Facile access to versatile aza-macrolides through iridium-catalysed cascade allyl-amination/macrolactonization†
Abstract
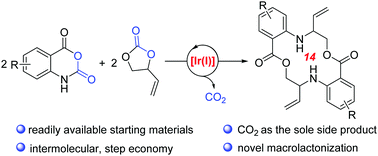
Direct access to benzo-fused aza-macrolides was successfully achieved through iridium-catalysed intermolecular decarboxylative coupling of vinylethylene carbonates with isatoic anhydrides under relatively mild reaction conditions. Notably, this reaction proceeded through sequential allyl-amination/macrolactonization upon extrusion of CO2. Moreover, favourable fluorescence properties could be observed in the title macrocyclic products.


 Please wait while we load your content...
Please wait while we load your content...