Molecular balloon, Pd6L8 cages: recognition of alkyl sulfate surfactants†
Haeri
Lee‡
,
Dongwon
Kim‡
,
Hyejin
Oh
and
Ok-Sang
Jung
 *
*
Department of Chemistry, Pusan National University, Busan 46241, Republic of Korea. E-mail: oksjung@pusan.ac.kr; Fax: +82 51-5163522; Tel: + 82 51-5103240
First published on 7th February 2020
Abstract
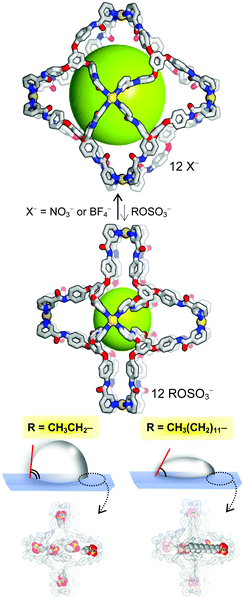
The unique molecular balloon system of [Pd6L8](NO3)12 (an inner cavity of 19 × 21 × 25 Å3 ⇄ 13 × 13 × 13 Å3) was carried out via the anion exchange of nitrate with alkyl sulfates.
Reversible molecular encapsulation via the control of a trigger plays a major role in important chemical recognition processes including biological signal transduction and enzyme catalysis.1 Recently, chemical scientists have attempted to exploit intermolecular interactions as an alternative means of significant control over the threshold of encapsulation.2 Such interactions tend to mimic the supramolecular system that brings changes to the encapsulated substrates via the desired hydrophobic/hydrophilic pores.3 The encapsulated substrate can be activated by the influence of weak interactions or external stimuli.4 Thus, research on the construction of desirable tailor-made large cages has been a hot issue, owing to diverse task-specific functions such as guest indicators, catalysts, solvent reservoirs, region-selectivity controllers, and drug-delivery systems.5 Among various cage compounds, elegant cationic palladium(II) coordination cages have exhibited molecular recognition depending on the counteranionic nature and confined space.6 Furthermore, post-modification of coordination cages for control of their physicochemical properties has attracted particular attention for its provision of facile variation of function.7 However, to date, it remains difficult to include various guest molecules in coordination cages, due to the fixed size of the cavity. Both the internal space and the functional sites of such coordination cages have been known to be important factors for encapsulation and confinement effects.8 A series of alkyl sulfate salts of CnH2n+1OSO3− have been used in ubiquitous detergents for dish-washing liquids, shower gels, shampoos, hair conditioners, fabric softeners, cosmetics, medicines, toothpaste emulsifiers, and fire-extinguishing agents.9 Thus, recognition of and analytical techniques for alkyl sulfate surfactants are significant issues, owing to their widespread use.10
Herein we present an unprecedented novel approach to the control of the uptake of alkyl sulfate surfactants based on flexible host–guest interaction between alkyl sulfates and large coordination cages. For the aggregates encapsulated via hydrogen bonds between sulfate groups and cage skeletons, the contact angles were measured according to the length of the alkyl sulfates. Electrostatic interaction between Pd2+⋯−OSO3R (3.92(1)–4.23(2) Å) forced to face to each other inside cage (Table S2, ESI†). A new C3-symmetric N-donor ligand, 1,3,5-tris(4-nicotinamide-phenoxy)benzene (L), was synthesized in three steps in a reasonable yield starting from phloroglucinol. The L was employed in the self-assembly of palladium(II) ions for the coordination cages [Pd6L8]12+(X−)12 (X− = NO3−, 1·12NO3; X− = BF4−, 1·12BF4) (Scheme 1). The reaction was monitored by 1H NMR spectra that showed a significant downfield shift compared with that of the free L (Fig. S1–S5 and S8–S11, ESI†). High-resolution ESI-mass data at 1711.2797 ([1·12NO3–4NO3]4+, calcd 1711.276), 1356.6252 ([1·12NO3–5NO3]5+, calcd 1356.623) for 1·12NO3 and at 1761.2153 ([1·12BF4–4BF4]4+, calcd 1761.306), 1391.6473 ([1·12BF4–5BF4]5+, calcd 1391.661), 1145.2067 ([1·12BF4–6BF4]6+, calcd 1145.203) for 1·12BF4 were consistent with the formation of the coordination cages. Their crystal structures were investigated via single-crystal X-ray structure determination. The crystalline solids of the cages are insoluble in common organic solvents such as chloroform, acetonitrile, and tetrahydrofuran, but are soluble in Me2SO. Subsequent reactions with the addition of tetrabutylammonium alkyl sulfates (ROSO3−NBu4+, R = ethyl-, octyl-, decyl-, dodecyl-, and tetradecyl-), [Pd6(ROSO3)6L8](ROSO3)6 (2·12ROSO3) were attempted. It is noteworthy that the crystalline solid of 2·12ROSO3 has a much lower solubility in common organic solvents including N,N-dimethylformamide and Me2SO. In a trial entailing X-ray diffraction data collection, we could calculate unit cell parameters for 2·12C2H5OSO3 and 2·12C14H29OSO3 that were similar to those of 2·12C8H17OSO3 and 2·12C12H25OSO3, due to weak diffraction. The IR spectra for 2·12ROSO3 showed the S–O vibrational frequencies of the sulfate group (1361 and 1064 cm−1) and C–H from the aliphatic chains (1925–2852 cm−1) (Fig. S15, ESI†). Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) results showed that the coordinate cages of 1·12NO3 were stable up to 367 °C and that the solvate molecules were evaporated at 350 °C (two-step weight loss for solvate molecules: found, 4.7%; calcd, 9.3%, found, 24%; calcd, 27%). 1·12BF4 decomposed at 297 °C, up to the point, the solvate molecules were evaporated (two-step weight loss for solvate molecules: found, 8.1%; calcd, 7.9%, found, 20%; calcd, 25%). 2·12C12H25OSO3 started to be decomposed at temperatures above 420 °C, and the solvate molecules were fully evaporated by 300 °C (found, 23%; calcd, 22%).
In the crystal structures of the Pd6L8 cages, the geometry of the palladium(II) ion was a typical square-planar arrangement with four N-donors from four L (Pd–N = 1.97(2)–2.048(6) Å). The diameter of all of the present cages was ∼4 nm. However, the dimensions of the inner cavity for each cage were significantly different depending on the anions (19 × 21 × 25 Å3 and 13 × 13 × 13 Å3 for 1·12NO3 and 2·12C12H25OSO3, respectively) (Scheme 1 and Fig. S19, ESI†). The encapsulated alkyl sulfates were bound with skeletal cages via hydrogen bonds (RO3S–O⋯H–NCO = 2.11(1)–2.66(6) Å) for 2·12C8H17OSO3 and 1.98(2)–2.38(1) Å for 2·12C12H25OSO3 following the conformational change in the amide bonds, which was a driving force of the cage contraction (Fig. S19, ESI†). The solvent-accessible void volume was calculated as 63.0% (11695.6/18556.0 Å3) for 1·12NO3, 50.2% (26491.5/52684.0 Å3) for 2·12C8H17OSO3, and 45.3% (23932.4/52813.0 Å3) for 2·12C12H25OSO3 based on PLATON.11 For the 2·12C8H17OSO3 and 2·12C12H25OSO3 structures, a half amount of alkyl sulfates were found on the electron density map, which showed them to exist at the apical position of the palladium(II) inside the cages (Fig. 1b), whereas the opposite position was occupied by the Me2SO solvate molecules (Me2S = O⋯Pd(II) = 2.96(2) Å for 2·12C8H17OSO3 and 2.88(3) Å for 2·12C12H25OSO3).
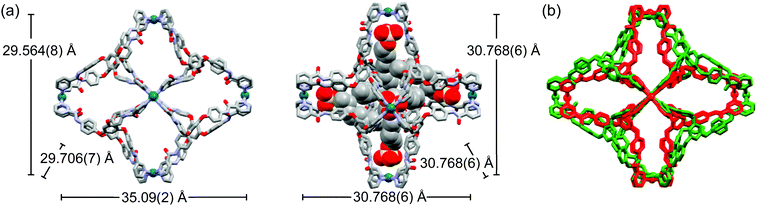 | ||
| Fig. 1 Crystal structures and Pd⋯Pd distances for 1·12NO3 (a, left) and 2·C8H15OSO3 (a, right). Overlay (b) of 1·12NO3 (green) and 2·C8H15OSO3 (red). | ||
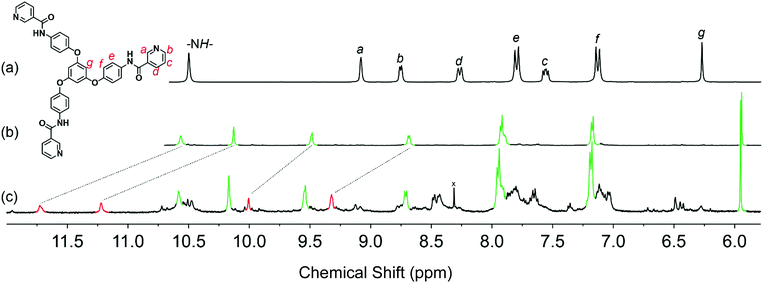
In 1H NMR spectra, the addition of sodium dodecyl sulfate to the Me2SO-d6 solution of 1·12NO3 produced significant chemical shifts in the protons of the amide and pyridyl moieties, indicating the contracted cages’ strong hydrogen bonds between the sulfate groups and the cage-skeletal structures (Fig. 2c). That is, the intermolecular interactions between the coordination cages and alkyl sulfates resulted in downfield shifts. The gradual addition up to 12 equiv. of alkyl sulfate to the 1·12NO3 solution attained the equilibrium state with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 integral ratio of contracted and expanded cages in the solution state along with a small amount of free L owing to partial dissociation of the cages in the Me2SO solution. However, either addition of an excessive amount of alkyl sulfate or rapid addition results in precipitation from the solution, owing to the low solubility. Of course, crystalline solids of 2·12ROSO3 are rarely soluble in common organic solvents. The significant solubility difference seems to come from low solvation energy between cage and solvent molecules, presumably owing to suitable interactions between cages and alkyl sulfates. The present IR spectra support our hypothesis that the anion exchange is reversible between NO3− and alkyl sulfate (Fig. S21, ESI†).
3 integral ratio of contracted and expanded cages in the solution state along with a small amount of free L owing to partial dissociation of the cages in the Me2SO solution. However, either addition of an excessive amount of alkyl sulfate or rapid addition results in precipitation from the solution, owing to the low solubility. Of course, crystalline solids of 2·12ROSO3 are rarely soluble in common organic solvents. The significant solubility difference seems to come from low solvation energy between cage and solvent molecules, presumably owing to suitable interactions between cages and alkyl sulfates. The present IR spectra support our hypothesis that the anion exchange is reversible between NO3− and alkyl sulfate (Fig. S21, ESI†).
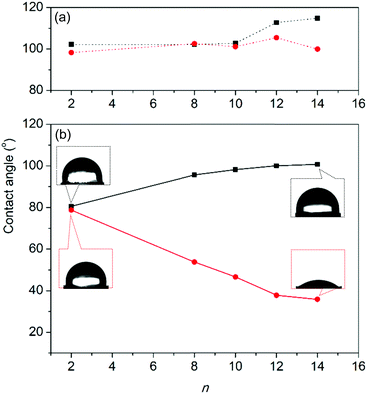
In order to investigate the recognition and adsorption properties of the flexible cages, the contact angles of a droplet on the surface of fine-ground microcrystals (20–40 μm) were measured (Fig. S22, ESI†). The crystalline surface was prepared by spreading the ground microcrystals on a glass tape and subsequently flattening them onto slide glass. As shown in Fig. 3 and Table S3 (ESI†), the contact angles for a drop of water to 2·12C14H29OSO3 (114.87°) were larger than those of cages containing 2·12C2H5OSO3 with ethyl analog (102.20°). Furthermore, contact angles increase according to the carbon number (n) of alkyl sulfates, thus indicating that the surface of the long chain crystals is more hydrophobic. In the present experimentation, the contact angles slightly decreased to 80.54° (n = 2) ∼100.80° (n = 14) after 10 min. By contrast, for a drop of 20% ethanol aqueous solution, the contact angles drastically decreased (from 99.94° to 35.84° on 2·12C14H29OSO3) after 10 min, as depicted in Fig. 3. The alcohol aqueous drop induced water adsorption into the void of the microcrystalline structure, as caused by hydrophilic structure transformation.12 Furthermore, the reason it takes several minutes to wet the surface is that adsorption occurs in the heterogeneous phase. This process is an advanced method for surface modification of crystals via anion exchange by alkyl sulfate surfactants. Furthermore, the wettability of 2 crystals can be utilized as a molecular ruler for measurement of the length of alkyl sulfate chains.
In conclusion, we demonstrated an unusual system for contraction and expansion of flexible Pd6L8 cages via inclusion and release of alkyl sulfate surfactants. Hydrogen bonds between surfactants and cage structures are the main driving force of cage transformation. Furthermore, the microcrystal surface of the Pd6L8 cages presents the opportunity for tremendous modification of physical properties according to the chain length of sulfate anions. In particular, the relationship between surfactant recognition and hydrophobic properties has been demonstrated by dynamic contact angles showing surfactant-enhanced spread. That is, these cages can be an unprecedented recognizer for CnH2n+1OSO3− surfactants as well as a unique molecular ruler of alkyl chains via contact angles. This strategy, which involves an uncommon synergic cage effect, might find important applications for spatial and chain-length control entailing the release and retention of molecules.
This work was supported by National Research Foundation of Korea (NRF) grants funded by the Korean Government [MEST] (2016R1A2B3009532 and 2016R1A5A1009405). X-ray crystallography at the PLS-II 2D-SMC beamline was supported in part by MSIP and POSTECH.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) C. Edlinger, T. Einfalt, M. Spulber, A. Car, W. Meier and C. G. Palivan, Nano Lett., 2017, 17, 5790 CrossRef CAS PubMed; (b) G. Grossi, M. D. E. Jepsen, J. Kjems and E. S. Andersen, Nat. Commun., 2017, 8, 1 CrossRef CAS PubMed.
- (a) V. H. Pérez-Luna and O. González-Reynoso, Gels, 2018, 4, 61 CrossRef PubMed; (b) E. M. M. D. Valle, Process Biochem., 2004, 39, 1033 CrossRef; (c) J. Rebek, Jr., Angew. Chem., Int. Ed., 2005, 44, 2068 CrossRef PubMed.
- (a) D. K. Singh, B. V. V. S. P. Kumar and M. Eswaramoorthy, Nanoscale, 2015, 7, 13358 RSC; (b) Y. Jang, R. Natarajan, Y. H. Ko and K. Kim, Angew. Chem., Int. Ed., 2014, 53, 1003 CrossRef CAS PubMed; (c) D. Prochowicz, A. Kornowicz and J. Lewiński, Chem. Rev., 2017, 117, 13461 CrossRef CAS PubMed.
- (a) A. J. Harnoy, I. Rosenbaum, E. Tirosh, Y. Ebenstein, R. Shaharabani, R. Beck and R. J. Amir, J. Am. Chem. Soc., 2014, 136, 7531 CrossRef CAS PubMed; (b) I. K. Jeong, G. H. Gao, Y. Li, S. W. Kang and D. S. Lee, Macromol. Biosci., 2013, 13, 946 CrossRef CAS PubMed; (c) Y. Y. Xu, G. Li, W. Zhuang, H. C. Yu, Y. Hu and Y. Wang, J. Mater. Chem. B, 2018, 6, 7495 RSC.
- (a) D. Shetty, et al. , ACS Appl. Mater. Interfaces, 2018, 10, 17359 CrossRef CAS PubMed; (b) M. Hatano, T. Sakamoto, T. Mizuno, Y. Goto and K. Ishihara, J. Am. Chem. Soc., 2018, 140, 16253 CrossRef CAS PubMed; (c) S. Kawano, T. Fukushima and K. Tanaka, Angew. Chem., Int. Ed., 2018, 130, 15043 CrossRef; (d) J. Guo, Y.-W. Xu, K. Li, L.-M. Xiao, S. Chen, K. Wu, X.-D. Chen, Y.-Z. Fan, J.-M. Liu and C.-Y. Su, Angew. Chem., Int. Ed., 2017, 56, 3852 CrossRef CAS; (e) I. Ortiz-Rivera, M. Mathesh and D. A. Wilson, Acc. Chem. Res., 2018, 51, 1891 CrossRef CAS.
- (a) T. Murase, Y. Nishijima and M. Fujita, J. Am. Chem. Soc., 2012, 134, 162 CrossRef CAS; (b) W. Cullen, M. C. Misuraca, C. A. Hunter, N. H. Williams and M. D. Ward, Nat. Chem., 2016, 8, 231 CrossRef CAS; (c) S. Löffler, J. Lübben, L. Krause, D. Stalke, B. Dittrich and G. H. Clever, J. Am. Chem. Soc., 2015, 137, 1060 CrossRef; (d) Y. Nishioka, T. Yamaguchi, M. Yoshizawa and M. Fujita, J. Am. Chem. Soc., 2007, 129, 7000 CrossRef CAS PubMed; (e) T. Y. Kim, R. A. S. Vasdev, D. Preston and J. D. Crowley, Chem. – Eur. J., 2018, 24, 14878 CrossRef CAS PubMed; (f) A. M. Castilla, T. K. Ronson and J. R. Nitschke, J. Am. Chem. Soc., 2016, 138, 2342 CrossRef CAS; (g) J. E. M. Lewis, A. B. S. Elliott, C. J. McAdam, K. C. Gordon and J. D. Crowley, Chem. Sci., 2014, 5, 1833 RSC.
- (a) M. Han, R. Michel, B. He, Y.-S. Chen, D. Stalke, M. John and G. H. Clever, Angew. Chem., Int. Ed., 2013, 52, 1319 CrossRef CAS; (b) R. Custelcean, Chem. Soc. Rev., 2014, 43, 1813 RSC; (c) J. Mosquera, S. Zarra and J. R. Nitschke, Angew. Chem., Int. Ed., 2014, 53, 1556 CrossRef CAS; (d) L. M. Mesquita, J. Anhäuser, D. Bellaire, S. Becker, A. Lützen and S. Kubik, Org. Lett., 2019, 21(16), 6442 CrossRef CAS; (e) L. Escobar, D. Villarón, E. C. Escudero-Adán and P. Ballester, Chem. Commun., 2019, 55, 604 RSC.
- C. M. Hong, D. M. Kaphan, R. G. Bergman, K. N. Raymond and F. D. Toste, J. Am. Chem. Soc., 2017, 139, 8013 CrossRef CAS PubMed.
- (a) M. Tsumadori, CD Proceedings 6th World Surfactant Congress CESIO, 2004, 196; (b) D. A. Korolchenko, E. N. Degaev and A. F. Sharovarnikov, ICMEA 2015, 2015, 23 Search PubMed; (c) R. Vanyúr, L. Biczók and Z. Miskolczy, Colloids Surf., A, 2007, 299, 256 CrossRef.
- (a) K. M. Ko, B. H. Chon, S. B. Jang and H. Y. Jang, J. Ind. Eng. Chem., 2014, 20, 228 CrossRef CAS; (b) J. Huang. and H. R. Matthews, Anal. Biochem., 1990, 188, 114 CrossRef CAS.
- A. L. Spek, Acta Crystallogr., 2009, D65, 148 CrossRef.
- (a) J. F. Wall and C. F. Zukoski, Langmuir, 1999, 15, 7432 CrossRef CAS; (b) Y. Yuan and T. R. Lee, Surface Sciences, Springer, New York, 2013, vol. 51, p. 3 Search PubMed; (c) X. Wang, J. Venzmer and E. Bonaccurso, Langmuir, 2016, 32, 8322 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and crystal structure determination. CCDC 1888048–1888051. HR-ESI-TOF-Mass spectra, TGA/DSC curves, NMR spectra, IR spectra and SEM images. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9cc09742b |
| ‡ Haeri Lee and Dongwon Kim contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |