Open Access Article
Open Access ArticleCrosslinkable polymeric contrast agent for high-resolution X-ray imaging of the vascular system†
Ngoc An
Le‡
 a,
Willy
Kuo‡
a,
Willy
Kuo‡
 bcd,
Bert
Müller
bcd,
Bert
Müller
 d,
Vartan
Kurtcuoglu
d,
Vartan
Kurtcuoglu
 *bc and
Bernhard
Spingler
*bc and
Bernhard
Spingler
 *a
*a
aDepartment of Chemistry, University of Zurich, 8057 Zurich, Switzerland. E-mail: spingler@chem.uzh.ch
bInstitute of Physiology, University of Zurich, 8057 Zurich, Switzerland. E-mail: vartan.kurtcuoglu@uzh.ch
cNational Centre of Competence in Research, Kidney.CH, 8057 Zurich, Switzerland
dBiomaterials Science Center, Department of Biomedical Engineering, University of Basel, 4123 Allschwil, Switzerland
First published on 27th April 2020
Abstract
A contrast agent for X-ray micro computed tomography (μCT), called XlinCA, that combines reliable perfusion and permanent retention and contrast properties, was developed for ex vivo imaging. The new imaging agent XlinCA features high molecular weight, low viscosity and a high iodine content.
Qualitative and quantitative assessment of vascular physiology, pathology and angiogenesis under various circumstances such as cancer, myocardial infarction, stroke, atherosclerosis, vasculitis, and inflammation requires accurate three-dimensional (3D) structural data.1 A large number of different 3D imaging techniques can provide morphometric parameters including vessel volume, connectivity, number, thickness, thickness distribution, separation, and degree of anisotropy.1b,d,2 These parameters are not only useful for investigating diseases that affect the vasculature, but are also crucial for proper evaluation of pro- and anti-angiogenic therapies in preclinical models.3
Micro computed tomography (μCT) allows for non-destructive 3D imaging with isotropic quality and micrometer resolution. The geometric magnification used in cone-beam μCT allows for continuously variable effective pixel sizes and for hierarchical imaging on low-resolution animal scale and high-resolution organ scale in a single device.4 Whole small animals and organs can be imaged in their entirety in their native hydrated state, minimizing sample distortion through sample preparation artefacts, dehydration, realignment artefacts or optical distortion.
Ex vivo high-resolution X-ray imaging is not limited by movement during respiratory and cardiac cycles,5 anaesthetic tolerance or the dose of ionizing radiation.6 In addition, organs can be extracted and imaged at smaller fields of view, resulting in a corresponding increase of resolution in cone-beam μCT. The density difference between blood and soft tissue is, however, too small to be captured with standard absorption contrast using laboratory sources. Radiopaque X-ray contrast agents featuring heavy atomic elements have to be injected into the vasculature to provide the necessary contrast.7
Currently available contrast agents have various limitations when used for ex vivo vascular imaging. Standard clinical angiography contrast agents, such as iopamidol and iohexol, are small molecular compounds capable of passing through blood vessel walls, which also leads to a loss of contrast between the vascular lumen and the surrounding tissue within a few minutes.8 This issue can be addressed by the use of blood pool contrast agents, which are large nanoparticles or polymeric compounds that can achieve vascular circulation times on the scale of hours.9 In an ex vivo setting, blood pool contrast agents tend to sediment and aggregate, and can slowly leak out of the vasculature as well.10 These limitations make both types of angiography contrast agents unsuitable for capillary resolution ex vivo vascular imaging.
For such application, the current gold standard consists of vascular casting with plastic resins, e.g. Microfil (Microfil, Flow Tech, Carver, MA), PU4ii (vasQtec, Zurich, Switzerland) and μAngiofil (Fumedica AG, Muri, Switzerland).11 These hydrophobic resins are incapable of passing through the hydrated endothelial cell layer and polymerize after injection. They are thus permanently retained within the vasculature and provide excellent contrast. Perfusion of these resins is, however, technically challenging. The plastic resin may polymerize prematurely before the vasculature has been fully perfused, leading to incompletely filled blood vessels. Water inclusions and gas bubble formation are frequently occurring artefacts that cause disconnected vessel segments.11a,12 Well-optimized injection techniques, closure of blood vessels via ligation in order to divert all flow to the organ of interest and high perfusion pressures are, therefore, required to obtain consistent perfusion results.11a While some of these problems can be attributed to the high viscosity of the polymerizing plastic resins, they are inherent limitations shared by hydrophobic casting materials.
While plastic resins can provide reasonable vascular filling, extensive practice is required to reliably prevent frequent sample preparation failures and incomplete vessel filling.13 Reliable vessel filling is, however, absolutely required for the quantitative characterization of pathological processes and the comparison of vascular phenotypes, as the resulting structural data is otherwise dominated by sample preparation artefacts and not representative of the true vascular structure.
In this paper, we address the shortcomings of current X-ray contrast agents in high resolution ex vivo imaging of the vascular network with μCT. We have developed a crosslinkable polymeric contrast agent, XlinCA, that combines the simple and reliable perfusion of the hydrophilic angiography contrast agents with the permanent retention and high contrast of the hydrophobic vascular casting resins. We demonstrate its application for capillary-level imaging of a brain hemisphere and vascular imaging of an entire mouse with a non-optimized transcardial perfusion. XlinCA was designed to fulfil a specific set of criteria to resolve issues encountered in ex vivo vascular imaging with current contrast agents:
- Highly water-soluble, so as to avoid incomplete vascular filling and interrupted vessel segments.
- Higher molecular weight than 65 kDa, for the prevention of immediate leakage through blood vessel walls.
- Crosslinkable with free primary amine groups as targets for glutaraldehyde fixation, which enables crosslinking via imine formation,14 in order to prevent leakage over time.
- High X-ray attenuation coefficient with a high iodine content,15 for reducing the required contrast agent concentration, viscosity and osmolarity.
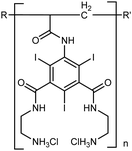
After considering all the above criteria, we designed the crosslinkable polymeric contrast agent XlinCA represented schematically in Fig. 1. The theoretical iodine content is 49.5 m/m%, which is comparable to standard small molecule angiography iodine contrast agents and considerably higher than what could be achieved by other typical approaches, such as linkage to polyethylene glycol (PEG).16
The contrast agent was synthesized through a multi-step process, see Fig. S1 (ESI†). Starting from the commercially available 5-amino-2,4,6-triiodoisophthalic acid 1, the acryloyl group was added through reaction with acrylic anhydride and a catalytic amount of sulfuric acid. The key step in this synthesis was the polymerization of 2 to give polymer 3 by reversible addition–fragmentation chain transfer (RAFT) polymerization.17 Trithiocarbonate 7 was chosen as RAFT agent for polymerization of 2 due to the reported compatibility with various acrylamide derivatives.18 We found that dimethylformamide (DMF) is the most suitable solvent for RAFT polymerization of 2. It provides high solubility of both starting materials as well as polymeric products and allows a high degree of conversion of the monomers. The optimal reaction temperature was 70 °C. The concentrations of monomer, RAFT agent and AIBN as radical initiator were optimized (Table S2, ESI†). The average molecular weight of the synthesized polymer increases with the ratio of monomer to RAFT agent.17d The optimal ratio of monomer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RAFT agent
RAFT agent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AIBN was found to be 400
AIBN was found to be 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Lower ratios of RAFT and AIBN to 2 led to higher conversion degrees, but lowered the molecular weight of 3, while higher ratios led to precipitates of both starting material and product. The molecular weight of 3 could not be measured with gel permeation chromatography (GPC) even in warm DMF due to low solubility and the tendency of the polymer towards aggregation. However, the molecular weight and polydispersity index of 3 could be estimated indirectly from the GPC result of the final product 6, which is water soluble and stable against aggregation. Based on the ratio of the molecular weights of the monomers and the average molecular weight of 6 (Fig. S6, ESI†), we calculated the average molecular weight of 3 to be 30
1. Lower ratios of RAFT and AIBN to 2 led to higher conversion degrees, but lowered the molecular weight of 3, while higher ratios led to precipitates of both starting material and product. The molecular weight of 3 could not be measured with gel permeation chromatography (GPC) even in warm DMF due to low solubility and the tendency of the polymer towards aggregation. However, the molecular weight and polydispersity index of 3 could be estimated indirectly from the GPC result of the final product 6, which is water soluble and stable against aggregation. Based on the ratio of the molecular weights of the monomers and the average molecular weight of 6 (Fig. S6, ESI†), we calculated the average molecular weight of 3 to be 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 Da. Ethylenediamine was added to the polymer 3 after activation of the carboxylic groups with oxalyl chloride. The obtained 5 dissolved easily in dilute hydrochloric acid solution to give the contrast agent XlinCA (6), which is water soluble and can be crosslinked with aldehydes due to the presence of the amine groups.
400 Da. Ethylenediamine was added to the polymer 3 after activation of the carboxylic groups with oxalyl chloride. The obtained 5 dissolved easily in dilute hydrochloric acid solution to give the contrast agent XlinCA (6), which is water soluble and can be crosslinked with aldehydes due to the presence of the amine groups.
The ability to be crosslinked not only enables polymerization of XlinCA after injection into the vasculature, but also facilitates the increase of the molecular weight of the contrast agent by pre-crosslinking with glutaraldehyde (Fig. S7, ESI†). In order to obtain the desired molecular weight of 65 kDa, required to prevent diffusion through the blood vessel walls, XlinCA was pre-crosslinked with different amounts of 25% glutaraldehyde prior to perfusion into the mouse. The highest amount that could be introduced without inducing gelation was found to be 30 μL of 25% glutaraldehyde solution per 1 g of XlinCA dissolved in 4 mL water. This ratio led to a limited increase in viscosity through an increase in the molecular weight of the polymer by pre-crosslinking. The pre-crosslinked contrast agent was dialysed with a 100 kDa dialysis membrane to remove all compounds below the size threshold and lyophilised. In this form it should be used within a week, as its solubility reduces over time. After the contrast agent was administered into the vasculature, further crosslinking of the contrast agent with glutaraldehyde led to gelation of the contrast agent, preventing any leakage and loss of contrast over time (Fig. S7, ESI†).
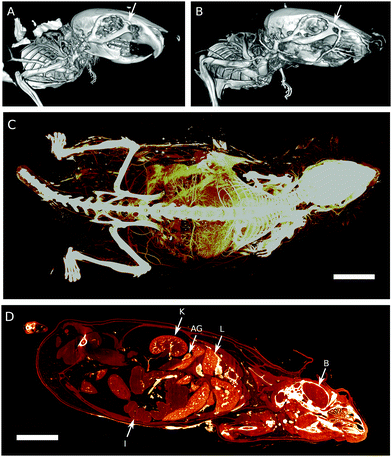
Mice perfused with vascular casting resin PU4ii and polymeric contrast agent XlinCA were compared by using low-resolution μCT with a voxel size of 80 μm. In the PU4ii-perfused mouse, numerous water inclusions and gas bubbles could be observed in the descending aorta, vena cava and larger vessels in the kidneys and the liver. Correspondingly, one liver lobe, the adrenal glands and part of the kidneys were only partially filled with PU4ii. Certain large blood vessels, such as the naso-frontal vein and anterior vein, remained completely devoid of PU4ii (Fig. 2A), while their counterparts in the mouse perfused with XlinCA were filled in their entirety (Fig. 2B and Fig. S8, ESI†). In order to evaluate completeness of the vessel filling further, the XlinCA-perfused mouse was scanned with a voxel size of 20 μm. Brain, heart, lungs, liver, kidneys and adrenal glands appeared well-perfused, see Fig. 2C, D and Fig. S9, S10 (ESI†). No discontinuous vessel segments could be found, as expected for a water-soluble compound. Vascular filling with XlinCA was not entirely complete. Small vessels posterior to the kidneys were ill-defined. Parts of the capillaries were missing in the renal medulla of the kidney (Fig. S10, ESI†) and the spleen. Leftover blood seen in the histological examination indicated that this was caused by incomplete flushing of blood prior to contrast agent injection, and thus not due to any contrast agent-related property.
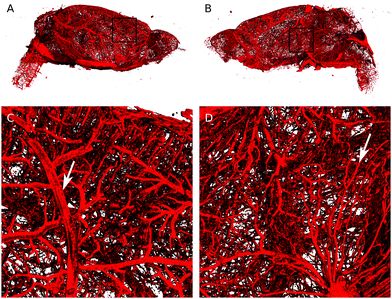
The brain of the XlinCA-perfused mouse was removed and the right brain hemisphere was scanned with a voxel size of 4.4 μm, as the smaller field of view allowed for scans with approximately twice the resolution compared to a full brain. There were no large areas of missing vasculature as has been reported for optimized Microfil perfusions.11a Neither vessel discontinuities through insufficient filling nor gas bubbles could be identified (Fig. 3). Independent of the choice of vascular casting agent, non-optimized transcardial perfusion techniques cannot entirely outperform optimized perfusion techniques in specific organs. On equal terms, however, XlinCA provided more complete and reliable filling of multiple organs in an entire mouse with a simple transcardial injection, without requiring clamping or ligation of the descending aorta and vena cava (Fig. S11, ESI†). The procedure is thus much simpler to perform and allows multiple organs to be harvested and used in the analysis of the vasculature, which reduces the number of animals required and the variance in multi-organ correlation studies.
Factors such as injection volume and flow rate did not have to be optimized, as crosslinking can be initiated at any time after perfusion is complete. In contrast, Microfil polymerizes within approximately 20 minutes,11a limiting the perfusion volume before viscosity and thus resistance to flow increases. With the time constraints removed, flow rate and thus perfusion pressure are no longer factors that require optimization. Perfusion pressure in our experiment was 150 mmHg, which is consistent with the pressure used in the transcardial perfusion reported by Chugh et al.,12b but lower pressures can be used without risk of premature polymerization and resulting incomplete filling. Higher perfusion pressures are still of advantage in the prior perfusion steps required for flushing the vasculature of remaining blood, however.
In conclusion, we have shown that our new imaging agent XlinCA circumvents the limitations of current vascular casting with plastic resins. As XlinCA is a water-soluble compound, water inclusions are inherently avoided. The contrast agent is free of microparticles, thereby preventing blockage of capillaries by solid aggregates. Crosslinking can be initiated after perfusion is complete, allowing perfusion to be conducted for any desired period of time without an increase in viscosity limiting the amount of contrast agent volume that can be injected. The flow rate does not need optimization, and perfusion pressure ceases to be an important factor for contrast agent filling.
Vascular casting with XlinCA thus requires not only considerably less technical expertise and time, but also permits perfusion of both the brain and the whole mouse in a single procedure. We envision that these features will enable hierarchical imaging of organs in their original spatial context and identification of areas with vascular abnormalities, which can then be excised for higher resolution imaging. The vasculature of more than one organ of the same animal could be analysed, which would reduce the number of animals required and reduce the variance in multi-organ correlation studies.
This work was financially supported by University of Zurich and the Swiss National Science Foundation through NCCR Kidney.CH, R'Equip 133802 and grant 205321_153523. We thank Dr Thomas Fox for recording the 1H-NMR of XlinCA (6) and Dr Georg Schulz for support for the high resolution μCT scans.
Conflicts of interest
A patent application for the polymeric contrast agent described in this publication has been filed by the University of Zurich.References
- (a) Z. Starosolski, C. A. Villamizar, D. Rendon, M. J. Paldino, D. M. Milewicz, K. B. Ghaghada and A. V. Annapragada, Sci. Rep., 2015, 5, 10178 CrossRef CAS PubMed; (b) J. Ehling, J. Babickova, F. Gremse, B. M. Klinkhammer, S. Baetke, R. Knuechel, F. Kiessling, J. Floege, T. Lammers and P. Boor, J. Am. Soc. Nephrol., 2016, 27, 520–532 CrossRef CAS PubMed; (c) J. López-Guimet, L. Peña-Perez, R. S. Bradley, P. Garcia-Canadilla, C. Disney, H. Geng, A. J. Bodey, P. J. Withers, B. Bijnens, M. J. Sherratt and G. Egea, Theranostics, 2018, 8, 6038–6052 CrossRef PubMed; (d) J. Epah, K. Palfi, F. L. Dienst, P. F. Malacarne, R. Bremer, M. Salamon, S. Kumar, H. Jo, C. Schürmann and R. P. Brandes, Theranostics, 2018, 8, 2117–2133 CrossRef CAS PubMed.
- L. Zagorchev, P. Oses, Z. W. Zhuang, K. Moodie, M. J. Mulligan-Kehoe, M. Simons and T. Couffinhal, J. Angiogenes Res., 2010, 2, 7 CrossRef PubMed.
- (a) J. Ehling, B. Theek, F. Gremse, S. Baetke, D. Mockel, J. Maynard, S. A. Ricketts, H. Grull, M. Neeman, R. Knuechel, W. Lederle, F. Kiessling and T. Lammers, Am. J. Pathol., 2014, 184, 431–441 CrossRef PubMed; (b) L. Ayala-Dominguez and M. E. Brandan, Biomed. Phys. Eng. Express, 2018, 4, 062001 CrossRef; (c) S. Gu, J. Xue, Y. Xi, R. Tang, W. Jin, J.-J. Chen, X. Zhang, Z.-M. Shao and J. Wu, Quant. Imaging Med. Surg., 2019, 9, 418–426 CrossRef PubMed.
- (a) L. A. Walton, R. S. Bradley, P. J. Withers, V. L. Newton, R. E. B. Watson, C. Austin and M. J. Sherratt, Sci. Rep., 2015, 5, 10074 CrossRef CAS PubMed; (b) E. L. Ritman, Proc. SPIE, 2011, 7898, 78980I CrossRef; (c) T. Salditt, T. Aspelmeier and S. Aeffner, Biomedical Imaging: Principles of Radiography, Tomography and Medical Physics, De Gruyter, Berlin, Boston, 1st edn, 2017 CrossRef.
- R. J. Alfidi, W. J. Mac Intyre and J. R. Haaga, Am. J. Roentgenol., 1976, 127, 11–15 CrossRef CAS PubMed.
- (a) J. Lerch, L. Gazdzinski, J. Germann, J. Sled, R. M. Henkelman and B. Nieman, Front. Neuroinform., 2012, 6, 6 CrossRef PubMed; (b) S. K. Carlson, K. L. Classic, C. E. Bender and S. J. Russell, Mol. Imaging Biol., 2007, 9, 78–82 CrossRef PubMed.
- (a) B. Müller, J. Fischer, U. Dietz, P. J. Thurner and F. Beckmann, Nucl. Instrum. Methods Phys. Res., Sect. B, 2006, 246, 254–261 CrossRef; (b) E. Eggl, K. Mechlem, E. Braig, S. Kulpe, M. Dierolf, B. Günther, K. Achterhold, J. Herzen, B. Gleich, E. Rummeny, P. B. Noël, F. Pfeiffer and D. Muenzel, Sci. Rep., 2017, 7, 42211 CrossRef CAS PubMed.
- M. Bourin, P. Jolliet and F. Ballereau, Clin. Pharmacokinet., 1997, 32, 180–193 CrossRef CAS PubMed.
- (a) F. Hallouard, N. Anton, P. Choquet, A. Constantinesco and T. Vandamme, Biomaterials, 2010, 31, 6249–6268 CrossRef CAS PubMed; (b) L. Nebuloni, G. A. Kuhn and R. Müller, Acad. Radiol., 2013, 20, 1247–1255 CrossRef PubMed; (c) C. Gao, Y. Zhang, Y. Zhang, S. Li, X. Yang, Y. Chen, J. Fu, Y. Wang and X. Yang, Polym. Chem., 2020, 11, 889–899 RSC.
- W. Kuo, G. Schulz, B. Müller and V. Kurtcuoglu, Developments in X-Ray Tomography XII, San Diego, California, USA, 2019, p. 111130Q Search PubMed.
- (a) S. Ghanavati, L. X. Yu, J. P. Lerch and J. G. Sled, J. Neurosci. Meth., 2014, 221, 70–77 CrossRef PubMed; (b) T. Krucker, A. Lang and E. P. Meyer, Microsc. Res. Tech., 2006, 69, 138–147 CrossRef CAS PubMed; (c) L. Schaad, R. Hlushchuk, S. Barre, R. Gianni-Barrera, D. Haberthur, A. Banfi and V. Djonov, Sci. Rep., 2017, 7, 41842 CrossRef CAS PubMed.
- (a) S. Grabherr, V. Djonov, K. Yen, M. J. Thali and R. Dirnhofer, Am. J. Roentgenol., 2007, 188, 832–838 CrossRef PubMed; (b) B. P. Chugh, J. P. Lerch, L. X. Yu, M. Pienkowski, R. V. Harrison, R. M. Henkelman and J. G. Sled, NeuroImage, 2009, 47, 1312–1318 CrossRef PubMed; (c) S. X. Vasquez, F. Gao, F. Su, V. Grijalva, J. Pope, B. Martin, J. Stinstra, M. Masner, N. Shah, D. M. Weinstein, R. Farias-Eisner and S. T. Reddy, PLoS One, 2011, 6, e19099 CrossRef CAS PubMed; (d) L. Fan, S. Wang, X. He, E. Gonzalez-Fernandez, C. Lechene, F. Fan and R. J. Roman, Physiol. Rep., 2019, 7, e14065 CrossRef PubMed.
- D. S. Perrien, M. A. Saleh, K. Takahashi, M. S. Madhur, D. G. Harrison, R. C. Harris and T. Takahashi, BMC Nephrol., 2016, 17, 24 CrossRef PubMed.
- (a) D. T. Cheung and M. E. Nimni, Connect. Tissue Res., 1982, 10, 187–199 CrossRef CAS PubMed; (b) I. Migneault, C. Dartiguenave, M. J. Bertrand and K. C. Waldron, Biotechniques, 2004, 37, 790–802 CrossRef CAS PubMed.
- H. Lusic and M. W. Grinstaff, Chem. Rev., 2013, 113, 1641–1666 CrossRef CAS PubMed.
- E. Jin and Z.-R. Lu, Biomaterials, 2014, 35, 5822–5829 CrossRef CAS PubMed.
- (a) J. Chiefari, Y. K. Chong, F. Ercole, J. Krstina, J. Jeffery, T. P. T. Le, R. T. A. Mayadunne, G. F. Meijs, C. L. Moad, G. Moad, E. Rizzardo and S. H. Thang, Macromolecules, 1998, 31, 5559–5562 CrossRef CAS; (b) C. Boyer, V. Bulmus, T. P. Davis, V. Ladmiral, J. Liu and S. Perrier, Chem. Rev., 2009, 109, 5402–5436 CrossRef CAS PubMed; (c) D. J. Keddie, Chem. Soc. Rev., 2014, 43, 496–505 RSC; (d) S. Perrier, Macromolecules, 2017, 50, 7433–7447 CrossRef CAS.
- G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2012, 65, 985–1076 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis of XlinCA and further experimental details. See DOI: 10.1039/c9cc09883f |
| ‡ These authors contributed equally to this work respectively. |
| This journal is © The Royal Society of Chemistry 2020 |