On-resin synthesis of cyclic peptides via tandem N-to-S acyl migration and intramolecular thiol additive-free native chemical ligation†
Abstract
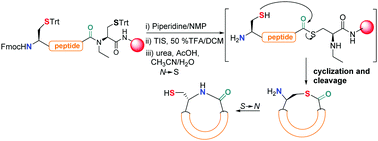
On-resin intramolecular native chemical ligation (NCL) assisted by N-ethylcysteine using Fmoc/SPPS to obtain cyclic peptides is described. N-terminal cysteine-containing peptides were subjected to NCL conditions leading to cyclization–cleavage reactions and consecutive S → N shift, rendering cyclic peptides in good yields and purities. The compounds were evaluated against P. falciparum 3D7.
- This article is part of the themed collection: Celebrating Latin American Talent in Chemistry


 Please wait while we load your content...
Please wait while we load your content...