Pre-lab proteolysis for dried serum spots – a paper-based sampling concept targeting low abundant biomarkers†
Øystein
Skjærvø
 ,
Trine Grønhaug
Halvorsen
,
Trine Grønhaug
Halvorsen
 and
Léon
Reubsaet
and
Léon
Reubsaet
 *
*
Department of Pharmaceutical Chemistry, School of Pharmacy, University of Oslo, P.O. Box 1068 Blindern, NO-0316 Oslo, Norway. E-mail: leon.reubsaet@farmasi.uio.no
First published on 29th November 2019
Abstract
Paper-based sampling of biological matrices in combination with mass spectrometry has proven to be a promising technique for bottom-up analysis of proteins. However, these techniques have been challenging for determining low abundance proteins (pg mL−1 to low ng mL−1) due to low sampling volumes and adsorption of proteins to the sampling material. Here we are introducing a paper-based sampling concept in chip format with in-device proteolysis combined with selective proteotypic epitope peptide extraction. The sampling concept is demonstrated with the low abundant biomarker proGRP and showed promising performance from 500 pg mL−1 to 1000 ng mL−1 (R2 = 0.99) of proGRP spiked to human serum samples (all RSDs within 26%) in a five point concentration curve (n = 3). LOD and LOQ were determined to 150 pg mL−1 and 500 pg mL−1, respectively. The combination of paper-based sampling with in-device proteolysis and subsequent proteotypic epitope peptide immunocapture showed promising performance for other clinical applications.
1 Introduction
During the recent years new paper-based sampling techniques such as in-device immunocapture1 and in-device proteolysis2,3 have been introduced to simplify sampling and analysis of proteins in biological matrices sampled on paper. In addition to the paper-based devices, other sampling means with proteolysis,4,5 immunocapture6–8 or plasma separation membranes9 have been demonstrated. These devices allow easy and integrated sample preparation and have shown to significantly simplify and shorten the laboratory labor and time associated with the paper-based sampling methodology in protein analysis. Other efforts on improving sample preparation with respect to cleaner sample extracts after sample preparation for protein analyses (buffered solution or venipuncture as sampling) have also been introduced: by specifically capturing and enriching a proteotypic peptide (post proteolysis) rather than capturing intact protein before it is digested has shown to yield clean sample extracts. This has been described using both peptide capture by anti-protein antibodies,10–12 and molecular imprinted polymers (MIPs; also called plastic antibodies),13 as well as for peptide capture using Stable Isotope Standards and Capture by Anti-Peptide Antibodies (SISCAPA).14 Recently we introduced a paper-based sampling concept in a chip format15 that holds the potential to be immobilized with trypsin3 to digest proteins in-device during the sampling stage. This device simplifies the workflow related to these analysis by integrating proteolysis within the sampling device and has also shown to be feasible with proteins needing reduction and alkylation.2,3 Combining the wax printed device introduced in chip format to hold liquid static in the immobilized sampling material (with immobilized trypsin) carry the potential to simplify and shorten conventional laboratory time for protein analysis. The latter combined with proteotypic peptide enrichment could make for a promising workflow providing highly clean sample extracts for mass spectrometric determinations. Therefore, the aim of this study was to explore paper-based smart sampling with integrated proteolysis combined with (immobilized with monoclonal antibodies (mAb)) for a fast and high performing targeted sampling method for low abundant proteins in serum. For the demonstration, a serum marker for small-cell lung cancer pro-gastrin-releasing peptide (ProGRP) was used as model analyte, however, the concept could readily be adapted to other proteins as well.2 Experimental
2.1 Chemicals and reagents
Acetonitrile (99.9%, (ACN)), sodium phosphate dodecahydrate (99–102%), sodium phosphate monobasic (99–102%) were purchased from Merck (Darmstadt, Germany). Ethanolamine (≥99%) and ammonium bicarbonate (BioUltra, ≥99.5%, (ABC)) were purchased from Fluka (Milwaukee, WI, USA). Formic acid (≥98%, (FA)), benzamidine (≥95%), N,N-dimethylformamide anhydrous (DMF), 3-(trimethoxysilyl)propyl methacrylate (γ-MAPS, 98%), 1-heptanol, 2,2-diphenyl-1-picrylhydrazyl hydrate (DPPH), 2-hydroxyethyl methacrylate (HEMA, 97%, containing 200–220 ppm monomethyl ether hydroquinone), initiator 2,2′-azobis(2-methylpropionitrile) (98%, (AIBN)) and trypsin from bovine pancreas TPCK treated (≥10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 BAEE units per mg protein) were purchased from Sigma-Aldrich. 2-Vinyl-4,4-dimethylazlactone (VDM) was purchased from Polysciences Inc. (Warrington, PA, USA). Water used in this experiment was filtrated through a Merck Millipore Milli-Q integral 3 water dispenser (resistivity: 18.2 MΩ cm−1). ProGRP isoform 1 (cloned) and anti-ProGRP (monoclonal antibody (mAb) M18) were provided by the Central Laboratory, Norwegian Radium Hospital, Oslo University Hospital (Oslo, Norway). Tosyl-activated M280 Dynabeads was purchased from Life Technologies (Oslo, Norway). The peptide internal standard [NH2-ALGNQQPSWDSEDSSNF(K*)-COOH] (purity: >95%), labeled 13C/15N at K*, was bought from Innovagen (Lund, Sweden).
000 BAEE units per mg protein) were purchased from Sigma-Aldrich. 2-Vinyl-4,4-dimethylazlactone (VDM) was purchased from Polysciences Inc. (Warrington, PA, USA). Water used in this experiment was filtrated through a Merck Millipore Milli-Q integral 3 water dispenser (resistivity: 18.2 MΩ cm−1). ProGRP isoform 1 (cloned) and anti-ProGRP (monoclonal antibody (mAb) M18) were provided by the Central Laboratory, Norwegian Radium Hospital, Oslo University Hospital (Oslo, Norway). Tosyl-activated M280 Dynabeads was purchased from Life Technologies (Oslo, Norway). The peptide internal standard [NH2-ALGNQQPSWDSEDSSNF(K*)-COOH] (purity: >95%), labeled 13C/15N at K*, was bought from Innovagen (Lund, Sweden).
2.2 Preparation of sampling chips
Pre-cut DMPK-C circles (6.0 mm in diameter) were silanized and polymerized as previously described by Skjærvø et al.3 with minor modifications. The polymerized circles were further immobilized with trypsin and installed in wax printed sampling cartridges. The polymerization, immobilization and fabrication of the wax printed sampling cartridges is presented in S1.2.3 Preparation of antibody immobilized magnetic beads
Immobilization of the monoclonal antibody M18 to magnetic Dynabeads was performed with minor modifications according to a previously described procedure by Paus and Nustad.16 The procedure in short is described in S2.2.4 Sample preparation
2.5 LC-MS method
Sample loading (5 μL) on a Thermo Fischer Scientific (Waltham, MA, USA) Acclaim™ PepMap™ 100 C18 trap column (L: 5.0 mm × 300 μm ID, dp: 5.0 μm) was performed over five min at 10.0 μL min−1 (water/ACN/FA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98
98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
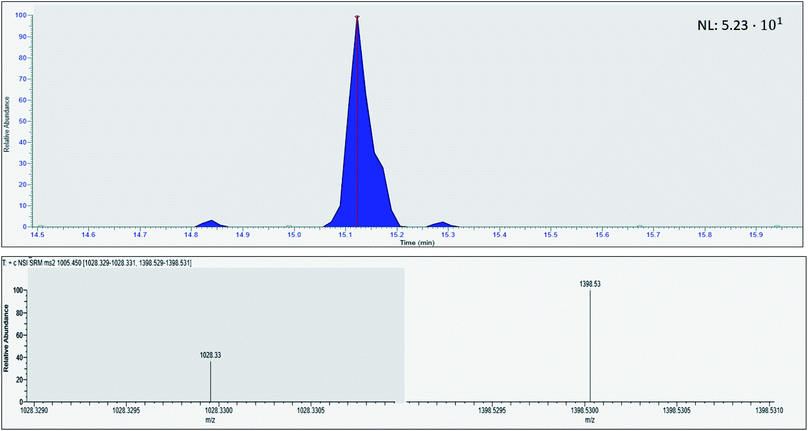
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 v/v)). Mobile phase A (MP A) consisted of water/ACN/FA (95/5/0.1 v/v/v). The chromatographic separation was carried out at 250 nL min−1 over 15 minutes from 5–70% mobile phase B (MP B, ACN/water/FA (95/5/0.1 v/v)) followed by 10 and 15 minutes with 70% MP B and starting conditions, respectively. The chromatography was performed at 35 °C on an Acclaim™ PepMap™ 100 C18 (ID: 75.0 μm, L: 150 mm, dp: 3.0 μm) separation column coupled to a Dionex (Sunnyvale, CA, USA) Ultimate 3000 RSLC nano system. Detection was performed using a Thermo Fischer TSQ Quantiva operated in SRM positive mode nano ESI with 2.25 kV applied voltage. The MS parameters were set as followed: 350 °C ion transfer tube, 35 V collision energy, 1.5 mTorr CID gas, 1 second scan cycle and Q1 and Q3 resolution at 0.7 and 0.7 at FWHM, respectively. Quantification of target ions was carried out by measuring precursor ion m/z 1005.45 and product ion m/z 1028.33 and m/z 1398.53 for the A-peptide (AA-sequence: ALGNQQPSWDSEDSSNFK) and precursor ion m/z 1009.70 and product ion m/z 1036.50 and m/z 1406.70 for the internal standard (AA-sequence: [NH2-ALGNQQPSWDSEDSSNF(K*)-COOH])
0.1 v/v)). Mobile phase A (MP A) consisted of water/ACN/FA (95/5/0.1 v/v/v). The chromatographic separation was carried out at 250 nL min−1 over 15 minutes from 5–70% mobile phase B (MP B, ACN/water/FA (95/5/0.1 v/v)) followed by 10 and 15 minutes with 70% MP B and starting conditions, respectively. The chromatography was performed at 35 °C on an Acclaim™ PepMap™ 100 C18 (ID: 75.0 μm, L: 150 mm, dp: 3.0 μm) separation column coupled to a Dionex (Sunnyvale, CA, USA) Ultimate 3000 RSLC nano system. Detection was performed using a Thermo Fischer TSQ Quantiva operated in SRM positive mode nano ESI with 2.25 kV applied voltage. The MS parameters were set as followed: 350 °C ion transfer tube, 35 V collision energy, 1.5 mTorr CID gas, 1 second scan cycle and Q1 and Q3 resolution at 0.7 and 0.7 at FWHM, respectively. Quantification of target ions was carried out by measuring precursor ion m/z 1005.45 and product ion m/z 1028.33 and m/z 1398.53 for the A-peptide (AA-sequence: ALGNQQPSWDSEDSSNFK) and precursor ion m/z 1009.70 and product ion m/z 1036.50 and m/z 1406.70 for the internal standard (AA-sequence: [NH2-ALGNQQPSWDSEDSSNF(K*)-COOH])
3 Results and discussion
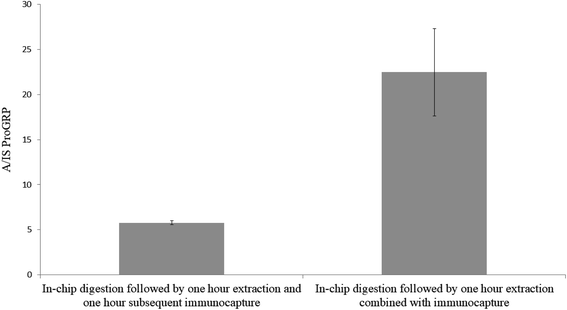
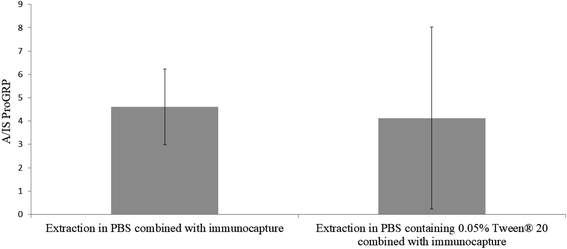
3.1 Extraction
3.3 Extended tryptic digestion
Since the protein digestion starts instantly in-device while the sample (proGRP spiked serum) is drying, sufficiently long interaction time could potentially be a problem since 15 μL sample dries relatively fast and the proteins will therefore face short digestion time. Extending reaction time potentially gains a complete digestion of the target protein and thus more of the proteotypic peptide of interest. In order to evaluate whether or not the in-device tryptic digestion was sufficient for analysis of protein concentrations in the pg mL−1 to ng mL−1 range, the possibility to extend the protein digestion time was assessed. To achieve this, the following was added subsequently to the sample wells after sampling: (1) PBS in various volumes to extend the drying time (elaborated in Section 3.4) or (2) trypsin immobilized beads in volumes 5 μL, 10 μL and 15 μL to increase the enzyme-to-protein ratio. Addition of buffer to extend in-device reaction time has been elaborated in previous work15 and were not further investigated surpassing 30 μL as elaborated in Section 3.4. The investigation showed (S4, Fig. S2†) that none of the additions of extra trypsin beads served any gains in the production of the A-peptide. Therefore, a tentative conclusion was made that the immobilized pHEMA–VDM sampler yielded sufficient protein digestion as is.3.4 Sample dilution
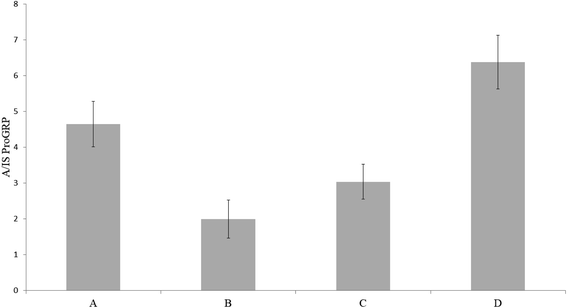
Since all initial experiments had been carried out with proGRP spiked to a matrix composed of 50% serum and 50% PBS, it was of interest to investigate if sample dilution was necessary in order to obtain viable protein digestion. To investigate this, a concentration of 2 μg mL−1 proGRP with an effective sampling volume of 15 μL was chosen. The sampling variants were as following; (A) 30 μL serum![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PBS (50
PBS (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v), (B) 15 μL serum with a subsequent addition of 15 μL PBS (while the sample spot still was wet), (C) 15 μL serum with an addition of 15 μL PBS after the serum had dried in order to extend the trypsin interaction time and (D) 15 μL serum without additions. There was no gain in either diluting the sample at the time of sampling, after the sample had dried (both to extend the drying time and thus digestion time) or use of a less complex matrix (diluted serum) (Fig. 3). The results indicated that the trypsin-to-protein ratio was sufficiently high for a sufficient digestion within 30 minutes (drying time of 15 μL serum sample). Interestingly, extending the digestion time by adding PBS either as an addition to the serum sample or after the serum had dried had a negative impact on the signal intensity. A possible reason for this might be an additional sample dilution leading to the peptides in the device is being wicked toward parts of the wax printed cartridge to adsorb. If the latter occurred, it is assumed that not all of the sample gets extracted enough for subsequent immunocapture of the A-peptide. These theories were not further investigated. All in all, further experiments were carried out solely with spiked, undiluted serum.
50 v/v), (B) 15 μL serum with a subsequent addition of 15 μL PBS (while the sample spot still was wet), (C) 15 μL serum with an addition of 15 μL PBS after the serum had dried in order to extend the trypsin interaction time and (D) 15 μL serum without additions. There was no gain in either diluting the sample at the time of sampling, after the sample had dried (both to extend the drying time and thus digestion time) or use of a less complex matrix (diluted serum) (Fig. 3). The results indicated that the trypsin-to-protein ratio was sufficiently high for a sufficient digestion within 30 minutes (drying time of 15 μL serum sample). Interestingly, extending the digestion time by adding PBS either as an addition to the serum sample or after the serum had dried had a negative impact on the signal intensity. A possible reason for this might be an additional sample dilution leading to the peptides in the device is being wicked toward parts of the wax printed cartridge to adsorb. If the latter occurred, it is assumed that not all of the sample gets extracted enough for subsequent immunocapture of the A-peptide. These theories were not further investigated. All in all, further experiments were carried out solely with spiked, undiluted serum.
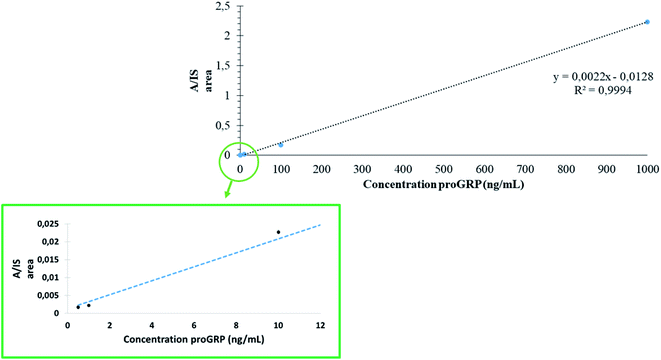
3.5 Quantitative performance
4 Conclusion
A simple paper-based sampling device in chip format integrated with in-device proteolysis is here demonstrated in combination with selective proteotypic epitope peptide immunoextraction for simple and accessible sampling and semi-automated sample preparation. The combination showed a high degree of performance from pg mL−1 to μg mL−1 with minimal time manual sample preparing labor for the low abundant serum biomarker proGRP. The entire work flow was performed within 2.5 hours and demonstrated satisfactory correlation and precision. The sampling chips showed promising potential in combination with proteotypic epitope peptide immunoextraction for future clinical applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Elisabeth Paus at Radium Hospitalet, Oslo University Hospital for kindly donating proGRP standards and monoclonal M18 antibodies.References
- Ø. Skjærvø, E. J. Solbakk, T. G. Halvorsen and L. Reubsaet, Talanta, 2019, 195, 764–770 CrossRef.
- Ø. Skjærvø, C. Rosting, T. G. Halvorsen and L. Reubsaet, Analyst, 2017, 142, 3837–3847 RSC.
- Ø. Skjærvø, T. G. Halvorsen and L. Reubsaet, Analyst, 2018, 143, 3184–3190 RSC.
- Y. Liu, H. Wang, Q. Liu, H. Qu, B. Liu and P. Yang, Lab Chip, 2010, 10, 2887–2893 RSC.
- Y. Liu, W. Zhong, S. Meng, J. Kong, H. Lu, P. Yang, H. H. Girault and B. Liu, Chem.–Eur. J., 2006, 12, 6585–6591 CrossRef CAS.
- C. Zhang, T. Glaros and N. E. Manicke, J. Am. Chem. Soc., 2017, 139, 10996–10999 CrossRef CAS.
- K. Sato, M. Tokeshi, T. Odake, H. Kimura, T. Ooi, M. Nakao and T. Kitamori, Anal. Chem., 2000, 72, 1144–1147 CrossRef CAS PubMed.
- S. Fang, H. Tian, X. Li, D. Jin, X. Li, J. Kong, C. Yang, X. Yang, Y. Lu, Y. Luo, B. Lin, W. Niu and T. Liu, PLoS One, 2017, 12, e0175050 CrossRef.
- B. J. Bills and N. E. Manicke, Clinical Mass Spectrometry, 2016, 2, 18–24 CrossRef.
- M. C. S. Levernæs, O. K. Brandtzaeg, S. F. Amundsen, L. Reubsaet, E. Lundanes, T. G. Halvorsen and S. R. Wilson, Anal. Chem., 2018, 90, 13860–13866 CrossRef.
- R. M. Schoenherr, L. Zhao, R. G. Ivey, U. J. Voytovich, J. Kennedy, P. Yan, C. Lin, J. R. Whiteaker and A. G. Paulovich, Proteomics, 2016, 16, 2141–2145 CrossRef CAS.
- L. Maren, F. Bassem, O. Inger, A. S. Sazan, P. Elisabeth, R. Léon and H. Trine Grønhaug, Exploring peptide capture by anti-protein antibodies for LC-MS-based biomarker determination, 2018 Search PubMed.
- C. Rossetti, O. G. Ore, B. Sellergren, T. G. Halvorsen and L. Reubsaet, Anal. Bioanal. Chem., 2017, 409, 5631–5643 CrossRef CAS.
- M. Razavi, N. Leigh Anderson, M. E. Pope, R. Yip and T. W. Pearson, New Biotechnol., 2016, 33, 494–502 CrossRef CAS PubMed.
- Ø. Skjærvø, T. G. Halvorsen and L. Reubsaet, Anal. Chim. Acta, 2019, 1089, 56–65 CrossRef.
- E. Paus and K. Nustad, Clin. Chem., 1989, 35, 2034–2038 CAS.
- L. F. Qualtiere, A. G. Anderson and P. Meyers, J. Immunol., 1977, 119, 1645 CAS.
- M. Agarkhed, C. O'Dell, M.-C. Hsieh, J. Zhang, J. Goldstein and A. Srivastava, AAPS PharmSciTech, 2018, 19, 79–92 CrossRef CAS.
- G. J. Dimitriadis, Anal. Biochem., 1979, 98, 445–451 CrossRef CAS.
- R. G. Couston, M. W. Skoda, S. Uddin and C. F. van der Walle, mAbs, 2013, 5, 126–139 CrossRef.
- C. Rossetti, M. C. S. Levernæs, L. Reubsaet and T. G. Halvorsen, J. Chromatogr. A, 2016, 1471, 19–26 CrossRef CAS.
- M. C. S. Levernæs, M. N. Broughton, L. Reubsaet and T. G. Halvorsen, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2017, 1055–1056, 51–60 CrossRef.
- H. Sjögren, C. A. Ericsson, J. Evenäs and S. Ulvenlund, Biophys. J., 2005, 89, 4219–4233 CrossRef.
- B. Winther, M. Nordlund, E. Paus, L. Reubsaet and T. G. Halvorsen, J. Sep. Sci., 2009, 32, 2937–2943 CrossRef CAS.
- S. B. Torsetnes, M. S. Levernæs, M. N. Broughton, E. Paus, T. G. Halvorsen and L. Reubsaet, Anal. Chem., 2014, 86, 6983–6992 CrossRef CAS PubMed.
- M. S. Nordlund, J. Bjerner, D. J. Warren, K. Nustad and E. Paus, Tumor Biol., 2008, 29, 204–210 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ay01976f |
| This journal is © The Royal Society of Chemistry 2020 |