Open Access Article
Open Access ArticleA diamino-substituted carbodiphosphorane as strong C-donor and weak N-donor: isolation of monomeric trigonal-planar L·ZnCl2†
Alexander
Kroll
,
Henning
Steinert
 ,
Lennart T.
Scharf
,
Thorsten
Scherpf
,
Bert
Mallick
and
Viktoria H.
Gessner
,
Lennart T.
Scharf
,
Thorsten
Scherpf
,
Bert
Mallick
and
Viktoria H.
Gessner
 *
*
Chair of Inorganic Chemistry II, Faculty of Chemistry and Biochemistry, Ruhr University Bochum, Universitätsstrasse 150, 44780 Bochum, Germany. E-mail: viktoria.gessner@rub.de
First published on 15th June 2020
Abstract
The isolation, structural characterization and coordination chemistry of a di(amino)-substituted carbodiphosphorane (CDP) are reported. Compared to the analogue, dianionic bis(iminophosphoryl)methandiides, the CDP is a stronger C-, but much weaker N-donor which led to the isolation of solely C-coordinated metal complexes amongst an unusual monomeric trigonal-planar L·ZnCl2 complex.
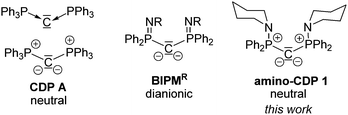
Bisylidic compounds, such as carbodiphosphoranes (CDP) or carbodicarbenes (CDC), have received intense research interest over the past two decades. Although the strong Lewis basicity and donor properties of CDPs were early recognized,1 recent computational studies by Frenking and co-workers2 emphasized the unusual bonding situation in these compounds and led to a renaissance in their chemistry.3 CDPs – above all the parent hexaphenylcarbodiphosphorane A (Fig. 1) – are strong donor ligands with higher donor strengths than those of other L-type ligands including carbenes. Accordingly, they were capable of stabilizing electron-deficient main group compounds, such as borenium4 or phosphorus cations5 and were applied as bases in Frustrated Lewis Pairs (FLP).6 While the coordination chemistry of CDP A has been studied intensively, derivatives with further donor functions have been neglected for a long time. Alcarazo and coworkers reported on a mono-pyridyl substituted CDP and heterobimetallic gold and copper complexes thereof,7 while a symmetric bis(pyridyl) system was used by Zhao, Frenking, Zhu and coworkers for the preparation of uranium carbene complexes8 and by Sundermeyer et al. for a series of different transition metals.9 Most interestingly, Sundermeyer et al. very recently also reported on the generation of CDPs incorporating pyrrolidine, tetramethylguanidine, or tris(dimethylamino)phosphazene substituents. These CDPs revealed to be superbases with outstandingly high basicities.10 However, no coordination chemistry was reported.
In the course of our research program on carbanionic and ylidic ligands, we became interested in the properties and coordination chemistry of bis(amino)-substituted CDPs, which were already described by Appel in 1981, but neglected ever since.11 These amino-CDPs such as 1 (Fig. 1) are structurally closely related to bis(iminophosphoryl)methandiides, BIPMR, which have been used as pincer ligands in a series of carbene complexes with unusual bonding situations and reactivities.12 Given this similarity, we were interested to study the differences in the electronic structure as well as the coordination chemistry between CDP 1, hexaphenyl compound A and the related BIPM ligands.
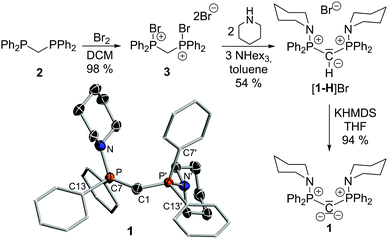
Bis(amino)-CDP 1 was prepared in a three-step procedure starting from dppm (2) (Fig. 2). Bromination to 3 and subsequent amination with two equiv. of piperidine in the presence of three equiv. of trihexylamine directly led to the monodeprotonated precursor [1-H]Br, which was isolated as an off-white solid in 54% yield. The use of trihexylamine as base is crucial to facilitate purification and separation of the more soluble ammonium salt [HNHex3]Br from [1-H]Br. Deprotonation to the amino-substituted CDP 1 was tested with a set of different bases under varying reaction conditions. Best results were obtained with sodium or potassium hexamethyldisilazide (HMDS) at room temperature providing 1 as a yellow solid in yields of up to 94%. Similar to other bisylides and related ligands, 1 is highly sensitive towards air and moisture and is readily protonated by traces of water or any acidic protons including thoroughly dried THF. 1 is characterized by the absence of the signal for the proton at the PCP unit (δH = 2.07 ppm for [1-H]Br) in the 1H NMR spectrum and by a singlet at 12.5 ppm in the 31P{1H} NMR spectrum (δP = 39.7 ppm for [1-H]Br). The central carbon atom appears as triplet at δC = 14.5 ppm in the 31C NMR spectrum. This shift is comparable to other CDPs (e.g. δC = 10.8 ppm for Me3PCPMe3)13 and slightly down-field shifted compared to [1-H]Br (δC = 6.90 ppm). The 1JPC coupling constant of 172 Hz in 1 is larger than that reported for A14 thus indicating a higher s-character in the P–C bond of 1 and hence less stabilized lone pairs at the central carbon atom.
Single crystals of 1 (Scheme 1) and [1-H]Br (see ESI†) were grown by slow diffusion of pentane into concentrated solutions in diethyl ether. 1 crystallizes as a C2-symmetric molecule with a disorder of the piperidinyl and one phenyl substituent. The P–C1 bond length amounts to 1.632(2) Å and is thus considerably shorter than the one in precursor 1-HBr, but comparable to CDP A (Table 1). The bond shortening can be attributed to the increased electrostatic interaction within the P+–C2−–P+ linkage upon deprotonation. Interestingly, the P–C bond in BIPMTol and in related methandiides is slightly longer than that of 1 and other CDPs (Fig. 1).15–17 This can be explained by the lower positive charge at the iminophosphoryl moiety compared to the aminophosphonium group. Consistent with the two lone pairs of electrons at C1, 1 exhibits a bent P–C–P moiety with a large angle of 136.5(3)°.14,17
| [1-H]Br | 1 | CDP Aa |
BIPMTol
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
|
|
|---|---|---|---|---|
| a Values taken from ref. 14. b Values taken from ref. 15; bond lengths are average values. c At the BP86/def2-TZVP//BP86/def2-SVP level. | ||||
| δ P [ppm] | 39.7 | 12.5 | −2.5 | 15.3 |
| δ C [ppm], 1JPC [Hz] | 6.90, 143.5 | 14.5, 172.2 | 13.4, 130 | n.d. |
| C1–P [Å] | 1.7091(9) | 1.632(2) | 1.635(5) | 1.674(3) |
| CPh–P [Å] | 1.805(1) | 1.804(3) | 1.831(5) | 1.830(3) |
| 1.814(1) | 1.840(14) | |||
| P–N [Å] | 1.668(1) | 1.707(11) | — | 1.641(3) |
| P–C–P [°] | 115.8(1) | 136.5(3) | 131.7(3) | 131.5(2) |
| q C(NBO)c | n.d. | −1.44 | −1.38 | −1.28 |
| q N(NBO)c | n.d. | −0.83 | — | −0.95 |
Overall, bisylide 1, the parent CDP A and the dianionic BIPMTol show similar structural features. Surprisingly, the calculated charge (BP86/def2svp) at the central carbon atom is more negative in the neutral CDPs than in the dianionic methandiide. This is presumably the result of stronger negative hyperconjugation/back-bonding into the σ*(P–N) orbitals of BIPMTol compared to 1. Accordingly, the nitrogen atoms in the methandiide have higher negative charge than 1. This suggests that 1 is a stronger carbon but a weaker nitrogen donor than the BIPM ligands. Comparison of the NBO charges in 1 with those of A, suggests that 1 is a slightly stronger carbon donor.
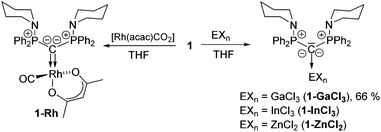
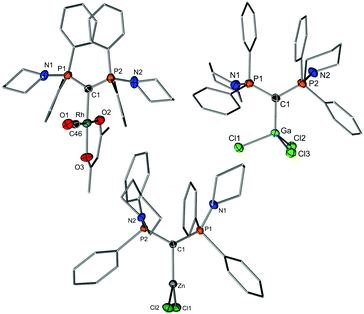
To further evaluate the donor strength of the CDP, we determined its Tolman electronic parameter (TEP). To this end, the Rh(acac)CO complex of 1 was synthesized and the C–O stretching frequency was recorded by IR spectroscopy (1921 cm−1), thus giving a TEP of 2036.5 cm−1.18 This value confirms that 1 is clearly more electron-rich than carbenes or phosphines and is in the range of other CDPs (see Table S1, ESI†).19 The Rh complex was also analyzed by XRD analysis (Fig. 3) thus confirming the expected coordination of the CDP ligand to the rhodium center. The Rh–C bond lengths (2.092(3) and 2.009(1) Å; two molecules in the asymmetric unit) are similar to the one found in an analogous NHC complex with IDipp (1.975 Å)20 and shorter than in the cyclometallated complex A-Rh(cod) with a metallated phenyl group (Rh–C: 2.202(3) Å).21 It should be noted that the transition metal chemistry of CDPs is surprisingly little studied. Except for group 10 and 11 complexes only few complexes have been reported, the majority with pyridine-substituted CDPs in which the coordination is supported by further donor sites.8,9,22
Since the correlation of the CO stretching frequencies with the donor strength of a ligand was found to be less reliable for strong donor ligands,23 we also probed the donor capacity of 1 by means of a further method. Gandon and coworkers showed that the pyramidalization of GaCl3 in the corresponding Lewis base adducts L·GaCl3 can also be used as measure for the donor strength.24 The stronger the donor properties of the ligand L, the higher the p-character in the Ga–Cl bond and hence the smaller the Cl–Ga–Cl angles. To determine the TEP value from the gallium complex, 1·GaCl3 was synthesized by mixing 1 with GaCl3 in THF and could be isolated in crystalline form in 66% yield. The NMR spectra showed patterns typical for a symmetric CDP coordination. The crystal structure (Fig. 3) confirms the coordination of the CDP ligand solely via the carbon center with a Ga–C bond length of 1.986(2) Å. This bond is shorter than the Ga–C bond in GaCl3 carbene complexes but comparable to those of other CDPs or carbodicarbenes.24 The sum of the Cl–Ga–Cl angles amounts to 313.31(2)°. This value is slightly lower than the one reported for the parent CDP A (315.40(3)°), thus making 1 a slightly stronger donor. Correlation with the TEP according to literature24 gives a TEP value of 2031.8 cm−1 for 1 thus confirming its high donor capacity already derived from the rhodium complex. This places 1 far beyond carbene ligands and in between CDP A and the 6-membered cyclic CDP [C3H6(Ph2P)2C] (see Table S1, ESI†).24
Both, the Rh and Ga complexes confirm the propensity of 1 to act as a strong C-donor. We further probed the coordination chemistry of 1 by reactions with a variety of metal halides to test, whether coordination of the ligand via the N-donor sites can be enforced either by increasing the size of the metals or the Lewis acidity. However, monitoring of the complexation reactions always showed 31P NMR signals at approx. 40 ppm (similar to 1-Rh and 1-GaCl3) and symmetric NMR patterns in the 1H and 13C NMR spectra, thus suggesting no coordination of the N-donors. This also holds true for YCl3 as well as lanthanide halides (NdCl3, CeI3), with which pincer-type complexes were readily formed with the BIPM ligands.25 Isolation of the complexes was often prevented by competing protonation of the ligand or the complexes, which readily react under protonative cleavage of the C–M bond. However, isolation was successful for the reaction of 1 with 1 equiv. of InCl3 (see ESI†) and ZnCl2 (Fig. 3). Both complexes could be isolated in crystalline form and structurally characterised.
As already suggested by the symmetric NMR patterns of these complexes in solution, no coordination of the N-donors is observed in the solid state. The remarkably low donor strength is impressively demonstrated by the structure of 1·ZnCl2, in which the zinc center adopts an unusual trigonal planar geometry (sum of angles: 359.9(1)°). This geometry is highly surprising, since zinc has a high preference for a 4-coordinate tetrahedral coordination environment and usually strongly binds to N-donors. To the best of our knowledge, no trigonal planar L·ZnCl2 complex has ever been reported. A survey of the CCDC database only yielded classical tetrahedral structures for C-, N-, O- and S-donors with ZnCl2. Here, either the coordination of a second donor ligand or dimerization via a bridging chlorido ligand was observed. For example, in case of NHC coordinated ZnCl2, dimeric complexes or monomers with additional coordinating solvent molecules were observed.26 Other trigonal planar geometries were only found in complexes with bulky anionic substituents (e.g. alkyl, aryl, OR or SR moieties).27 It is noteworthy, that in case of a BIPMH complex, N,N-coordination of ZnCl2 was reported,28 while a dimeric structure was observed with ZnMe2 and a cyclic CDP ligand.29 The C–Zn bond length of 1·ZnCl2 is 1.994(2) Å and thus slightly shorter than in NHC·ZnCl2(THF) (e.g. 2.045(4) with IDipp26c). The Zn–Cl distances of 2.222(1) and 2.227(1) Å are in the range of tetra-coordinate ZnCl2 structures thus indicating no lengthening as a consequence of a possible π donation from 1 to the zinc center.
To gain further insights into the electronic structure of 1-ZnCl2, we performed DFT calculations. The HOMO was found to be localized at the carbon atom and represents the π-symmetric lone pair at C1 (Fig. 4). No distinct polarization towards zinc is observed. The Zn–C σ-bond is represented by the HOMO−1 and HOMO−7, which are also strongly polarized towards C1. Consistently, NBO analysis yields two remaining lone pairs at C1 suggesting an overall highly electrostatic nature of the Zn–C interaction best described by a dative C → Zn interaction. Accordingly, the Wiberg bond index only amounts to 0.21. A C → Zn π-donation is not possible due to a non-coplanar arrangement of the PCP and ZnCl2 moiety (P1–C1–Zn–Cl2:125.3(1)°) which excludes any π-overlap. Calculations suggest that this arrangement is favoured over a planar arrangement by 23.4 kJ mol−1. This is presumably due to steric effects, minimizing the repulsion between the ZnCl2 moiety and the phenyl substituents. It is noteworthy that in the co-planar arrangement between ZnCl2 and the two P atoms, the C1 atom pyramidalizes, thus also preventing π-donation to the metal (see Fig. S28 and S29, ESI†). This bonding situation contrasts with the one found in the rhodium complex 1-Rh. Here, the π-symmetric orbital is delocalized over the metal and the co-ligands (Fig. S32 and S33, ESI†), indicating at least partial π-interaction. Accordingly, the WBI of the metal carbon bond is significantly higher (0.52) compared to the zinc complex. NBO analysis only shows a σ bond and a remaining lone pair at carbon, which however is involved in secondary interactions with orbitals at the metal and co-ligands. Comparison of both complexes 1-Rh and 1-ZnCl2, indicates that the CDP 1 can exhibit quite different bonding situations, but highly π-acidic metal centers are required to enforce the π-bonding.
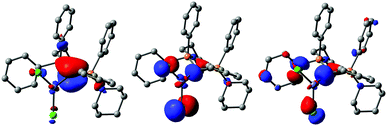 | ||
| Fig. 4 Plot of the HOMO (left), HOMO−1 (middle) and HOMO−7 (right) in 1·ZnCl2 at the BP86/def2-TZVP//BP86/def2-SVP level.; isosurface value 0.05. | ||
In conclusion, we reported on the preparation and isolation of a diamino-substituted carbodiphosphorane, which is closely related to the often employed dianionic BIPM pincer ligands. While DFT as well as experimental studies showed that 1 is an extremely strong C-donor, the donor ability of the nitrogen side-arms was found to be small. Thus, in contrast to the BIPM ligands, 1 was found to only bind to neutral metals and main group elements via the carbon atom. Several complexes could be isolated amongst the first trigonal planar ZnCl2 adduct. We are currently testing the coordination ability of 1 toward cationic species.
This work was supported by the European Research Council (Starting Grant No. 677749, YlideLigands) and the Fonds der Chemischen Industrie (fellowship to L. T. S.).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) F. Ramírez, N. B. Desai, B. Hansen and N. McKelvie, J. Am. Chem. Soc., 1961, 83, 3539 CrossRef; (b) A. T. Vincent and P. J. Wheatley, J. Chem. Soc., Dalton Trans., 1972, 617 RSC; (c) W. C. Kaska, D. K. Mitschell and R. F. Reichelderfer, J. Organomet. Chem., 1973, 47, 391 CrossRef CAS.
- (a) R. Tonner and G. Frenking, Chem. – Eur. J., 2008, 14, 3260 CrossRef CAS PubMed; (b) R. Tonner and G. Frenking, Chem. – Eur. J., 2008, 14, 3273 CrossRef CAS PubMed; (c) R. Tonner and G. Frenking, Angew. Chem., Int. Ed., 2007, 46, 8695 CrossRef CAS PubMed; (d) R. Tonner, F. Öxler, B. Neumüller, W. Petz and G. Frenking, Angew. Chem., Int. Ed., 2006, 45, 8038 CrossRef CAS PubMed.
- For reviews, see: (a) M. Alcarazo, Dalton Trans., 2011, 40, 1839 RSC; (b) W. Petz, Coord. Chem. Rev., 2015, 291, 1 CrossRef CAS.
- B. Inés, M. Patil, J. Carreras, R. Goddard, W. Thiel and M. Alcarazo, Angew. Chem., Int. Ed., 2011, 50, 8400 CrossRef PubMed.
- M. Y. T. Tay, Y. Lu, R. Ganguly and D. Vidović, Angew. Chem., Int. Ed., 2013, 52, 3132 CrossRef CAS PubMed.
- M. Alcarazo, C. Gomez, S. Holle and R. Goddard, Angew. Chem., Int. Ed., 2010, 49, 5788 CrossRef CAS PubMed.
- M. Alcarazo, K. Radkowski, G. Mehler, R. Goddard and A. Fürstner, Chem. Commun., 2013, 49, 3140 RSC.
- (a) W. Su, S. Pan, X. Sun, S. Wang, L. Zhao, G. Frenking and C. Zhu, Nat. Commun., 2018, 9, 1 CrossRef PubMed; (b) W. Su, S. Pan, X. Sun, L. Zhao, G. Frenking and C. Zhu, Dalton Trans., 2019, 48, 16108 RSC.
- M. Klein, X. Xie, O. Burghaus and J. Sundermeyer, Organometallics, 2019, 38, 3768 CrossRef CAS.
- S. Ullrich, B. Kovačević, B. Koch, K. Harms and J. Sundermeyer, Chem. Sci., 2019, 10, 9483 RSC.
- (a) R. Appel and K. Waid, Z. Naturforsch., B: Anorg. Chem., Org. Chem., 1981, 36B, 131 CAS; (b) R. Appel, U. Baumeister and F. Knoch, Chem. Ber., 1983, 116, 2275 CrossRef CAS.
- (a) V. H. Gessner, J. Becker and K.-S. Feichtner, Eur. J. Inorg. Chem., 2015, 1841 CrossRef CAS; (b) T. Cantat, N. Mézailles, A. Auffrant and P. Le Floch, Dalton Trans., 2008, 1957 RSC; (c) S. T. Liddle, D. P. Mills and A. J. Wooles, Chem. Soc. Rev., 2011, 40, 2164 RSC.
- O. Gasser and H. Schmidbaur, J. Am. Chem. Soc., 1975, 97, 6281 CrossRef CAS.
- (a) G. E. Hardy, J. I. Zink, W. C. Kaska and J. C. Baldwin, J. Am. Chem. Soc., 1978, 100, 8002 CrossRef; (b) M. Gruber, W. Bauer, H. Maid, K. Schöll and R. R. Tykwinski, Inorg. Chim. Acta, 2017, 468, 152 CrossRef CAS.
- K.-S. Feichtner and V. H. Gessner, Dalton Trans., 2014, 43, 14399 RSC.
- (a) A. Kasani, R. P. Kamalesh Babu, R. McDonald and R. G. Cavell, Angew. Chem., Int. Ed., 1999, 38, 1483 CrossRef CAS; (b) C. M. Ong and D. W. Stephan, J. Am. Chem. Soc., 1999, 121, 2939 CrossRef CAS; (c) M. Demange, L. Boubekeur, A. Auffrant, N. Mézailles, L. Ricard, X. Le Goff and P. Le Floch, New J. Chem., 2006, 30, 1745 RSC.
- Note that for CDP A a series of different structures with varying P-C-P angles have been reported. See: (a) P. J. Quinlivan and G. Parkin, Inorg. Chem., 2017, 56, 5493 CrossRef CAS PubMed; (b) A. T. Vincent and P. J. Wheatley, J. Chem. Soc., Dalton Trans., 1972, 617 RSC.
- L. Chen, P. Ren and B. P. Carrow, J. Am. Chem. Soc., 2016, 138, 6329 Search PubMed.
- R. Tonner and G. Frenking, Organometallics, 2009, 28, 3901 CrossRef CAS.
- M. S. Datt, J. J. Nair and S. Otto, J. Organomet. Chem., 2005, 690, 3422 CrossRef CAS.
- K. Kubo, N. D. Jones, M. J. Ferguson, R. McDonald and R. G. Cavell, J. Am. Chem. Soc., 2005, 127, 5314 CrossRef CAS PubMed.
- J. Sundermeyer, K. Weber, K. Peters and H. G. von Schnering, Organometallics, 1994, 13, 2560 CrossRef CAS.
- D. Cremer and E. Kraka, Dalton Trans., 2017, 46, 8323 RSC.
- A. El-Hellani, J. Monot, S. Tang, R. Guillot, C. Bour and V. Gandon, Inorg. Chem., 2013, 52, 11493 CrossRef CAS PubMed.
- For example: (a) M. Gregson, E. Lu, J. McMaster, W. Lewis, A. J. Blake and S. T. Liddle, Angew. Chem., Int. Ed., 2013, 52, 13016 CrossRef CAS PubMed; (b) S. T. Liddle, J. McMaster, J. C. Green and P. L. Arnold, Chem. Commun., 2008, 1747 RSC; (c) D. P. Mills, L. Soutar, W. Lewis, A. J. Blake and S. T. Liddle, J. Am. Chem. Soc., 2010, 132, 14379 CrossRef CAS PubMed; (d) A. Buchard, A. Auffrant, L. Ricard, X. F. Le Goff, R. H. Platel, C. K. Williams and P. Le Floch, Dalton Trans., 2009, 10219 RSC.
- (a) S. Naumann, F. G. Schmidt, W. Frey and M. R. Buchmeiser, Polym. Chem., 2013, 4, 4172 RSC; (b) C. Fliedel, S. Mameri, S. Dagorne and T. Avilés, Appl. Organometal. Chem., 2014, 28, 504 CrossRef CAS; (c) A. Doddi, C. Gemel, R. W. Seidel, M. Winter and R. A. Fischer, Polyhedron, 2013, 52, 1103 CrossRef CAS.
- (a) A. J. Arduengo III, H. V. R. Dias, F. Davidson and R. L. Harlow, J. Organomet. Chem., 1993, 462, 13 CrossRef; (b) K. Thiele, H. Görls and W. Seidel, Z. Anorg. Allg. Chem., 1998, 624, 555 CrossRef CAS; (c) D. J. Darensbourg, S. A. Niezgoda, J. D. Draper and J. H. Reibenspies, Inorg. Chem., 1999, 38, 1356 CrossRef CAS PubMed; (d) M. Bochmann, G. Bwembya, R. Grinter, J. Lu, K. J. Webb, D. J. Williamson, M. B. Hursthouse and M. Mazid, Inorg. Chem., 1993, 32, 532 CrossRef CAS; (e) M. Bochmann, K. Webb, M. Harman and M. B. Hursthouse, Angew. Chem., Int. Ed. Engl., 1990, 29, 638 CrossRef; (f) M. Bochmann, K. Webb, M. B. Hursthouse and M. Mazid, J. Chem. Soc., Dalton Trans., 1991, 2317 RSC.
- S. Marks, T. K. Panda and P. W. Roesky, Dalton Trans., 2010, 39, 7230 RSC.
- H. Schmidbaur, T. Costa and B. Milewski-Mahrla, Chem. Ber., 1981, 114, 1428 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Full details and references for synthetic, spectroscopic, crystallographic and computational studies. CCDC 1993242–1993247. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cc02496a |
| This journal is © The Royal Society of Chemistry 2020 |