Open Access Article
Open Access ArticleMulti-modal control over the assembly of a molecular motor bola-amphiphile in water†
Fan
Xu
,
Lukas
Pfeifer
 ,
Marc C. A.
Stuart
,
Marc C. A.
Stuart
 ,
Franco King-Chi
Leung‡
,
Franco King-Chi
Leung‡
 * and
Ben L.
Feringa
* and
Ben L.
Feringa
 *
*
Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG, Groningen, The Netherlands. E-mail: b.l.feringa@rug.nl; kingchifranco.leung@polyu.edu.hk
First published on 14th May 2020
Abstract
We report multi-modal-control over the assembly behaviour of a first-generation molecular motor bola-amphiphile in water by light, pH and the choice of counter-ions. These findings open up opportunities for the development of materials that reconfigurate enabling complex functions in response to different stimuli.
The variety of noncovalent interactions between assembled units found in nature provides a basis for designing artificial supramolecular systems.1 The intrinsic dynamics, tunability of properties and stimuli responsiveness of synthetic supramolecular systems is controlled by the precise design of organic molecules,2 mesogenic materials,3 polymers,4 and a variety of organic–inorganic hybrid composites,5 allowing modulation of, for example, assembly and functionality.6 Recent advances in supramolecular chemistry and soft materials design have enabled the creation of various supramolecular systems responsive to external stimuli,7 especially in aqueous media.1a,h,2a,6d Encoding specific responses to different stimuli into self-assembling systems is a promising approach to achieve more complex dynamic and adaptive behaviour.8 Besides making use of multi-stimuli responsive polymers4a,8 or responsive organic–inorganic composites,5,9 it has also been demonstrated that supramolecular assemblies of small organic molecules in water can be controlled independently by external stimuli like light,10 pH,11 and ions.12 Notably, a few of these systems can be controlled by various external stimuli simultaneously.13 For example, the supramolecular assemblies of polyanionic dendritic peptide amphiphiles, reported by Besenius et al.,13b were controlled systematically by pH and ionic strength. Among the various stimuli, light offers a non-invasive way with high spatial as well as temporal precision for manipulating the structures of molecular assemblies.6c,14 However, implementing responsiveness to other stimuli into photo-responsive supramolecular systems based on organic molecules13c to realize multi-modal control over their assembly in water remains highly challenging.
Taking advantage of rotary motors as light-driven multistate switches, we have previously developed a co-assembling supramolecular system, composed of a second-generation molecular motor and a lipid (1,2-dioleoyl-sn-glycero-3-phosphocholine), allowing upon irradiation,15 transformations from nanotubes into vesicles and multi-lamellar vesicles (in a fully reversible cycle) whereby comparable packing parameters were identified for both structures (1/2 < P < 1).16 In the present study, multi-modal control over the self-assembly morphology of a first-generation molecular motor is reported using light, pH and ions as triggering elements. This class of molecular motors offers advantages due to the distinct geometric differences between cis- and trans-isomer, leading to more pronounced changes of the packing parameter upon irradiation, and the absence of thermal back-isomerization. In the present study, the morphology of the molecular motor bola-amphiphile (MBA) aggregates could be switched between sheet-like structures and a mixture of micelles and vesicles upon irradiation (Scheme 1). By contrast, morphology transitions from sheet-like assemblies to discs and ultimately micelles were found with increasing pH. Moreover, the addition of NaCl resulted in the formation of vesicles, while CaCl2 led to macroscopic aggregates.
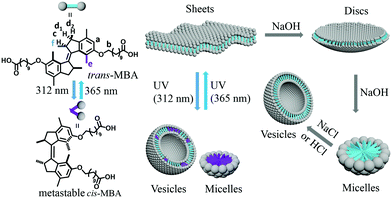 | ||
| Scheme 1 Schematic illustration of the photo-isomerization of MBA and multi-modal control over its assembly behaviour. | ||
The structures of trans- and cis-isomers of MBA are shown in Scheme 1. Although the rotation of first-generation motors passes through four states,17a the metastable trans-isomer is not discussed in this study due to its short half-life (t1/2 < 30 s) at room temperature.17b Hence, trans-MBA in this paper refers to the stable trans-isomer. Stable trans- and metastable cis-isomer could be interconverted by irradiation with 312 nm and 365 nm light, respectively, while the stable cis-isomer could be obtained through thermal helix inversion from the metastable cis-isomer with a half-life of ∼20 h.17a,c The stable and metastable cis-isomer are expected to have the same assembly morphology, due to their related geometry. Therefore, we mainly focus on the photo-induced morphology transition between stable trans-MBA and metastable cis-MBA in the present study. Two carboxylic acid groups are connected to the motor core through alkyl-linkers whose relative orientation significantly changes upon cis–trans isomerization, potentially leading to distinct differences in self-assembly behaviour. The carboxylic acid functionality has recently also been used as the end group on a motor amphiphile forming electrostatic interactions with counter-ions, such as Ca2+ to allow the formation of macroscopic string micro-actuators.10b,18a In the present study, we use this group as a cation binding as well as pH-responsive unit to gain additional handles for multi-modal control over MBA's assembly behaviour in addition to photochemical cis–trans isomerization.
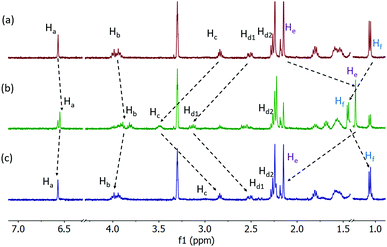
The synthesis and characterization of trans-MBA and stable cis-MBA are summarized in Scheme S1 (ESI†). The photo-responsive behaviour was studied by UV/Vis absorption and 1H NMR spectroscopy. Irradiation of the trans-isomer with 312 nm light resulted in a decrease of the absorption bands at 286 nm and 312 nm with concomitant formation of a new band around 350 nm, indicating the formation of the metastable cis-isomer (Fig. S1a, ESI†). After irradiating for 18 min, no further changes were observed, meaning that the photostationary state (PSS) had been reached. Subsequent irradiation with 365 nm light induced the opposite spectral changes caused by the back-isomerization to trans-MBA (Fig. S1b, ESI†). An isosbestic point at 329 nm was observed during both irradiation processes, confirming the clean formation of metastable cis- and trans-isomer, respectively. As shown in the 1H NMR spectra in Fig. 1, irradiation of a sample of trans-MBA with 312 nm light induced the formation of a new set of signals (e.g. Ha = 6.55 ppm, He = 1.21 ppm) belonging to the metastable cis-isomer. The cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans ratio at PSS was found to be 63
trans ratio at PSS was found to be 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37. Subsequent irradiation at 365 nm led to full recovery of the initial spectrum, demonstrating that the cis–trans isomerization of MBA can be precisely controlled by light.
37. Subsequent irradiation at 365 nm led to full recovery of the initial spectrum, demonstrating that the cis–trans isomerization of MBA can be precisely controlled by light.
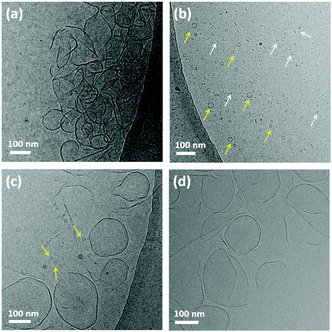
To investigate the critical aggregation concentration (CAC) of trans-MBA a Nile Red fluorescence assay (NRFA), which probes the internal hydrophobicity of assemblies,18 was performed. The results revealed a CAC of 4.0 × 10−6 M for trans-MBA (Fig. S2a, ESI†). The structure of aggregates of trans-MBA in water was imaged using cryogenic transmission electron microscopy (cryo-TEM) to accurately capture their solution-state morphology (see ESI†). Since self-assembly of trans-MBA is sensitive to pH and ionic strength (vide infra), irradiation experiments were carried out in sodium borate buffer (pH = 9.3, 0.1 M). As shown in Fig. 2a, trans-MBA was found to form sheet-like assemblies (P ≈ 1). After irradiating with 312 nm light for 10 min to form metastable cis-MBA, vesicles with a diameter of around 20 nm and micelles with 5–6 nm diameter were observed in addition to sheet-like structures (Fig. 2b). As a comparison, we studied the aggregate morphology of stable cis-MBA, whose CAC (1.0 × 10−5 M, Fig. S2b, ESI†) was distinct from that of trans-MBA (4.0 × 10−6 M), implying possible different assembly morphologies between trans- and cis-isomers. Indeed, the stable cis-isomer showed the formation of micelles with 5–6 nm diameter (P ≤ 1/3) (Fig. S4, ESI†). Due to its related geometry, metastable cis-MBA is expected to have the same assembly structure as stable cis-MBA. Therefore, the presence of micelles in the sample of trans-MBA after irradiation (mixture with metastable cis-MBA) can be attributed to the self-assembly of the metastable cis isomer. The formation of vesicles (1/2 < P < 1), we tentatively assigned to the co-assembly of both stable trans- and metastable cis-molecules. The remaining sheet-like assemblies (Fig. S5, ESI†) hint at an excess of self-assembling trans-MBA present in the mixture. This was supported by 1H NMR measurement revealing a 6% share of metastable cis-MBA in the mixture (Fig. S7b, ESI†). Upon subsequent irradiation with 365 nm light for 10 min (metastable cis-MBA to trans-MBA isomerization), the original sheet-like assemblies were recovered, albeit with some small vesicles remaining in the mixture (Fig. 2c). 1H NMR, however, revealed that there was no remaining cis-MBA (Fig. S7c, ESI†). This phenomenon had also been observed in earlier studies in our group.15 It was found that molecular motors could recover their original assembly morphologies only upon being subjected to conditions allowing them to reorganize. We therefore facilitated reorganisation by repeating the assembly preparation process (heating–cooling cycle) using the sample shown in Fig. 2c. Indeed, after this treatment, only sheet-like structures were observed (Fig. 2d).
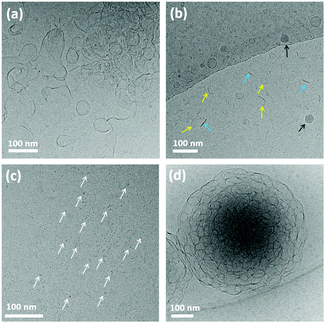
Besides reversible control by irradiation, the assembly structure of trans-MBA also showed significant changes upon altering pH. As shown in Fig. 3, trans-MBA formed sheet-like structures (P ≈ 1) at pH 8.8 (Fig. 3a) and disc-like structures (1/2 < P < 1) with a diameter of 30–40 nm and a thickness of 2–3 nm at pH 9.8 (Fig. 3b), as well as tiny micelles (P ≤ 1/3) with a diameter of 4–5 nm at pH 11 (Fig. 3c). Therefore, as pH goes up structures with increasingly smaller packing parameters are formed, which we attribute to the increasing concentration of deprotonated molecules at higher pH of the solution. It has been demonstrated that the head group area of long-chain fatty acids increases significantly upon dissociation of the carboxylic acid group.19 Thus, the ratio of molecules with larger head group area rises with increasing pH. Based on the equation of molecular packing parameters for amphiphiles,16 this increase of head group area leads to a decrease of the packing parameter, causing the self-assembly to change from sheets to micelles. In our case, the morphology transformations of trans-MBA were also in accordance with this analysis. Interestingly, when trace amounts of an aq. HCl stock solution (0.1 M) were added to the sample at pH 11 to decrease the pH to 9.4, the morphology changed from micelles to vesicles and sponge-like structures as congeries of vesicles (Fig. 3d). In this case, however, both pH as well as the concentration of NaCl were altered and the observed transformation of morphology could be the result of changing either one or both of these conditions.
In order to explore a possible new handle for control over the assembly morphologies of trans-MBA, the counter-ion effect of Na+, K+, Ca2+, and Zn2+ on the self-assembly structures formed by trans-MBA was studied by Nile Red fluorescence assay. Therefore, the blue shift of Nile Red in trans-MBA dicarboxylate solution (5.0 × 10−6 M) was determined in the presence of various concentrations of the chloride salts (1.0 × 10−6–5.0 × 10−1 M) (Fig. S3, ESI†). A gradual blue shift was observed with enhanced concentrations of NaCl, KCl, ZnCl2 and CaCl2, reflecting an increase of the internal hydrophobicity of the trans-MBA assemblies. Interestingly, the blue shift induced by increasing the concentration of CaCl2 was significantly larger compared to other chloride salts, indicating that Ca2+ ions induce aggregate formation of trans-MBA more effectively.18a Based on the results of NRFA, Ca2+ and Na+ were chosen for additional studies of the control of assembly morphology. Vesicles with diameters of 100–200 nm were observed in aq. 0.1 M NaCl solution (Fig. 4a) contrasting with the micelles observed in samples at high pH discussed earlier. The addition of NaCl likely reduces the repulsion between the deprotonated head groups due to charge screening.19 As a consequence, the decrease in head group area results in an increase of the packing parameter of MBA leading to the observed change in assembly structure. However, in 0.1 M aq. CaCl2 solution trans-MBA formed macroscopic precipitates. In order to study the assembly structure in more detail, a sample of trans-MBA dicarboxylate at a lower CaCl2 concentration (5.0 × 10−3 M) was characterized by cryo-TEM; micrometre-sized aggregates were observed as shown in Fig. 4b. The presence of Ca in these aggregates was confirmed by EDX analysis (Fig. S6, ESI†). The preferential induction of aggregate formation observed for Ca2+ may be related to the stronger interaction of Ca2+ with carboxylate groups.18a
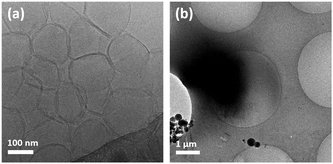 | ||
| Fig. 4 Cryo-TEM images of trans-MBA dicarboxylate (7.0 × 10−3 M) in (a) 0.1 M NaCl solution and (b) 5.0 × 10−3 M CaCl2 solution. | ||
In summary, we realized multi-modal control over the assembly behaviour of a first-generation molecular motor in water. Transitions between sheet-like structures and a mixture of micelles and vesicles, indicating large changes in packing parameter, could be controlled by light in a reversible process. Dramatic changes of assembly morphology from sheet-like assemblies to discs and ultimately micelles were achieved by adjusting pH. Furthermore, the addition of NaCl led to the formation of vesicles, while CaCl2 induced macroscopic aggregates. To the best of our knowledge, this is the first example of multi-responsive assembly of a molecular motor-based bola-amphiphile in water. This study significantly enhances our understanding of the self-assembly behaviour of multi-responsive systems in water and thereby paves the way to future applications like adaptive materials, delivery systems or supramolecular materials capable to perform various distinct tasks.
This work was financially supported by the Netherlands Organization for Scientific Research (NWO-CW), the European Research Council (ERC; advanced grant no. 694345 to B. L. F.), the European Commission (MSCA-IF No. 793082 to L. P.), the Dutch Ministry of Education, Culture and Science (Gravitation program no. 024.001.035), the Chinese Scholarship Council (CSC; 201707040064) and the Croucher Foundation (Croucher Postdoctoral Fellowship Startup Allowance to F. K. C. L).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) J. M. Lehn, Supramol. Chem., John Wiley & Sons, Weinheim, 1995 Search PubMed; (b) T. Aida, E. W. Meijer and S. I. Stupp, Science, 2012, 335, 813–817 CrossRef CAS PubMed; (c) E. Krieg, M. M. C. Bastings, P. Besenius and B. Rybtchinski, Chem. Rev., 2016, 116, 2414–2477 CrossRef CAS PubMed; (d) E. Mattia and S. Otto, Nat. Nanotechnol., 2015, 10, 111–119 CrossRef CAS PubMed; (e) L. Voorhaar and R. Hoogenboom, Chem. Soc. Rev., 2016, 45, 4013–4031 RSC; (f) F. Würthner, C. R. Saha-Möller, B. Fimmel, S. Ogi, P. Leowanawat and D. Schmidt, Chem. Rev., 2016, 116, 962–1052 CrossRef PubMed; (g) T. F. A. De Greef, M. M. J. Smulders, M. Wolffs, A. P. H. J. Schenning, R. P. Sijbesma and E. W. Meijer, Chem. Rev., 2009, 109, 5687–5754 CrossRef CAS PubMed; (h) B. N. S. Thota, L. H. Urner and R. Haag, Chem. Rev., 2016, 116, 2079–2102 CrossRef CAS PubMed.
- (a) L. L. K. Taylor, I. A. Riddell and M. M. J. Smulders, Angew. Chem., Int. Ed., 2019, 58, 1280–1307 CrossRef CAS PubMed; (b) J. Raeburn, A. Z. Cardoso and D. J. Adams, Chem. Soc. Rev., 2013, 42, 5143–5156 RSC.
- (a) R. Eelkema and B. L. Feringa, Org. Biomol. Chem., 2006, 4, 3729–3745 RSC; (b) T. Kato, J. Uchida, T. Ichikawa and B. Soberats, Polym. J., 2018, 50, 149–166 CrossRef CAS; (c) D. B. Amabilino, D. K. Smith and J. W. Steed, Chem. Soc. Rev., 2017, 46, 2404–2420 RSC.
- (a) F. D. Jochum and P. Theato, Chem. Soc. Rev., 2013, 42, 7468–7483 RSC; (b) X. Yan, F. Wang, B. Zheng and F. Huang, Chem. Soc. Rev., 2012, 41, 6042–6065 RSC.
- M. Grzelczak, L. M. Liz-Marzán and R. Klajn, Chem. Soc. Rev., 2019, 48, 1342–1361 RSC.
- (a) O. J. G. M. Goor, S. I. S. Hendrikse, P. Y. W. Dankers and E. W. Meijer, Chem. Soc. Rev., 2017, 46, 6621–6637 RSC; (b) K. Sato, M. P. Hendricks, L. C. Palmer and S. I. Stupp, Chem. Soc. Rev., 2018, 47, 7539–7551 RSC; (c) E. Busseron, Y. Ruff, E. Moulin and N. Giuseppone, Nanoscale, 2013, 5, 7098–7140 RSC; (d) X. Ma and H. Tian, Acc. Chem. Res., 2014, 47, 1971–1981 CrossRef CAS PubMed; (e) T. Muraoka and K. Kinbara, Chem. Commun., 2016, 52, 2667–2678 RSC; (f) C. Heinzmann, C. Weder and L. M. de Espinosa, Chem. Soc. Rev., 2016, 45, 342–358 RSC.
- (a) C. D. Jones and J. W. Steed, Chem. Soc. Rev., 2016, 45, 6546–6596 RSC; (b) B. L. Feringa and W. R. Browne, Molecular Switches, Wiley-VCH, Weinheim, 2011 CrossRef.
- J. Zhuang, M. R. Gordon, J. Ventura, L. Li and S. Thayumanavan, Chem. Soc. Rev., 2013, 42, 7421–7435 RSC.
- (a) O. Chovnik, R. Balgley, J. R. Goldman and R. Klajn, J. Am. Chem. Soc., 2012, 134, 19564–19567 CrossRef CAS PubMed; (b) J. H. Schenkel, A. Samanta and B. J. Ravoo, Adv. Mater., 2014, 26, 1076–1080 CrossRef CAS PubMed.
- (a) W. A. Velema, M. C. A. Stuart, W. Szymanski and B. L. Feringa, Chem. Commun., 2013, 49, 5001–5003 RSC; (b) J. Chen, F. K. C. Leung, M. C. A. Stuart, T. Kajitani, T. Fukushima, E. van der Giessen and B. L. Feringa, Nat. Chem., 2018, 10, 132–138 CrossRef CAS PubMed.
- (a) T. J. Moyer, J. A. Finbloom, F. Chen, D. J. Toft, V. L. Cryns and S. I. Stupp, J. Am. Chem. Soc., 2014, 136, 14746–14752 CrossRef CAS PubMed; (b) B. F. Lin, K. A. Megley, N. Viswanathan, D. V. Krogstad, L. B. Drews, M. J. Kade, Y. Qian and M. V. Tirrell, J. Mater. Chem., 2012, 22, 19447–19454 RSC.
- S. Shin, S. Lim, Y. Kim, T. Kim, T. Choi and M. Lee, J. Am. Chem. Soc., 2013, 135, 2156–2159 CrossRef CAS PubMed.
- (a) H. A. M. Ardona and J. D. Tovar, Chem. Sci., 2015, 6, 1474–1484 RSC; (b) M. Von Gröning, I. de Feijter, M. C. A. Stuart, I. K. Voets and P. Besenius, J. Mater. Chem. B, 2013, 1, 2008–2012 RSC; (c) F. K. C. Leung, T. Kajitani, M. C. A. Stuart, T. Fukushima and B. L. Feringa, Angew. Chem., Int. Ed., 2019, 8503, 10985–10989 CrossRef PubMed; (d) E. Krieg, E. Shirman, H. Weissman, E. Shimoni, S. G. Wolf, I. Pinkas and B. Rybtchinski, J. Am. Chem. Soc., 2009, 131, 14365–14373 CrossRef CAS PubMed.
- S. Yagai and A. Kitamura, Chem. Soc. Rev., 2008, 37, 1520–1529 RSC.
- D. J. van Dijken, J. Chen, M. C. A. Stuart, L. Hou and B. L. Feringa, J. Am. Chem. Soc., 2016, 138, 660–669 CrossRef CAS PubMed.
- J. N. Israelachvili, S. Marcelja, R. G. Horn and J. N. Israelachvili, Q. Rev. Biophys., 1980, 13, 121–200 CrossRef CAS PubMed.
- (a) T. van Leeuwen, G. H. Heideman, D. Zhao, S. J. Wezenberg and B. L. Feringa, Chem. Commun., 2017, 53, 6393–6396 RSC; (b) M. M. Pollard, A. Meetsma and B. L. Feringa, Org. Biomol. Chem., 2008, 6, 507–512 RSC; (c) D. Zhao, T. van Leeuwen, J. Cheng and B. L. Feringa, Nat. Chem., 2016, 9, 250–256 CrossRef PubMed.
- (a) F. K. C. Leung, T. van den Enk, T. Kajitani, J. Chen, M. C. A. Stuart, J. Kuipers, T. Fukushima and B. L. Feringa, J. Am. Chem. Soc., 2018, 140, 17724–17733 CrossRef CAS PubMed; (b) F. Tantakitti, J. Boekhoven, X. Wang, R. V. Kazantsev, T. Yu, J. Li, E. Zhuang, R. Zandi, J. H. Ortony, C. J. Newcomb, L. C. Palmer, G. S. Shekhawat, M. O. de la Cruz, G. C. Schatz and S. I. Stupp, Nat. Mater., 2016, 15, 469–476 CrossRef CAS PubMed.
- Y. Yan, W. Xiong, X. Li, T. Lu, J. Huang, Z. Li and H. Fu, J. Phys. Chem. B, 2007, 111, 2225–2230 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0cc02177f |
| ‡ Present address: State Key Laboratory of Chemical Biology & Drug Discovery, Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Hong Kong, China. |
| This journal is © The Royal Society of Chemistry 2020 |