Surfactant-free synthesis of ultralong silver nanowires for durable transparent conducting electrodes†
Abstract
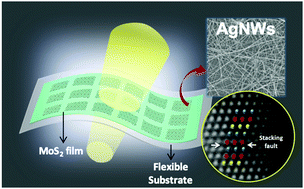
The present study employed the surfactant-free growth of ultralong (∼50 μm) silver nanowires (AgNWs) with a high aspect ratio (more than 1000) by galvanic replacement. AgNW conducting films were fabricated as electrodes using drop-casting. The AgNW film had 90% transmittance to 550 nm light with a sheet resistance of 232 Ω sq−1. Further, the flexibility test of the transparent flexible AgNW/MoS2 device array indicated that the variation of the current was within 5% when a strain of 0.5% was applied to the device; additionally, the device showed a 13% decrease in current after experiencing 50 000 bending cycles. This study indicated that the ultralong AgNWs synthesized using galvanic replacement have potential for applications as transparent and flexible electrodes.


 Please wait while we load your content...
Please wait while we load your content...