Enhanced catalytic activity of copper complexes in microgels for aerobic oxidation of benzyl alcohols†
Abstract
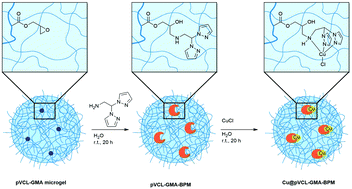
Catalytically active copper bis(pyrazolyl)methane complexes have been anchored into pVCL-GMA microgels on specified positions within the microgel network. Functionalized microgels act as nanoreactors providing a tailored environment and stabilization for the copper complexes thus increasing the product yield. The oxidation of benzyl alcohols to their respective aldehydes was chosen as a test reaction to show the enhancement of catalytic activity due to the immobilization of the copper complex compared to the copper salt and the molecular copper complex.


 Please wait while we load your content...
Please wait while we load your content...