A new approach to study human perivascular adipose tissue of the internal mammary artery by fiber-optic Raman spectroscopy supported by spectral modelling†
Zuzanna
Majka‡
 ab,
Krzysztof
Czamara‡
ab,
Krzysztof
Czamara‡
 a,
Piotr
Wegrzyn
c,
Radoslaw
Litwinowicz
c,
Joanna
Janus
a,
Piotr
Wegrzyn
c,
Radoslaw
Litwinowicz
c,
Joanna
Janus
 ab,
Stefan
Chlopicki
ab,
Stefan
Chlopicki
 ad and
Agnieszka
Kaczor
ad and
Agnieszka
Kaczor
 *ab
*ab
aJagiellonian Centre for Experimental Therapeutics (JCET), Jagiellonian University, 14 Bobrzynskiego Str., 30-348 Krakow, Poland. E-mail: agnieszka.kaczor@uj.edu.pl
bFaculty of Chemistry, Jagiellonian University, 2 Gronostajowa Str., 30-387 Krakow, Poland
cDepartment of Cardiovascular Surgery and Transplantology, John Paul II Hospital, Jagiellonian University Medical College, 80 Pradnicka Str., 31-202 Krakow, Poland
dChair of Pharmacology, Jagiellonian University Medical College, 16 Grzegorzecka Str., 31-531 Krakow, Poland
First published on 2nd October 2020
Abstract
Perivascular adipose tissue (PVAT) regulates vascular function and represents a novel therapeutic target in vascular diseases. In this work, a new approach based on fiber-optic Raman spectroscopy and spectral modelling was used to characterize the chemical content of the PVAT of the internal mammary artery (IMA) of patients with advanced coronary atherosclerosis (n = 10) undergoing coronary bypass surgery. Our results showed a high degree of lipid unsaturation and low carotenoid content in the PVAT of the IMA of patients with more advanced coronary artery disease. Moreover, the spectral modelling of the IMA's PVAT composition indicated that glyceryl trioleate was a major PVAT lipid and for patients with relatively low levels of β-carotene, it was accompanied by arachidonic acid and glyceryl trilinolenate. In summary, our proof-of-concept study suggests that carotenoid content and lipid unsaturation degree may reflect the PVAT functional status and a Raman-based assessment of the PVAT of the IMA could prove useful as a novel diagnostic tool to rapidly define the PVAT phenotype in a grafted artery in patients undergoing coronary bypass.
Introduction
Paradoxical as it may sound, the major cause of death of people in the 21st century is related to lifestyle diseases. The obesity and hypercholesterolemia epidemic significantly contributes to the development of cardiovascular diseases, including atherosclerosis, a pathology responsible for the majority of cardiovascular events.1 It is well known that the endothelial function determines the health of the cardiovascular system, while its dysfunction leads to cardiovascular diseases.2,3 However, recent studies have demonstrated that the adipose tissue surrounding the arteries, i.e. the perivascular adipose tissue (PVAT), exhibits paracrine activity and can exert significant influence on the vascular status including endothelial function.4–8 A number of PVAT-dependent mechanisms that regulate vascular function have been identified including, for example, nitric oxide (NO), angiotensin II, angiotensin 1–7, leptin, adiponectin, hydrogen sulphide, and methyl palmitate.9 A dysfunctional PVAT produces a plethora of pro-inflammatory adipokines, cytokines and chemokines that are involved in the progression of cardiovascular diseases and in the development of obesity8,10–12 and atherosclerosis.9,13–17PVAT is a heterogeneous and dynamic tissue that changes its phenotype in response to physiological or pathophysiological stimuli. Regional differences in PVAT in various vascular beds have been described and may even occur in the same vessel.18 In fact, in mice the abdominal aortic PVAT resembles the white adipose tissue (WAT) with large lipid droplets,19 whilst the thoracic aortic PVAT is morphologically similar to the brown adipose tissue (BAT) due to the highly vascularized architecture and closely packed mitochondria.20
Given the vasoprotective effects of BAT,21,22 therapeutic approaches to stimulate or expand BAT are nowadays considered as a strategy for the prevention of atherosclerosis and cardiovascular events. Interestingly, recent reports indicate that BAT activation can be achieved efficiently by a carotenoid-rich diet.23–26 Carotenoids are antioxidants and precursors of retinoid synthesis (retinol, retinaldehyde and retinoic acid), stored mainly in the adipose tissue.27,28 The most recognizable carotenoid is β-carotene being the key substrate for the synthesis of retinoids, which are transcriptional activators of the uncoupling protein 1 (UCP1) gene.23 In BAT, UCP1 expression is molecularly responsible for non-shivering thermogenesis and has an effect on the process of ‘beiging’ of WAT, i.e. processes that have a direct impact on reducing the progression of obesity and atherosclerosis.29 Due to their anti-inflammatory potential, the effects of carotenoids on the onset and development of cardiovascular diseases have been widely studied. Yet, numerous clinical reports regarding the role of carotenoid supplementation on cardiovascular diseases gave discordant results.30 Most of the reports indicated that carotenoid supplementation was associated with a decreased risk of incidence of coronary heart disease,31 the prevalence of atherosclerosis in the carotid and femoral arteries, atherosclerotic lesions in the carotid arteries,32 risk for strokes,33,34 risk for subarachnoid and/or intracerebral hemorrhage,34,35 acute myocardial infarction (AMI),36 cardiovascular diseases (CVD) mortality risk,37–40 sudden cardiac death,41 and congestive heart failure.42 However, other studies report no evidence to support the effective primary or secondary prevention of cardiovascular diseases with carotenoids.30
To the best of our knowledge, changes in the carotenoid content and lipid characteristics of PVAT in atherosclerosis were not investigated, partially due to the lack of tools to study the chemical content of PVAT including carotenoids in situ. A powerful method to investigate carotenoids as well as lipids is Raman spectroscopy, an unbiased method enabling the detailed characterization of the sample composition. When set up with a fiber-optic probe, Raman spectroscopy is capable of investigating tissues in situ and in vivo;43,44 however, there is scarce evidence of its application in studying adipose tissue, limited to a general characterization18,45,46 and changes upon atherosclerosis.47
Therefore, in this proof of concept work, we have applied a novel strategy of studying PVAT in human vessels with the application of fiber-optic Raman spectroscopy and spectral modelling. The aim of the study was to propose a method for the reliable and rapid evaluation of the phenotype of PVAT that in the future can be adopted intraoperatively in hospital conditions. The PVAT surrounding the human internal mammary artery (IMA) was analysed in the distal fragments of arteries taken from patients with coronary artery disease (n = 10) undergoing coronary bypass surgery. We applied spectral modelling to resolve the chemical composition of the PVAT of IMA. The results show that the PVAT chemical composition, in particular, carotenoid content and lipid unsaturation may become the biomarkers of the PVAT functional status18 of the internal mammary artery in coronary artery bypass in patients with coronary artery disease. Indeed, we demonstrated that PVAT carotenoid content and lipid unsaturation seemed to be related to the CCS Angina Grading Scale suggesting that these two Raman-based markers detected in rapid in situ measurements might reflect the PVAT functional status.
Experimental
General characteristics of patients
The study shows the results of the investigation of a group of 10 male patients of age 57–73 with advanced atherosclerosis. All patients were diagnosed with coronary artery disease (CAD) and were subjected to coronary artery bypass surgery. All patients showed hypercholesterolemia and hypertension, and some of them suffered from other co-morbidities; in particular, 6 had confirmed type 2 diabetes mellitus. Information about the patients are given in Table 1. The Ethical Committee of Regional Medical Chamber approved the study (document number L.dz.OIL/KBL/12/2018) and all patients have given consent for the experiment.| Characteristics | Value |
|---|---|
| a Mean value with standard deviation. | |
| Gender (%) | |
| Male | 10 (100%) |
| Female | 0 (0%) |
| Age (years) | 65.6 ± 5.13a |
| Smoking (%) | |
| Smokers | 4 (40%) |
| Non-smokers | 6 (60%) |
| Body mass index (kg m −2 ) | 27.82 ± 3.93a |
| Blood glucose level (mmol L −1 ) | 6.56 ± 1.41a |
| Diagnosis (%) | |
| Coronary artery atherosclerosis | 10 (100%) |
| Arterial hypertension | 10 (100%) |
| Hypercholesterolemia | 10 (100%) |
| Type 2 diabetes mellitus | 6 (60%) |
| Acute myocardial infarction | 3 (30%) |
| Obesity | 3 (30%) |
| Canadian Cardiovascular Society Scale (%) | |
| II | 5 (50%) |
| III | 5 (50%) |
| Number of bypass grafts | |
| 1 | 1 (10%) |
| 2 | 1 (10%) |
| 3 | 5 (50%) |
| 4 | 3 (30%) |
| Atrial fibrillation (%) | 1 (10%) |
| Drug administered before bypass graft | |
| Statins | 10 (100%) |
| ACE inhibitors | 6 (60%) |
| Beta-blockers | 10 (100%) |
| Insulin | 6 (60%) |
Preparation of IMA
Coronary artery bypass procedures (CABG) were performed in a theatre under general anaesthesia. Complete sternotomy was performed for all patients and every CABG surgery was conducted with cardiopulmonary bypass (CPB). For extracorporeal circulation, heparin was administered at a dose of 3–4 mg kg−1 with the monitoring of activating clotting time (ACT).48The internal mammary artery was harvested with an appropriate retractor using the open left pleura technique for easier exposure of the IMA.49 All the arteries were harvested with accompanying perivascular adipose tissue and vessel bunch from the left internal mammary artery to the left anterior descending coronary artery (LIMA–LAD) anastomosis. A short distal fragment not used for anastomosis and approximately 0.5 cm maximum in length was cut and collected in test tubes with 4% buffered formaldehyde for 10 minutes. After preserving, the arteries’ fragments with the tissue were put into another test tubes with phosphate buffered solution (PBS) and were passed on for further laboratory examinations.
In order to preserve the tissue in the unaltered form, the biopsies of IMA with the surrounding PVAT were kept in PBS usually less than 24 hours before Raman spectra were collected. For Raman measurements (description later), a PVAT piece of the size similar to the size of a sesame seed was cut off and put on a CaF2 slide. Then, such prepared samples were placed under the laser beam light. The samples were neither pressed down nor pulled out to flatten. In addition, none of the drying procedure was applied.
Instrumentation
Samples of the PVAT of the IMA were measured using a prototype of a portable WITec Alpha Cart system. The system is equipped with a low-noise CCD detector (Andor), a spectrograph (600 lines per mm grating), an air-cooled solid state laser with an excitation wavelength of 532 nm and a fiber-optic Raman probe tipped with an air objective (Zeiss, 10× magnification, NA = 0.23, WD = 11.1 mm). The setup has a diffraction-limited spectral resolution. With the used objective, the spot size is 1.2 microns laterally and 5 microns vertically. The spectra were recorded by averaging 30 accumulations with 1 s integration time, using the maximum laser power of ca. 28 mW at the sample position. For each measurement, the working distance of the air objective was adjusted to maximize the signal. The experimental setup is presented in Fig. S1 (ESI).† The samples were identified as the PVAT of the IMA based on the localization and Raman spectra that are unique for the adipose tissue.18 For each sample, at least 7 good quality spectra acquired from different locations of the sample were taken for analysis.Data analysis
Preprocessing was done using the WITec Project Plus software and included baseline correction using the autopolynomial of degree 3 and cosmic ray removal procedure. In the second stage, using the OPUS 7.2 program Raman spectra were normalized used vector normalization in the 400–1800 cm−1 spectral ranges. Furthermore, in the averaged spectra of various tissue fragments, the integral intensity of the bands at 1660, 1445 and 1519 cm−1 were calculated. The ratio of bands at 1660/1445 cm−1 was used to determine the unsaturation of lipids. The data were compared in the Origin Pro 9.1 program using the Student's test. If the p parameter was at most 0.05, the differences were identified as statistically significant. The assumption of normality of distribution was checked by the Shapiro–Wilk normality test (p > 0.05) and in one case the distribution was not normal (denoted by *: p < 0.05).Spectral modelling
For the PVAT of IMA composition and quantification of individual lipids were performed with the Solver add-in of Excel software (Microsoft Office 365). This approach enables us to reconstruct the band intensities of a given spectrum based on the database of the Raman spectra of various predicted components. The method requires appropriate spectral preprocessing. The average spectra of all patients and a database of saturated and unsaturated lipids, including palmitic acid, stearic acid, oleic acid, linoleic acid, linolenic acid, arachidonic acid, glyceryl tristearate, glyceryl trioleate, glyceryl trilinolenate and β-carotene, were subjected to slight corrections of band positions (make compatible correction, OPUS 7.2) and, subsequently, were baseline subtracted using the autopolynomial of degree 3 and offset corrected. In addition, vector normalization in the spectral range of 3100–400 cm−1 was done using the Unscrambler X 10.3 software (CAMO Software). To fit the experimental and modelled Raman spectra, the spectral range used to perform the calculations was cut in the 1200–1800 cm−1 spectral range. The modelling process was obtained by adding the intensities of the ingredients from the database of the above-mentioned standard spectra multiplied by the iteratively selected factors to fit the average Raman spectrum of each patient. To indicate the best fitting, the mean square error (MSE) was used.Results and discussion
Clinical characteristics of patients
The study shows the results of the investigation of the chemical composition of the IMA by fiber-optic Raman spectroscopy in a group of 10 male patients (at the age of 57–73 years) with advanced atherosclerosis. The detailed characteristics of the studied group of patients is given in Table S1 (ESI).† All patients were diagnosed with coronary artery disease (CCS II or III) and were undergoing coronary artery bypass surgery. Three patients were diagnosed with myocardial infarction upon admission to the hospital. Moreover, all individuals suffered from hypercholesterolemia and hypertension, 6 from type 2 diabetes mellitus, 4 with kidney diseases (3 with nephrolithiasis and 1 with chronic kidney disease). One patient also had mitral insufficiency and exhibited a suspicious lung lesion (in diagnostics) and one suffered from chronic obstructive pulmonary disease, asthma and bronchiectasis. According to BMI, 2 patients had normal weight (BMI < 25 m kg−2), 5 were overweight (BMI = 25–30 m kg−2) and 3 were moderately obese (BMI > 30 m kg−2, class I). According to the CCS scale, 5 patients were classified as CCS II and 5 patients as CCS III. The observations resulting from this division combined with spectral analysis are presented below.Lipid unsaturation and carotenoid content in PVAT of the IMA
Our previous results demonstrated that the basal unsaturation level of PVAT of the abdominal aorta and the mesenteric artery was considerably higher than that of the thoracic aorta and a significant increase of the unsaturation level of PVAT with age was observed showing that aging has a considerable impact on PVAT's chemical composition.18To evaluate the applicability of fiber-optic Raman spectroscopy to detect a possible PVAT dysfunction in patients with atherosclerosis, the adipose tissue surrounding the IMA was measured. The averaged Raman spectra of the PVAT of the IMA resected from the studied patients exhibit very high variability of the spectral profiles (Fig. S2, ESI†). The averaged Raman spectra of the PVAT of two most distinctive patients normalized in the 1800–400 cm−1 spectral range are presented in Fig. 1A.
The spectral signatures show the band characteristic of the adipose tissue i.e. due to triacylglycerols (bands at 1657, 1441, 1302, and 1270 cm−1) that can be roughly characterized as unsaturated fatty acids at least partially in the esterified form (the band due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration at 1746 cm−1). Strikingly, for some patients, these signals are accompanied by intense bands at 1519, 1158 and 1008 cm−1, undoubtedly arising from carotenoids, interestingly not found in the murine PVAT.18 The position of the band at 1519 cm−1 indicates the carotenoid with 11 conjugated double bonds in its structure that could be β-carotene, the most abundant carotenoid stored in the adipose tissue.50 The level of carotenoids and unsaturation of lipids in the PVAT were evaluated based on the Raman spectra by investigating the integral intensity of the carotenoid band at 1519 cm−1 and the ratio of bands at 1657/1441 cm−1, respectively, and the results are included in Table S1 (ESI†). The analysis reveals substantial individual variations of these two parameters between patients. Surprisingly, this heterogeneity was related to the severity of CAD (vide infra).
O stretching vibration at 1746 cm−1). Strikingly, for some patients, these signals are accompanied by intense bands at 1519, 1158 and 1008 cm−1, undoubtedly arising from carotenoids, interestingly not found in the murine PVAT.18 The position of the band at 1519 cm−1 indicates the carotenoid with 11 conjugated double bonds in its structure that could be β-carotene, the most abundant carotenoid stored in the adipose tissue.50 The level of carotenoids and unsaturation of lipids in the PVAT were evaluated based on the Raman spectra by investigating the integral intensity of the carotenoid band at 1519 cm−1 and the ratio of bands at 1657/1441 cm−1, respectively, and the results are included in Table S1 (ESI†). The analysis reveals substantial individual variations of these two parameters between patients. Surprisingly, this heterogeneity was related to the severity of CAD (vide infra).
To shed more light on the PVAT chemical composition, the spectral profile was resolved using a spectral modelling tool. The modelling of Raman spectra of the PVAT extracted from IMA enabled their decomposition and extraction of its main components. From the database of numerous model compounds (vide Data analysis), only glyceryl trioleate, β-carotene, glyceryl trilinoleate and arachidonic acid were found as PVAT components, although their relative content was highly variable in patients (1% intensity linoleic acid predicted for one patient was neglected). It is important to note that the percentage scale does not reflect the absolute amount of components, but their estimated relative intensity in the experimentally obtained Raman spectra. It is particularly important for β-carotene that has an extremely high scattering cross-section due to resonance at 532 nm and its amount in the tissue is certainly much lower than that of the lipids. Nevertheless, a modelling-based approach suggested quite a distinct content of glyceryl trioleate, β-carotene, glyceryl trilinoleate and arachidonic acid in the PVAT of studied patients.
It is important to note that the modelled spectra of PVA of the IMA did not fit perfectly to the experimental one (Fig. 1A) for patients with high carotenoid level, which could be related with the increased background in these experimental spectra. However, the degree of unsaturation of lipids calculated for modelled spectra versus experimental ones was in good agreement: 0.339 vs. 0.375 and 0.516 vs. 0.505 for patient 1 and 10, respectively, and a determined relative error did not exceed 10% (Table S2 in ESI†). The most interesting finding indicated by modelling was the fact that arachidonic acid was indicated as the PVAT component only for patients with lower carotenoid levels. The presence of highly unsaturated arachidonic acid might explain the higher unsaturation degree of PVAT-forming lipids (Fig. 1B) in these patients. However, arachidonic acid, the precursor of eicosanoids, may indicate the activation of eicosanoid formation in the PVAT of patients with low carotenoid levels. One could hypothesize that in PVAT, a reduced level of carotenoids is linked to inflammation. Moreover, there are some reports that the adipose tissue may consist of linolenic acid.51–53 Human population studies showed that the level of linolenic acid decreases with age51 and may be related to ischemic stroke52 and risk of cardiovascular disease.53 However, to verify this hypothesis, further studies are needed.
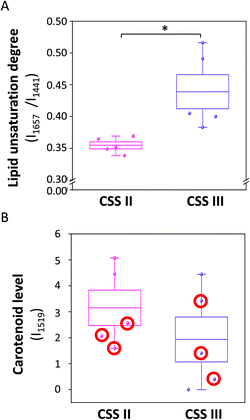
Lipid unsaturation in PVAT of the IMA is related to patients’ CCS Angina Grading Scale and type 2 diabetes influences the PVAT carotenoid level
To better understand the possible clinical significance of our finding, the relation between the lipid unsaturation degree and carotenoid level in the PVAT of the IMA and severity of atherosclerosis expressed as the CCS Angina Grading Scale were studied. The CCS scale, although imperfect, is a qualitative descriptor of the severity of exertional angina, one of the AMI predictors and presents the gold standard.54 Our study shows that for male individuals with atherosclerosis, the CCS Angina Grading Scale was related to the lipid unsaturation (Fig. 2A).Among the relatively small cohort of studied patients, five patients with lower lipid unsaturation in the PVAT of the IMA were assigned to CCS II during diagnosis, while five patients with higher values of lipid unsaturation were assigned to CCS III. The lipid unsaturation degree was significantly (26%) higher for patients with CCS III compared to that for patients with CCS II. In addition, the patients with CCS II had a more homogeneous distribution of the marker of PVAT lipid unsaturation and for CCS III group the heterogeneity was due to the two oldest patients, who had markedly higher levels of lipid unsaturation in PVAT; Table S1 (ESI).†
The carotenoid content was also distinct for CCS III vs. CCS II groups; however, the difference did not reach a statistical significance. However, it was clear that the obtained results regarding carotenoid content seemed to be linked with diabetes. As shown in Fig. 2B, patients in the diabetic group have markedly lower (45%) levels of accumulated carotenoids in the PVAT relative to patients not suffering from diabetes. Interestingly, in a 37,846 participant follow-up study, it was shown that higher dietary carotenoid intake was associated with reduced type 2 diabetes risk.55 It was also reported previously that the β-carotene concentration in the abdominal subcutaneous adipocytes was significantly (over 2-fold) lower for obese (BMI ≥ 30 kg m−2) individuals, both diabetic and non-diabetic3 (vide infra). Accordingly, our findings may support the low carotenoid level in patients with diabetes, while lipid unsaturation seemed to be more clearly linked to the progression of atherosclerosis to CCS III.
Conclusions
Antonopoulos et al.56 using computed tomography recently demonstrated that changes in the coronary perivascular adipose tissue were associated with the degree of inflammation in adjacent coronary plaques underscoring a key role of PVAT in vascular inflammation in humans. Here, we show, to the best of our knowledge, for the first time that the PVAT functional status might be detected by Raman spectroscopy based on the characterization of the PVAT chemical composition, in particular, by the level of carotenoids and lipid unsaturation. In contrast to the imaging techniques that can be time-consuming (Raman imaging), or require elaborate sample preparation (fluorescence imaging, SEM or TEM), application of Raman fiber probes and acquisition of single spectra provide rapid information about the condition of a native and unchanged PVAT in a grafted artery and could be done intraoperatively in future.Based on experimental and modelled Raman spectra, we demonstrated that the PVAT taken from the IMA of 10 patients undergoing cardiac bypass surgery had variable compositions in terms of carotenoid content and lipid unsaturation that may reflect the PVAT functional status. Lipid unsaturation was higher in patients classified as CCS III compared to those as CCS II, whereas the PVAT carotenoid level was low in patients with diabetes independent of the classification of patients as CCS II and CCS III.
The lipid unsaturation and carotenoid content of PVAT can be easily and rapidly measured based on Raman spectra in a semi-quantitative manner and application of fiber-optic probes makes Raman-based methodology suitable to study lipid unsaturation/carotenoid content in the PVAT of IMA or other grafted vessels for the purposes of intraoperative diagnostics. Although IMA is resistant to atherosclerosis development,10,57 its lipid unsaturation/carotenoid content might reflect the PVAT functional status of the patients. In fact, the adipose tissue represents a storage site for retinols58 and carotenoids27,28 and various carotenoid concentrations in human abdominal adipose tissue and plasma2,50,59,60 may represent a contributing factor to energy dissipation in brown and white adipocytes and a degree of inflammation of the adipose tissue.61,62 In turn, increased lipid unsaturation was linked to vascular inflammation;63 thus, both of these biomarkers (lipid unsaturation and carotenoid content) may have pathophysiological relevance.
Altogether, our study suggests that PVAT lipid unsaturation level and carotenoid content represent possible novel biomarkers of the PVAT functional status and their Raman-based assessment in the PVAT of IMA represents a novel diagnostic tool for patients undergoing coronary bypass surgery to rapidly define the PVAT phenotype in a grafted IMA. Further studies on larger groups of patients are necessary to relate our findings to the PVAT molecular phenotype, to validate this technique and to evaluate its possible diagnostic potential in the assessment of vascular inflammation and post-operative prognosis.
Compliance with ethical standards
The Ethical Committee of Regional Medical Chamber approved the study (document number L.dz.OIL/KBL/12/2018) and all patients have given consent for the experiment.Author contribution
S.C. conceived and A.K., K.C. and P.W. designed the experiments. Z.M., K.C. and J.J. performed the Raman experiments. P.W. and R.L. prepared and provided the human adipose tissue samples. K.C., Z.M. and A.K. analysed the data. A.K. drafted the manuscript and S.C. critically revised the manuscript for important intellectual content. All authors reviewed the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The study was supported by the grant from the National Science Centre Poland (NCN) (OPUS9 2015/17/B/ST4/03894 to AK). KC was supported by the Foundation for Polish Science (FNP, START2020 program). Assoc. Prof. PhD, M.D. Jacek Piątek, Assoc. Prof. PhD, M.D. Janusz Konstanty-Kalandyk, PhD, M.D. Tomasz Myrdko and M.D. Bogdan Suder are acknowledged for helpful support during CABG surgery and shearing arteries from patients for this study.References
- J. A. Finegold, P. Asaria and D. P. Francis, Int. J. Cardiol., 2013, 168, 934 CrossRef.
- J.-J. Chiu and S. Chien, Physiol. Rev., 2011, 91, 327 CrossRef.
- P. Rajendran, T. Rengarajan, J. Thangavel, Y. Nishigaki, D. Sakthisekaran, G. Sethi and I. Nishigaki, Int. J. Biol. Sci., 2013, 9, 1057 CrossRef CAS.
- E. C. Eringa, W. Bakker, Y. M. Smulders, E. H. Serné, J. S. Yudkin and C. D. A. Stehouwer, Microcirculation, 2007, 14, 389 CrossRef CAS.
- M. Gollasch and G. Dubrovska, Trends Pharmacol. Sci., 2004, 25, 647 CrossRef CAS.
- M. Gil-Ortega, B. Somoza, Y. Huang, M. Gollasch and M. S. Ferna, Trends Endocrinol. Metab., 2015, 26, 367 CrossRef CAS.
- T. Szasz and R. C. Webb, Clin. Sci., 2012, 122, 1 CrossRef CAS.
- M. S. Fernández-Alfonso, M. Gil-Ortega, C. F. García-Prieto, I. Aranguez, M. Ruiz-Gayo and B. Somoza, Int. J. Endocrinol., 2013, 402053, 10 Search PubMed.
- A. D. Van Dam, M. R. Boon, J. F. P. Berbée, P. C. N. Rensen and V. van Harmelen, Eur. J. Pharmacol., 2017, 816, 82 CrossRef CAS.
- S. B. Police, S. E. Thatcher, R. Charnigo, A. Daugherty and L. A. Cassis, Arterioscler. Thromb. Vasc. Biol., 2009, 29, 1458 CrossRef CAS.
- V. Guglielmi and P. Sbraccia, Eat. Weight Disord. - Stud. Anorexia, Bulim. Obes., 2018, 23, 3 CrossRef.
- M. Rydén, D. P. Andersson, S. Bernard, K. Spalding and P. Arner, J. Lipid Res., 2013, 54, 2909 CrossRef.
- S. N. Verhagen and F. L. J. Visseren, Atherosclerosis, 2011, 214, 3 CrossRef CAS.
- K. Tanaka and M. Sata, Front. Physiol., 2018, 9, 1 CAS.
- G. Hoeke, S. Kooijman, M. R. Boon, P. C. N. Rensen and J. F. P. Berbeé, Circ. Res., 2016, 118, 173 CrossRef CAS.
- J. F. P. Berbe, M. R. Boon, P. P. S. J. Khedoe, A. Bartelt, C. Schlein, A. Worthmann, S. Kooijman, G. Hoeke, I. M. Mol, C. John, C. Jung, N. Vazirpanah, L. P. J. Brouwers, P. L. S. M. Gordts, J. D. Esko, P. S. Hiemstra, L. M. Havekes, L. Scheja, J. Heeren and P. C. N. Rensen, Nat. Commun., 2015, 6, 1 Search PubMed.
- C. Lohmann, N. Schäfer, T. von Lukowicz, M. A. S. Stein, J. Borén, S. Rütti, W. Wahli, M. Y. Donath, T. F. Lüscher and C. M. Matter, Arterosclerosis, 2009, 207, 360 CrossRef CAS.
- K. Czamara, Z. Majka, A. Fus, K. Matjasik, M. Z. Pacia, M. Sternak, S. Chlopicki and A. Kaczor, Analyst, 2018, 143, 5999 RSC.
- Z. F. Huang Cao, E. Stoffel and P. Cohen, Hypertension, 2017, 69(5), 770–777 CrossRef.
- T. P. Fitzgibbons, S. Kogan, M. Aouadi, G. M. Hendricks, J. Straubhaar and M. P. Czech, Am. J. Physiol.: Circ. Physiol., 2011, 301, 1425 Search PubMed.
- E. E. Soltis and L. A. Cassis, Clin. Exp. Hypertens., 1991, 13, 277 CAS.
- M. Löhn, G. Dubrovska, B. Lauterbach, F. C. Luft, M. Gollasch and A. M. Sharma, FASEB J., 2002, 16, 1057 CrossRef.
- F. Tourniaire, E. Gouranton, J. von Lintig, J. Keijer, M. L. Bonet, J. Amengual, G. Lietz and J.-F. Lrier, Genes Nutr., 2009, 4, 179 CrossRef CAS.
- J. A. Wise, G. R. Kaats, H. G. Preuss and R. J. Morin, Int. J. Food Sci. Nutr., 2009, 60, 65 CrossRef CAS.
- L. Wang, M. Gaziano, E. P. Norkus, J. E. Burning and H. D. Sesso, Am. J. Clin. Nutr., 2008, 88, 747 CrossRef CAS.
- L. F. Andersen, D. R. Jacobs Jr., M. D. Gross, P. J. Schreiner, O. Dale Williams and D. H. Lee, Br. J. Nutr., 2006, 95(2), 358–365 CrossRef CAS.
- L. Kaplan, J. Lau and E. Stein, Clin. Physiol. Biochem., 1990, 8, 1 CAS.
- R. Parker, J. Nutr., 1989, 119, 101 CrossRef CAS.
- S. Hildebr, J. Stümer and A. Pfeifer, Front. Physiol., 2018, 9, 1 Search PubMed.
- M. M. Ciccone, F. Cortese, M. Gesualdo, S. Carbonara, A. Zito, G. Ricci, F. De Pascalis, P. Scicchitano and G. Riccioni, Mediators Inflammation, 2013, 782137, 1 Search PubMed.
- Y. Ito, M. Kurata, K. Suzuki, N. Hamajima, H. Hishida and K. Aoki, J. Epidemiol., 2006, 16, 1 CrossRef.
- A. Shaish, A. Harari, L. Hananshvili, H. Cohen, R. Bitzur, T. Luvish, E. Ulman, M. Golan, A. Ben-Amotz, D. Gavish, Z. Rotstein and D. Harats, Atherosclerosis, 2006, 189, 215 CrossRef CAS.
- S. K. Myung, W. Ju, B. Cho, S. W. Oh, S. M. Park, B. K. Koo and B. J. Park, Br. Med. J., 2013, 346, 12 CrossRef.
- D. A. Street, G. W. Comstock, R. M. Salkeld, W. Schüep and M. J. Klag, Circulation, 1994, 90, 1154 CrossRef CAS.
- G. Bjelakovic, D. Nikolova, L. L. Gluud, R. G. Simonetti and C. Gluud, Cochrane Database Syst. Rev., 2012, 133, 1 Search PubMed.
- A. N. Howard, N. R. Williams, C. R. Palmer, J. P. Cambou, A. E. Evans, J. W. Foote, P. Marques-Vidal, E. E. McCrum, J. B. Ruidavets, S. V. Nigdikar, J. Williams-Fajput and D. I. Thurnham, Int. J. Vitam. Nutr. Res., 1996, 66, 113 CAS.
- J. Karppi, S. Kurl, T. H. Mäkikallio, K. Ronkainen and J. A. Laukkanen, Int. J. Cardiol., 2013, 168, 1841 CrossRef.
- Q. Bin, X. Hu, Y. Cao and F. Gao, Thromb. Haemostasis, 2011, 105, 579 CrossRef CAS.
- H. D. Sesso, J. E. Buring, E. P. Norkus and J. M. Gaziano, Am. J. Clin. Nutr., 2004, 79, 47 CrossRef CAS.
- T. Hirvonen, J. Virtamo, P. Korhonen, D. Albanes and P. Pietinen, N. Engl. J. Med., 2000, 31, 2301 CAS.
- J. Karppi, J. A. Laukkanen, T. H. Mäkikallio, K. Ronkainen and S. Kurl, Nutr. Metab. Cardiovasc. Dis., 2012, 22, 921 CrossRef CAS.
- J. Karppi, J. A. Laukkanen, T. H. Mäkikallio, K. Ronkainen and S. Kurl, Atherosclerosis, 2013, 226, 172 CrossRef CAS.
- O. Stevens, I. E. Iping Petterson, J. C. C. Day and N. Stone, Chem. Soc. Rev., 2016, 45, 1919 RSC.
- I. Latka, S. Dochow, C. Krafft, B. Dietzek and J. Popp, Laser Photonics Rev., 2013, 7, 698 CrossRef CAS.
- P. Meksiarun, B. B. Andrian, H. Matsuyoshi and H. Sato, Sci. Rep., 2016, 1–8 Search PubMed.
- K. St-Arnaud, K. Aubertin, M. Strupler, W. J. Madore, A. A. Grosset, K. Petrecca, D. Trudel and F. Leblond, Med. Phys., 2018, 45, 328–339 CrossRef CAS.
- K. Czamara, Z. Majka, M. Sternak, M. Koziol, R. B. Kostogrys, S. Chlopicki and A. Kaczor, Int. J. Mol. Sci., 2020, 21, 1–14 Search PubMed.
- R. M. Bojar, Manual of Perioperative Care in Adult Cardiac Surgery, Willey – Blackwell, 2010 Search PubMed.
- L. H. Cohn and J. Bryne, Cardiac Surgery in the Adult, McGraw-Hill Co, 2008 Search PubMed.
- M. Osth, A. Ost, P. Kjolhede and P. Stralfors, PLoS One, 2014, 9, 1 CrossRef.
- C. Bolton-Smith, M. Woodward and R. Tavendale, Eur. J. Clin. Nutr., 1997, 51, 619–624 CrossRef CAS.
- C. S. Bork, S. K. Veno, S. Lundbye-Christensen, M. U. Jakobsen, A. Tjønneland, P. C. Calder, K. Overvad and E. B. Schmidt, PLoS One, 2018, 13, 1–13 CrossRef.
- A. Pan, M. Chen, R. Chowdhury, Q. Sun, H. Campos, D. Mozaffarian and F. B. Hu, Am. J. Clin. Nutr., 2012, 96, 1262–1273 CrossRef CAS.
- L. Campeau, Can. J. Cardiol., 2002, 18, 371 Search PubMed.
- I. Sluijs, E. Cadier, J. W. J. Beulens, D. L. van der A, A. M. W. Spijkerman and Y. T. van der Schouw, Nutr. Metab. Cardiovasc. Dis., 2015, 25, 376 CrossRef CAS.
- A. S. Antonopoulos, F. Sanna, N. Sabharwal, S. Thomas, E. K. Oikonomou, L. Herdman, M. Margaritis, C. Shirodaria, A.-M. Kampoli, I. Akoumianakis, M. Petrou, R. Sayeed, G. Krasopoulos, C. Psarros, P. Ciccone, C. M. Brophy, J. Digby, A. Kelion, R. Uberoi, S. Anthony, N. Alexopoulos, D. Tousoulis, S. Achenbach, S. Neubauer, K. M. Channon and C. Antoniades, Sci. Transl. Med., 2017, 9, 1 Search PubMed.
- J. Padilla, N. T. Jenkins, V. J. Vieira-Potter and M. H. Laughlin, Am. J. Physiol.: Integr. Comp. Physiol., 2013, 304, 543 Search PubMed.
- C. Tsutsumi, M. Okuno, L. Tannous, R. Piantedosi, M. Allan, D. S. Goodman and W. S. Blaners, J. Biol. Chem., 1992, 267, 1805 CAS.
- A. El-Sohemy, A. Baylin, E. Kabagambe, A. Ascherio, D. Spiegelman and H. Campos, Am. J. Clin. Nutr., 2002, 76, 172 CrossRef CAS.
- L. Kohlmeier and M. Kohimeier, Environ. Health Perspect., 1995, 103, 99 CAS.
- E. Gouranton, C. El Yazidi, N. Cardinault, M. J. Amiot, P. Borel and J.-F. Lrier, Food Chem. Toxicol., 2008, 46, 3832 CrossRef CAS.
- M. L. Bonet, J. A. Canas, J. Ribot and A. Palou, in Carotenoids in Nature: Biosynthesis, Regulation Function, 2016, p. 374 Search PubMed.
- K. Czamara, K. Majzner, A. Selmi, M. Baranska, Y. Ozaki and A. Kaczor, Sci. Rep., 2017, 7, 1 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: The experimental setup, detailed characteristics of studied patients, experimental and modelled values of lipid unsaturation degree and carotenoid level, Raman spectra of the PVAT of the IMA for all studied patients. See DOI: 10.1039/d0an01868f |
| ‡ Both authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |