Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cyanine dye mediated mitochondrial targeting enhances the anti-cancer activity of small-molecule cargoes†
Alexander R.
Nödling‡
 a,
Emily M.
Mills‡
a,
Xuefei
Li
a,
Davide
Cardella
a,
Edward J.
Sayers
a,
Emily M.
Mills‡
a,
Xuefei
Li
a,
Davide
Cardella
a,
Edward J.
Sayers
 b,
Shih-Hsiung
Wu
cd,
Arwyn T.
Jones
b,
Shih-Hsiung
Wu
cd,
Arwyn T.
Jones
 b,
Louis Y. P.
Luk
b,
Louis Y. P.
Luk
 a and
Yu-Hsuan
Tsai
a and
Yu-Hsuan
Tsai
 *a
*a
aSchool of Chemistry, Cardiff University, Cardiff CF10 3AT, UK. E-mail: tsaiy5@cardiff.ac.uk
bSchool of Pharmacy and Pharmaceutical Sciences, Cardiff University, Cardiff, CF10 3NB, UK
cInstitute of Biological Chemistry, Academia Sinica, Taipei 11529, Taiwan
dDepartment of Chemistry, National Taiwan University, Taipei 10617, Taiwan
First published on 25th March 2020
Abstract
Organelle-specific delivery systems are of significant clinical interest. We demonstrate the use of common cyanine dyes Cy3 and Cy5 as vectors for targeting and delivering cargoes to mitochondria in cancer cells. Specifically, conjugation to the dyes can increase cytotoxicity by up to 1000-fold.
Mitochondria are important subcellular organelles for energy production in eukaryotic cells and play critical roles in human health and disease.1 As dysregulation of mitochondria is associated with a variety of human diseases,2 vectors delivering therapeutics or biochemical probes to this organelle has significant clinical interest.3–5 A few molecular entities have been identified as being mitochondria targeting,4–7 including triphenylphosphonium cations8,9 and designer peptides.10,11 It is generally accepted that molecules combining hydrophobic and cationic motifs may exhibit some degree of mitochondrial targeting due to the negative membrane potential (ca. −90 to −150 mV in mitochondrial matrix compared to cytosol).8 Nevertheless, development of chemical entities that can both deliver cargo to mitochondria and report their subcellular location simultaneously is still in its infancy.
Cy3 and Cy5 (Fig. 1a) are commercially available cationic cyanine dyes. They have been commonly used to label molecules for cell fluorescence studies including as a Förster resonance energy transfer (FRET) pair.12 As a FRET pair, excitation of Cy3 (554 nm) leads to fluorescence emission by Cy5 (666 nm) when the two fluorophores are in close proximity. These dyes are relatively small and hydrophobic yet are highly delocalised cations. There is also evidence suggesting that they tend to locate to mitochondria when incubated with cells at concentrations over 1 μM,13–18 thus offering them as potential mitochondrial targeting agents. Indeed, other heptamethine dyes have found sporadic usage as mitochondrial probes or delivery vectors.19–21 However, the full potential of Cy3 and Cy5 for mitochondria targeting and delivery has not been explored. Here, we illustrate that Cy3 and Cy5 are mitochondria targeting as single entities or conjugates and can deliver a variety of structurally diverse molecules (e.g. peptide, heterocycle, metallocomplex) to mitochondria to exert cellular effects. Moreover, mitochondrial targeting and delivery capacity is more prominent in cancer cells.
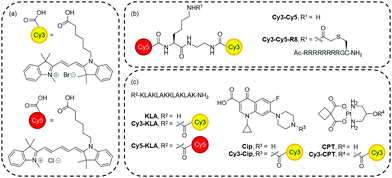 | ||
| Fig. 1 Structure of compounds used in this study. (a) Cy3 and Cy5. (b) Cy3–Cy5 and Cy3–Cy5-R8. (c) Dye conjugates. Synthetic schemes and procedures are in the ESI.† | ||
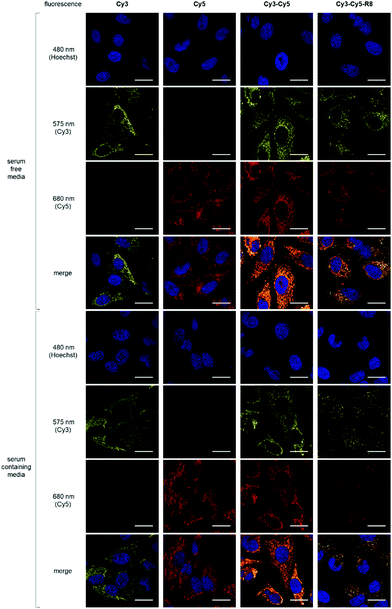
To examine their mitochondria targeting ability, HeLa cells were incubated with 10 μM Cy3, Cy5 or Cy3–Cy5 (Fig. 1a and b) followed by live-cell confocal imaging. Cy3–Cy5 was chosen to investigate if having two fluorophores would enhance uptake and targeting effect. This is of interest for designing Cy3–Cy5-based FRET pairs as theranostic targeting vectors. For all three constructs, the observed filamentous staining22 was highly indicative of mitochondrial accumulation (Fig. 2). The subcellular localisation pattern of these compounds was shown to be concentration independent (Fig. S1, ESI†), and there was no evidence of nuclear localisation (Pearson's correlation coefficients <0.15, Fig. 2 and Fig. S2, ESI†) or cytosolic staining. On the other hand, Pearson's coefficients >0.85 were observed with both Cy3 and Cy5 when cells were subsequently incubated with MitoTracker Green before imaging (Fig. S3, ESI†), confirming the selective mitochondrial localisation. However, sequential incubation of cells with Cy3–Cy5 and MitoTracker Green bizarrely showed a different fluorescent pattern when compared to cells that were treated with either molecule alone or as a non-covalent mixture (Fig. S4, ESI†). It is likely that the higher lipophilicity of Cy3–Cy5 promotes its propensity for aggregation.23–25 Indeed, lower Pearson's correlation coefficients were also obtained when treating cells with a mixture of Cy3 and Cy5 than either Cy3 or Cy5 alone with MitoTracker (Fig. S4, ESI†), suggesting hydrophobic interactions between these molecules. Hence, application of Cy3–Cy5 as a mitochondrial targeting vector is less desirable compared to either Cy3 or Cy5. Nevertheless, it is clear that all constructs preferentially accumulated in mitochondria.
As the mitochondrial membrane potential is the driving force for most cationic, lipophilic mitochondrial targeting molecules,8 we investigated if the sub-cellular localisation of cyanine dyes depends on the membrane potential. Fixation renders cells permeable to small molecules with a resulting collapse of the mitochondrial membrane potential. While MitoTracker Green contains functional groups for covalent crosslinking to mitochondria, such functionalities do not exist in Cy3. Indeed, little change of fluorescent pattern was observed for MitoTracker Green after fixing the cells with paraformaldehyde,26 whereas colocalisation of Cy3 with MitoTracker Green was lost after cell fixation (Fig. 3 and Fig. S5, ESI†). Similarly, when carbonyl cyanide m-chlorophenyl hydrazone (CCCP), a known mitochondrial depolarization agent,15 was added into cells treated with Cy3, the filamentous staining rapidly disappeared. Nevertheless, removal of CCCP from the culture media recovered the membrane potential and restored the filamentous mitochondrial staining for Cy3 after 1 h (Fig. 3). These results indicate that the membrane potential is critical for mitochondrial targeting of the tested cyanine dyes.
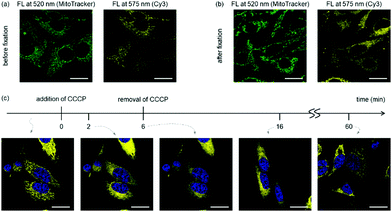 | ||
| Fig. 3 The membrane potential determines subcellular localisation of Cy3. (a and b) HeLa cells were stained with Mito-Tracker Green and Cy3 and imaged before (a) and after (b) fixation by paraformaldehyde. See Fig. S5 (ESI†) for the correlation analysis. (c) Cy3-stained HeLa cells in DMEM containing 10% FBS were treated with 20 μM CCCP for 6 min (from t = 0 to t = 6) and washed to remove CCCP. Cells were then incubated in fresh FBS-containing DMEM (from t = 6), and images were taken approximately 10 and 55 min afterwards (t = 16 and t = 60, respectively). Representative (N = 2) confocal microscopy images are shown here. FL = Fluorescence. | ||
A crucial factor in the design of drug delivery vectors is the knowledge of the respective uptake mechanism. Some small molecules can enter cells by passive diffusion, and this was confirmed for Cy3, Cy5 and Cy3–Cy5 by performing the uptake experiments at 4 °C (Fig. S6 and S7, ESI†), at which no ATP-dependent activity (e.g. endocytosis) takes place. We then examined if cyanine dyes can influence the subcellular localisation and/or the uptake mechanism of a molecule. We and others have explored the potential cell delivery capability of R8, a short cell-penetrating peptide of eight consecutive arginine residues.27–29 Small-molecule cargoes conjugated to R8 are normally taken up by cells via endocytosis at low concentration (<10 μM), but at higher conjugate concentration (≥10 μM) cytosolic delivery through passive diffusion of the conjugate can also be observed.30
Upon conjugation of R8 to cyanine dyes, the behaviour of the resulting Cy3–Cy5-R8 (Fig. 1b) was examined by confocal microscopy experiments using HeLa cells treated with different concentrations (1, 2.5 or 10 μM) of Cy3–Cy5-R8 at either 4 or 37 °C. At 4 °C, Cy3–Cy5-R8 was able to enter cells only at 10 μM but not at 2.5 μM (Fig. S6, ESI†), indicating passive diffusion of this conjugate only taking place at 10 μM but not lower concentrations. This observation is in agreement with the literature.30 While only punctuated fluorescence resembling endolysosomal localisation was observed with 2.5 μM Cy3–Cy5-R8 at 37 °C (Fig. S7 and S8, ESI†), both punctuate and filamentous fluorescence in the cytoplasm were observed when increasing the conjugate concentration to 10 μM (Fig. 2). However, the fluorescence intensity of Cy3–Cy5-R8 was influenced by the presence of serum in the culture media unlike the other constructs tested. This is likely due to binding of R8 to serum proteins, thus reducing the extracellular concentration of free conjugates and capacity for cell entry.31 Nevertheless, the filamentous fluorescence observed with 10 μM Cy3–Cy5-R8 at 37 °C is indicative of mitochondria accumulation, which is supported by moderate Pearson's correlation coefficients (0.4–0.8) of Cy3–Cy5-R8 and MitoTracker (Fig. S4, ESI†).
Our results indicate that conjugation to cyanine dyes affected the subcellular localisation of the conjugate but not the uptake mechanism. The mitochondria localisation observed with 10 μM Cy3–Cy5-R8 at 37 °C most likely resulted from the fraction of Cy3–Cy5-R8 that first translocated to the cytosol by passive diffusion through the plasma membrane. Once in the cytosol, the conjugate was directed to the mitochondria. The ability of cyanine dyes to alter the subcellular localisation of R8 is unique. It was reported that conjugation of a cell-penetrating peptide with a mitochondrial targeting triphenylphosphonium cation did not cause mitochondrial accumulation of the conjugate.32 Thus, cationic cyanine dyes may be superior mitochondrial targeting entities than triphenylphosphonium cations.
Having observed and studied the subcellular localisation properties and the cellular uptake mechanisms, we set to evaluate if Cy3 and Cy5 can be used to deliver different cargoes to mitochondria to mediate a cellular effect. Three different model cargoes were selected (Fig. 1c) to assess the effect of mitochondrial targeting by conjugation to a cyanine dye. These cargoes are a peptide (KLA), a heterocyclic compound (Cip), and a metallocomplex (CPT). They represent three important classes of drugs in clinical application, and the molecular targets of these cargoes are believed to be in mitochondria.33–39 KLAKLAKKLAKLAK (KLA) is a proapoptotic, antimicrobial peptide.33,34 Ciprofloxacin (Cip) is a fluoroquinolone with antibiotic activity but can also cause damage to mitochondrial DNA, showing cytotoxicity to eukaryotic cells.35–38CPT is a metal complex and a derivative of carboplatin (a widely used anti-cancer drug) that impairs mitochondrial function and leads to apoptosis.39 The synthesis of all drug conjugates proved to be rapid and straightforward through simple coupling of the free carboxylic acid of the cyanine dyes. Although conjugates containing both Cy3 and Cy5 may enhance their mitochondrial localisation, they may have higher propensity for aggregation. Therefore, we refrained from using conjugates containing two cyanine dyes for delivering mitochondrial-acting cargoes.
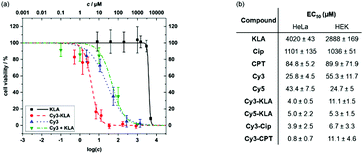
To assess the effect of mitochondrial targeting by conjugation to Cy3, we first determined the viability of HeLa cells after treatment with either Cy3, the unconjugated cargo, a mixture of the unconjugated cargo and Cy3, or the respective conjugate. The individual EC50 values were calculated by curve fitting (Fig. 4 and Fig. S9, ESI†). All conjugates containing a covalent linkage of Cy3 and the cargo molecule are more cytotoxic than either simple mixtures of Cy3 and the unconjugated cargo or the cargo alone. Compared to the parent cargo, toxicity increases from 100-fold (EC50 = 0.8 μM vs. 85 μM for Cy3-CPTvs.CPT) to 1000-fold were observed (EC50 = 4 μM vs. 4020 μM for Cy3-KLAvs.KLA). While Cy3 alone exhibits cytotoxicity (EC50 = 26 μM), a 5- to 30-fold enhancement was observed upon covalent conjugation to the cytotoxic cargo. Confocal microscopy studies of the cargo conjugates were performed to probe the dual functionality of the cyanine dyes to act as mitochondrial targeting/delivery vectors and as diagnostic tools to track cargo localisation (Fig. S10, ESI†). Clear colocalisation of Cy3-KLA with MitoTracker Green was observed upon sequential incubation of HeLa cells with MitoTacker and the conjugate (Pearson's correlation coefficient = 0.830), confirming the delivery of Cy3-KLA to the mitochondria. Although conjugation of a bioactive molecule to a vector may lead to a decrease in its biological activity40 (e.g. due to lowering the binding affinity to the cellular target), this does not seem to be the case for Cy3 conjugation which significantly increases the cytotoxicity (Fig. 4). Nevertheless, it would be interesting to investigate whether using a cleavable linker (e.g. disulphide)41 that releases the bioactive molecule upon reaching the subcellular target can further enhance the potency of this system.
Moreover, Cy3 conjugates were found to be more toxic in cancer than non-cancer cells (Fig. S9, ESI†). Specifically, EC50 values of Cy3-KLA are significantly lower (p < 0.01) in cancer cell lines (4.0, 1.7, 1.9 μM in HeLa, KB, MCF7) than non-cancer cell lines (11.1, 12.0 μM in HEK, 10T1/2). Up to 10-fold difference in potency was also observed with Cy3-CPT (EC50 = 0.8 μM in HeLa vs. 11.1 μM in HEK). Selectivity in different cells could be due to the more negative mitochondrial membrane potential in cancer cells than non-cancer cells.42–44 However, HEK and 10T1/2 cells are immortalised and should not be considered as healthy cells. Primary cells or in vivo studies are now required to further determine the exact therapeutic window for further translational studies of the conjugates between cancer and healthy cells. Lastly, Cy5 can also be used as a mitochondrial targeting and delivery vector as it decreases the EC50 of KLA upon forming the conjugated Cy5-KLA, demonstrating the general applicability of simple cationic cyanine dyes as targeting entity for bioactive molecule delivery.
In conclusion, our work demonstrated the use of common cyanine dyes as novel vectors for targeting drugs to mitochondria in cancer cells. In addition, due to the fluorescence properties of cyanine dyes, theranostics and novel molecules for photodynamic therapy can be derived and investigated. Nevertheless, our results also highlight how lipophilic cationic dyes as fluorescent labels may influence the cellular fate of labelled compounds in fluorescence studies.
We thank Mr Sanjay G Patel for the initial imaging works in this project and are grateful for the financial support from EPSRC (EP/P511122/1 to A.T. J., Y. H. T.), Wellcome Trust (202056/Z/16/Z to L. Y. P. L.), European Social Fund & Tenovus Cancer Care (Knowledge Economy Skills Scholarship to E. M. M.), and Welsh Government (Life Sciences Research Network Wales Scholarship to D. C.). Information on the original data underpinning the results presented here, including how to access them, can be found in the Cardiff University data catalogue at http://doi.org/10.17035/d.2020.0102334803.
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. P. Murphy and R. C. Hartley, Nat. Rev. Drug Discovery, 2018, 17, 865 CrossRef CAS PubMed.
- A. H. V. Schapira, Lancet, 2012, 379, 1825 CrossRef CAS.
- P. Gao, W. Pan, N. Li and B. Tang, Chem. Sci., 2019, 10, 6035 RSC.
- H. Zhu, J. Fan, J. Du and X. Peng, Acc. Chem. Res., 2016, 49, 2115 CrossRef CAS PubMed.
- A. Heller, G. Brockhoff and A. Goepferich, Eur. J. Pharm. Biopharm., 2012, 82, 1 CrossRef CAS PubMed.
- S. Rin Jean, D. V. Tulumello, S. P. Wisnovsky, E. K. Lei, M. P. Pereira and S. O. Kelley, ACS Chem. Biol., 2014, 9, 323 CrossRef CAS PubMed.
- R. W. Horobin, S. Trapp and V. Weissig, J. Controlled Release, 2007, 121, 125 CrossRef CAS PubMed.
- J. Zielonka, J. Joseph, A. Sikora, M. Hardy, O. Ouari, J. Vasquez-Vivar, G. Cheng, M. Lopez and B. Kalyanaraman, Chem. Rev., 2017, 117, 10043 CrossRef CAS PubMed.
- M. P. Murphy, Biochim. Biophys. Acta, 2008, 1777, 1028 CrossRef CAS PubMed.
- S. R. Jean, M. Ahmed, E. K. Lei, S. P. Wisnovsky and S. O. Kelley, Acc. Chem. Res., 2016, 49, 1893 CrossRef CAS PubMed.
- K. K. Maiti, W. S. Lee, T. Takeuchi, C. Watkins, M. Fretz, D. C. Kim, S. Futaki, A. Jones, K. T. Kim and S. K. Chung, Angew. Chem., Int. Ed., 2007, 46, 5880 CrossRef CAS PubMed.
- C. E. Rowland, C. W. Brown, I. L. Medintz and J. B. Delehanty, Methods Appl. Fluoresc., 2015, 3, 042006 CrossRef PubMed.
- I. Negwer, M. Hirsch, S. Kaloyanova, T. Brown, K. Peneva, H. J. Butt, K. Koynov and M. Helm, ChemBioChem, 2017, 18, 1814 CrossRef CAS PubMed.
- X. Jia, Q. Chen, Y. Yang, Y. Tang, R. Wang, Y. Xu, W. Zhu and X. Qian, J. Am. Chem. Soc., 2016, 138, 10778 CrossRef CAS PubMed.
- W. J. Rhee and G. Bao, Nucleic Acids Res., 2010, 38, e109 CrossRef PubMed.
- S. Tomcin, G. Baier, K. Landfester and V. Mailänder, Int. J. Nanomed., 2014, 9, 5471 CAS.
- S. Lorenz, S. Tomcin and V. Mailänder, Microsc. Microanal., 2011, 17, 440 CrossRef CAS PubMed.
- N. Chuard, K. Fujisawa, P. Morelli, J. Saarbach, N. Winssinger, P. Metrangolo, G. Resnati, N. Sakai and S. Matile, J. Am. Chem. Soc., 2016, 138, 11264 CrossRef CAS PubMed.
- S. Luo, X. Tan, S. Fang, Y. Wang, T. Liu, X. Wang, Y. Yuan, H. Sun, Q. Qi and C. Shi, Adv. Funct. Mater., 2016, 26, 2826 CrossRef CAS.
- Y. Liu, J. Zhou, L. Wang, X. Hu, X. Liu, M. Liu, Z. Cao, D. Shangguan and W. Tan, J. Am. Chem. Soc., 2016, 138, 12368 CrossRef CAS PubMed.
- S. M. Usama, B. Zhao and K. Burgess, Bioconjugate Chem., 2019, 30, 1175 CrossRef CAS PubMed.
- R. Ban-Ishihara, T. Ishihara, N. Sasaki, K. Mihara and N. Ishihara, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 11863 CrossRef CAS PubMed.
- J. Kang, O. Kaczmarek, J. Liebscher and L. Dähne, Int. J. Polym. Sci., 2010, 2010, 1 CrossRef.
- N. Fu, Y. Xiong and T. C. Squier, J. Am. Chem. Soc., 2012, 134, 18530 CrossRef CAS PubMed.
- L. I. Markova, V. L. Malinovskii, L. D. Patsenker and R. Häner, Chem. Commun., 2013, 49, 5298 RSC.
- B. Chazotte, Cold Spring Harb. Protoc., 2011, 2011, 990 Search PubMed.
- S. G. Patel, E. J. Sayers, L. He, R. Narayan, T. L. Williams, E. M. Mills, R. K. Allemann, L. Y. P. Luk, A. T. Jones and Y. H. Tsai, Sci. Rep., 2019, 9, 6298 CrossRef PubMed.
- M. Gallo, S. Defaus and D. Andreu, Arch. Biochem. Biophys., 2019, 661, 74 CrossRef CAS PubMed.
- C. P. Cerrato, K. Künnapuu and Ü. Langel, Expert Opin. Drug Delivery, 2017, 14, 245 CrossRef CAS PubMed.
- A. T. Jones and E. J. Sayers, J. Controlled Release, 2012, 161, 582 CrossRef CAS PubMed.
- M. Kosuge, T. Takeuchi, I. Nakase, A. T. Jones and S. Futaki, Bioconjugate Chem., 2008, 19, 656 CrossRef CAS PubMed.
- M. F. Ross, A. Filipovska, R. A. Smith, M. J. Gait and M. P. Murphy, Biochem. J., 2004, 383, 457 CrossRef CAS PubMed.
- B. Law, L. Quinti, Y. Choi, R. Weissleder and C. H. Tung, Mol. Cancer Ther., 2006, 5, 1944 CrossRef CAS PubMed.
- Z. M. Jeffrey, C. Mai, S.-H. Kim, B. Ng and P. D. Robbins, Cancer Res., 2001, 61, 7709 Search PubMed.
- J. W. Lawrence, D. C. Claire, V. Weissig and T. C. Rowe, Mol. Pharmacol., 1996, 50, 1178 CAS.
- J. Azéma, B. Guidetti, J. Dewelle, B. Le Calve, T. Mijatovic, A. Korolyov, J. Vaysse, M. Malet-Martino, R. Martino and R. Kiss, Bioorg. Med. Chem., 2009, 17, 5396 CrossRef PubMed.
- S. Kalghatgi, C. S. Spina, J. C. Costello, M. Liesa, J. R. Morones-Ramirez, S. Slomovic, A. Molina, O. S. Shirihai and J. J. Collins, Sci. Transl. Med., 2013, 5, 192ra85 Search PubMed.
- A. Hangas, K. Aasumets, N. J. Kekäläinen, M. Paloheinä, J. L. Pohjoismäki, J. M. Gerhold and S. Goffart, Nucleic Acids Res., 2018, 46, 9625 CrossRef CAS PubMed.
- V. Reshetnikov, S. Daum, C. Janko, W. Karawacka, R. Tietze, C. Alexiou, S. Paryzhak, T. Dumych, R. Bilyy, P. Tripal, B. Schmid, R. Palmisano and A. Mokhir, Angew. Chem., Int. Ed., 2018, 57, 11943 CrossRef CAS PubMed.
- Y.-H. Tsai, S. Essig, J. J. James, K. Lang and J. W. Chin, Nat. Chem., 2015, 7, 554 CrossRef CAS PubMed.
- E. K. Lei and S. O. Kelley, J. Am. Chem. Soc., 2017, 139, 9455 CrossRef CAS PubMed.
- S. Kim, L. Palanikumar, H. Choi, M. T. Jeena, C. Kim and J. H. Ryu, Chem. Sci., 2018, 9, 2474 RSC.
- S. Marrache and S. Dhar, Chem. Sci., 2015, 6, 1832 RSC.
- C. J. Zhang, Q. Hu, G. Feng, R. Zhang, Y. Yuan, X. Lu and B. Liu, Chem. Sci., 2015, 6, 4580 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterisation of compounds. See DOI: 10.1039/c9cc07931a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2020 |