Base-free Ni-catalyzed Suzuki-type cross-coupling reactions of epoxides with boronic acids†
Xiao-Yu
Lu
 *ab,
Lu-Yu
Yan
a,
Jin-Song
Li
a,
Jia-Mei
Li
a,
Hai-pin
Zhou
a,
Run-Chuang
Jiang
a,
Chuang-Chuang
Liu
a,
Ran
Lu
a and
Rong
Hu
a
*ab,
Lu-Yu
Yan
a,
Jin-Song
Li
a,
Jia-Mei
Li
a,
Hai-pin
Zhou
a,
Run-Chuang
Jiang
a,
Chuang-Chuang
Liu
a,
Ran
Lu
a and
Rong
Hu
a
aSchool of Materials and Chemical Engineering, ChuZhou University, Chu Zhou, 239000, China. E-mail: xiaoyulu@mail.ustc.edu.cn
bSchool of Chemistry and Chemical Engineering, AnHui University, He Fei, 230601, China
First published on 26th November 2019
Abstract
A Ni-catalyzed Suzuki-type cross-coupling of boronic acids with epoxides without an exogenous base and with broad substrate scope has been developed. The product selectivity of styrenyl epoxides is different from that of previous work. This methodology uses readily available starting materials to access a range of substituted alcohols, which are valuable feedstock chemicals.
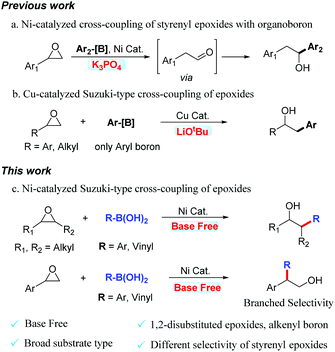
Transition metal-catalyzed Suzuki–Miyaura cross-couplings of organoboron compounds with organic electrophiles are among the most vital C–C bond-forming reactions in organic and medicinal synthesis. These transformations generally require the addition of a stoichiometric amount of exogenous base.1 This requirement restricts the functional-group compatibility of the reaction.2 Epoxides are crucial electrophiles in organic synthesis because they are readily available and highly useful for ring-opening reactions with various carbon-based nucleophiles.3 The alcohol products obtained from the ring-opening reactions of epoxides are valuable feedstock chemicals that play vital roles in synthetic and medicinal chemistry. Over the past few decades, various ring-opening reactions of epoxides with carbon-based nucleophiles, such as strongly nucleophilic organolithium reagents and Grignard reagents, have been investigated.4,5 Organoboron compounds are usually readily available and have a broad functional-group tolerance.6 In this context, Doyle reported nickel-catalyzed cross-coupling of styrenyl epoxides with arylboronic acids and obtained rearrangement products (Scheme 1a).7 Unfortunately, the use of the strong base K3PO4 was incompatible with many functional groups in this reaction. Recently, Fu realized the Cu-catalyzed cross-coupling of epoxides with organoboron compounds (Scheme 1b).8 The 1,2-disubstituted epoxides and alkenyl-boron are incompatible substrates. Unfortunately, using the strong base LiOtBu also proved to be incompatible with many polar functional groups.
Recently, nickel-catalyzed cross-coupling reactions have attracted great attention because of the low cost of nickel catalysts, and their excellent functional-group tolerance.9 To date, nickel-catalyzed Suzuki-type cross-coupling reactions of ubiquitous alkyl epoxides has not been reported. In this communication, we report an example of a Ni-catalyzed Suzuki-type cross-coupling of boronic acids with epoxides (Scheme 1c). In addition to monosubstituted and 1,1-disubstituted epoxides, 1,2-disubstituted epoxides also gave products with good reaction yields. Alkenyl boronic acids also undergo coupling to generate homoallylic alcohols. The selectivity of aromatic epoxides is different from Doyle's and Fu's work. The selectivity of the ring-opening reaction is excellent. This reaction does not use an exogenous base and has better substrate compatibility. This methodology utilizes readily available and inexpensive epoxides, and boronic acids to access a diverse array of synthetically valuable alcohols, which are valuable feedstock chemicals in synthetic and medicinal chemistry.
We began our study by selecting phenylboronic acid (1a) and cyclohexene oxide (2a) as the model reaction substrates (Table 1). On the basis of recent studies on the cross-coupling reaction of epoxides with organoboron compounds, we first examined analogous catalytic conditions with an exogenous base for the ring-opening coupling. Unfortunately, only a trace amount of the coupling product was observed (entry 1). Next, we tested other bases, such as K3PO4, K2CO3, LiOMe, NaOAc, and Et3N, to see whether they could accelerate the reaction. However, the reaction yield was still very low (see ESI†). We then used other solvents instead of N,N-dimethylacetamide (entry 5). However, the yields of the desired product remained very poor. Unexpectedly, under the same reaction conditions, we removed the exogenous base and obtained a moderate reaction yield (entry 6 vs. entry 1). In the absence of an exogenous base, we found that alcoholic solvents gave better reaction yields. Finally, we obtained the optimal reaction conditions after screening a series of alcoholic solvents (entry 9). Using other nickel catalysts, such as NiI2 and NiCl2(PPh3)2, significantly reduced the reaction yield (entry 10 and 11) compared to that of entry 9. Switching the ligand to 1,10-phenanthroline also decreased the reaction yield (entries 12). A control experiment indicated that the reaction completely shut down without the addition of a catalyst (entry 13). The use of phenylboronic acid ester instead of phenylboronic acid generated only a trace amount of the desired product (see ESI†).
| Entrya | Catalyst | Ligand | Base | Solvent | Yield% (d.r.) |
|---|---|---|---|---|---|
| a 1a (0.5 mmol), 2a (0.25 mmol), base (1.5 equiv.) in 0.8 mL solvent at 70 °C for 20 h. b No catalyst. The yield was determined by GC using Benzophenone as internal standard. The d.r. ratio was determined by GC. | |||||
| 1 | NiBr2·diglyme | dtbpy | LiOtBu | DMAc | Trace |
| 2 | NiBr2·diglyme | dtbpy | K3PO4 | DMAc | 2 |
| 3 | NiBr2·diglyme | dtbpy | K2CO3 | DMAc | 19(7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 4 | NiBr2·diglyme | dtbpy | LiOMe | DMAc | 6(8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 5 | NiBr2·diglyme | dtbpy | K2CO3 | THF | 16(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 6 | NiBr2·diglyme | dtbpy | — | DMAc | 30(8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 7 | NiBr2·diglyme | dtbpy | — | HOtBu | 47(9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 8 | NiBr2·diglyme | dtbpy | — | iPrOH | 65(8.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 9 | NiBr 2 ·diglyme | dtbpy | — | EtOH |
89(9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
| 10 | NiI2 | dtbpy | — | EtOH | 29(8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 11 | NiCl2(PPh3)2 | dtbpy | — | EtOH | 20(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 12 | NiBr2·diglyme | Phen | — | EtOH | 25(8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 13b | — | dtbpy | — | EtOH | 0 |
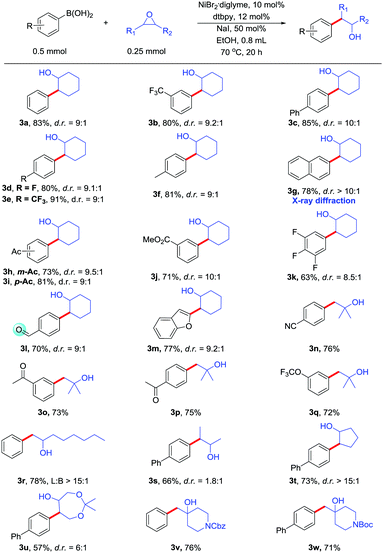
With optimized conditions in hand, we next evaluated the scope of the Suzuki-type cross-coupling of epoxides (Table 2). Many of the substituted boronic acids coupled with epoxides in moderate to good yields. Both electron-rich and electron-poor boronic acids afforded the desired products. Importantly, many functional groups are readily tolerated, including trifluoromethyl (3b, 3e), fluoride (3d), ester (3j), amide (3v, 3w), and ketal (3u). Even more reactive groups, such as aldehyde (3l), ketone (3h, 3i, 3o), were compatible in the reaction. These functional group is incompatible with Fu's method. Acyclic epoxides including monosubstituted, 1,1-disubstituted and 1,2-disubstituted epoxides also gave products with good reaction yields. Some functional groups, such as cyano (3n), ketone (3o, 3p), trifluoromethoxy (3q), are readily tolerated. Other cyclic epoxides (3t, 3u, 3v, 3w) can also participate in the coupling reaction. Benzofuran (3m) group can also be present in the reaction. The position of the substituent on the boronic acid does not affect the reaction. The configuration of the major isomer of 3g was determined by X-ray diffraction.
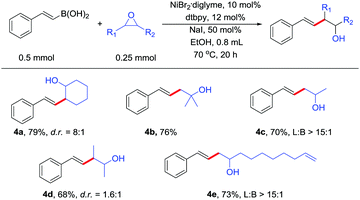
Transition metal-catalyzed Suzuki-type cross-coupling of alkenyl boron with alkyl epoxides has not been realized. The reaction can also be extended to alkenyl boronic acids. Alkenyl boronic acids underwent the reaction with a diverse array of substituted epoxides in high yields (Table 3). Therefore, the reaction can provide access to a diverse array of substituted homoallylic alcohols, which are valuable structural motifs in organic chemistry.
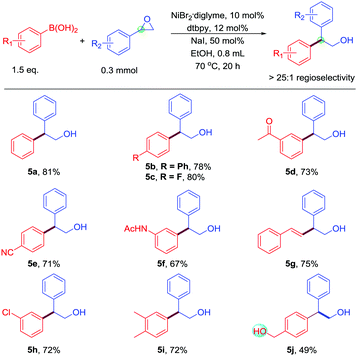
In the case of styrenyl epoxides, Doyle's work obtained rearrangement products,7 and Fu's work obtained a linear product.8 However, this work obtains the branched-chain products (Table 4). Moreover, the selectivity of this reaction is excellent; many of the substituted boronic acids coupled with styrenyl epoxides in good yields. Many functional groups are readily tolerated, including fluoride (5c), ketone (5d), cyano (5e), amide (5f), and chloride (5h). Alkenyl boronic acids underwent the reaction with styrenyl epoxides to give products in high yields. Even base-sensitive hydroxyl functional groups (5j) was compatible in the reaction. Therefore, this work complements the selectivity of the Suzuki-type reaction of styrenyl epoxides. The 1,1-disubstituted styrenyl epoxides, such as 2-methyl-2-phenyloxirane, cannot participate in this reaction.
Vinyl epoxide is a special type of epoxide, which is characterized by the conjugated reactivity of the epoxide and carbon–carbon double bond. Because of its unique structural features, vinyl epoxide can carry out SN2 and SN2′-type ring-opening/coupling reactions.10 We performed a reaction using alkenyl boronic acid with vinyl epoxide, which generated both the SN2 and SN2′-type products (Scheme 2). Therefore, this methodology can provide access to some special substituted homoallylic alcohols.
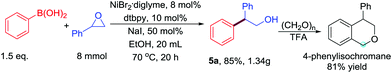
To demonstrate the scalability of the base-free Ni-catalyzed Suzuki-type cross-coupling of boronic acids with epoxides, we performed the reaction on a gram scale, which afforded the product in 85% yield (Scheme 3). Product 5a was efficiently transformed into 4-phenylisochromane through an Oxa-Pictet–Spengler reaction.
The monoalkyl-substituted chiral epoxide reacted smoothly at the less-substituted carbon center under the standard reaction conditions and obtained the desired products with almost no loss of enantiomeric excess (Scheme 4).
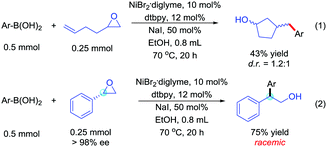
The mechanism of the reaction was investigated through several experiments. First, we observed the product in 61% yield when using iodohydrin instead of the epoxide and in the absence of sodium iodide. Using an epoxide starting material and in the absence of NaI, no production was observed. These results indicated that the epoxy group goes through a β-iodohydrin intermediate process.11 Then, we performed the reaction with an epoxide carrying a pendant homoallyl group. In this case, only the ring-closure isomer was isolated (Scheme 5, eqn (1)).12 Furthermore, the (R)-styrene oxide was used to study the stereochemistry, which led to a racemic product in 75% isolated yield (Scheme 5, eqn (2)). The above observations are consistent with a radical-type mechanism for the epoxides.13
In summary, we report an example of a Ni-catalyzed Suzuki-type cross-coupling of boronic acids with epoxides without an exogenous base. A variety of substituted epoxides are suitable reaction substrates. Alkenyl boronic acids also undergo coupling to generate homoallylic alcohols. The selectivity of aromatic epoxides is different from that of previous work. This Ni-catalyzed Suzuki-type cross-coupling reaction of epoxides has a wider range of substrate types than previous work. This reaction does not require the use of an exogenous base and has good functional-group compatibility. This methodology provides access to a diverse array of substituted alcohols, which are valuable feedstock chemicals in synthetic and medicinal chemistry.
This work was supported by 2017qd11, 16030801108 and KJ2019A0635.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) R. Jana, T. P. Pathak and M. S. Sigman, Chem. Rev., 2011, 111, 1417–1492 CrossRef CAS; (b) A. H. Cherney, N. T. Kadunce and S. E. Reisman, Chem. Rev., 2015, 115, 9587–9652 CrossRef CAS; (c) F.-S. Han, Chem. Soc. Rev., 2013, 42, 5270–5298 RSC; (d) A. Suzuki, Angew. Chem., Int. Ed., 2011, 50, 6722–6737 CrossRef CAS; (e) A. J. J. Lennox and G. C. Lloyd-Jones, Angew. Chem., Int. Ed., 2013, 52, 7362–7370 CrossRef CAS; (f) K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2005, 44, 4442–4489 CrossRef CAS PubMed.
- (a) C. A. Malapit, J. R. Bour, C. E. Brigham and M. S. Sanford, Nature, 2018, 563, 100–104 CrossRef CAS; (b) L. Chen, D. R. Sanchez, B. Zhang and B. P. Carrow, J. Am. Chem. Soc., 2017, 139, 12418–12421 CrossRef CAS; (c) T. J. A. Graham and A. G. Doyle, Org. Lett., 2012, 14, 1616–1619 CrossRef CAS.
- (a) P. Crotti and M. Pineschi, Aziridines and Epoxides in Organic Synthesis, Wiley-VCH Verlag GmbH & Co. KGaA, 2006, pp. 271–313 Search PubMed; (b) V. V. Fokin and P. Wu, Aziridines and Epoxides in Organic Synthesis, Wiley-VCH Verlag GmbH & Co. KGaA, 2006, pp. 443–477 Search PubMed; (c) P. A. S. Lowden, Aziridines and Epoxides in Organic Synthesis, Wiley-VCH Verlag GmbH & Co. KGaA, 2006, pp. 399–442 Search PubMed; (d) H. Ohno, Aziridines and Epoxides in Organic Synthesis, Wiley-VCH Verlag GmbH & Co. KGaA, 2006, pp. 37–71 Search PubMed; (e) B. Olofsson and P. Somfai, Aziridines and Epoxides in Organic Synthesis, Wiley-VCH Verlag GmbH & Co. KGaA, 2006, pp. 315–347 Search PubMed; (f) C. Molinaro and T. F. Jamison, J. Am. Chem. Soc., 2003, 125, 8076–8077 CrossRef CAS; (g) Y. Ikeda, H. Yorimitsu, H. Shinokubo and K. Oshima, Adv. Synth. Catal., 2004, 346, 1631 CrossRef CAS; (h) B. P. Woods, M. Orlandi, C.-Y. Huang, M. S. Sigman and A. G. Doyle, J. Am. Chem. Soc., 2017, 139, 5688–5691 CrossRef CAS; (i) X.-Y. Lu, J.-S. Li, S.-Q. Wang, Y.-J. Zhu, Y.-M. Li, L.-Y. Yan, J.-M. Li, J.-Y. Wang, H.-P. Zhou and X.-T. Ge, Chem. Commun., 2019, 55, 11123–11126 RSC; (j) K. L. Jensen, E. A. Standley and T. F. Jamison, J. Am. Chem. Soc., 2014, 136, 11145–11152 CrossRef CAS; (k) D. K. Nielsen, C.-Y. Huang and A. G. Doyle, J. Am. Chem. Soc., 2013, 135, 13605–13609 CrossRef CAS; (l) C.-Y. Huang and A. G. Doyle, J. Am. Chem. Soc., 2012, 134, 9541–9544 CrossRef CAS.
- C.-Y. Huang and A. G. Doyle, Chem. Rev., 2014, 114, 8153–8198 CrossRef CAS.
- (a) M. Alam, C. Wise, C. A. Baxter, E. Cleator and A. Walkinshaw, Org. Process Res. Dev., 2012, 16, 435–441 CrossRef CAS; (b) C. Bonini, L. Chiummiento, M. T. Lopardo, M. Pullez, F. Colobert and G. Solladie, Tetrahedron Lett., 2003, 44, 2695–2697 CrossRef CAS.
- For selected Suzuki-type cross-couplings (a) J. Zhou and G. C. Fu, J. Am. Chem. Soc., 2004, 126, 1340–1341 CrossRef CAS; (b) F. González-Bobes and G. C. Fu, J. Am. Chem. Soc., 2006, 128, 5360–5361 CrossRef; (c) N. A. Weires, E. L. Baker and N. K. Garg, Nat. Chem., 2015, 8, 75–79 CrossRef; (d) J. Wang, T. Qin, T.-G. Chen, L. Wimmer, J. T. Edwards, J. Cornella, B. Vokits, S. A. Shaw and P. S. Baran, Angew. Chem., Int. Ed., 2016, 55, 9676–9679 CrossRef CAS; (e) M. L. Duda and F. E. Michael, J. Am. Chem. Soc., 2013, 135, 18347–18349 CrossRef CAS; (f) Y. Takeda, Y. Ikeda, A. Kuroda, S. Tanaka and S. Minakata, J. Am. Chem. Soc., 2014, 136, 8544–8547 CrossRef CAS; (g) C. T. Yang, Z. Q. Zhang, Y. C. Liu and L. Liu, Angew. Chem., Int. Ed., 2011, 50, 3904 CrossRef CAS; (h) X.-Y. Yu, Q.-Q. Zhou, P.-Z. Wang, C.-M. Liao, J.-R. Chen and W.-J. Xiao, Org. Lett., 2018, 20, 421–424 CrossRef CAS; (i) J. C. Tellis, D. N. Primer and G. A. Molander, Science, 2014, 345, 433–436 CrossRef CAS; (j) P. Maity, D. M. Shacklady-McAtee, G. P. A. Yap, E. R. Sirianni and M. P. Watson, J. Am. Chem. Soc., 2013, 135, 280–285 CrossRef CAS.
- D. K. Nielsen and A. G. Doyle, Angew. Chem., Int. Ed., 2011, 50, 6056 CrossRef CAS.
- X. Y. Lu, C. T. Yang, J. H. Liu, Z. Q. Zhang, X. Lu, X. Lou, B. Xiao and Y. Fu, Chem. Commun., 2015, 51, 2388–2391 RSC.
- S. Z. Tasker, E. A. Standley and T. F. Jamison, Nature, 2014, 509, 299–309 CrossRef CAS.
- (a) J. He, J. Ling and P. Chiu, Chem. Rev., 2014, 114, 8037–8128 CrossRef CAS; (b) J. Kjellgren, J. Aydin, O. A. Wallner, I. V. Saltanova and K. J. Szab’o, Chem. – Eur. J., 2005, 11, 5260 CrossRef CAS; (c) J. P. Patterson, P. Cotanda, E. G. Kelley, A. O. Moughton, A. Lu, T. H. Epps 3rd and R. K. O'Reilly, Polym. Chem., 2013, 4, 2033 RSC; (d) X.-Y. Lu, J.-S. Li, J.-Y. Wang, S.-Q. Wang, Y.-M. Li, Y.-J. Zhu, R. Zhou and W.-J. Ma, RSC Adv., 2018, 8, 41561–41565 RSC.
- (a) Y. Zhao and D. J. Weix, J. Am. Chem. Soc., 2013, 136, 48–51 CrossRef; (b) Y. Zhao and D. J. Weix, J. Am. Chem. Soc., 2015, 137, 3237–3240 CrossRef CAS PubMed.
- S. Teng, M. E. Tessensohn, R. D. Webster and J. S. Zhou, ACS Catal., 2018, 8, 7439–7444 CrossRef CAS.
- Hammett analysis of the reaction was undertaken: see ESI;† (a) X. Lu, B. Xiao, Z. Zhang, T. Gong, W. Su, J. Yi, Y. Fu and L. Liu, Nat. Commun., 2016, 7, 11129 CrossRef PubMed; (b) E. Richmond and J. Moran, Synthesis, 2018, 499–513 CrossRef CAS; (c) X. Wang, Y. Dai and H. Gong, Top. Curr. Chem., 2016, 374, 43 CrossRef; (d) S. Joung, A. M. Bergmann and M. K. Brown, Chem. Sci., 2019 10.1039/C9SC04199K.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1967481. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9cc08079a |
| This journal is © The Royal Society of Chemistry 2020 |