Society of Chemistry 2020 |