A cerium-based fluorescent nanosensor for highly specific detection of glutathione over cysteine and homocysteine†
Tianlin
Wang
ab,
Zhanhui
Tao
b,
Chi
Qu
b,
Shuo
Wang
*c and
Yaqing
Liu
 *ab
*ab
aBeijing Advanced Innovation Center for Food Nutrition and Human Health, Beijing Technology and Business University, Beijing 100037, P. R. China
bState Key Laboratory of Food Nutrition and Safety, College of Food Engineering and Biotechnology, Tianjin University of Science and Technology, Tianjin 300457, P. R. China. E-mail: yaqingliu@tust.edu.cn
cTianjin Key Laboratory of Food Science and Health, School of Medicine, Nankai University, Tianjin, 300071, P. R. China. E-mail: wangshuo@nankai.edu.cn
First published on 21st October 2020
Abstract
Ever-increasing attention has been focused on constructing a sensing system for specific detection of glutathione (GSH) over cysteine (Cys) and homocysteine (Hcy), which usually interfere with the GSH detection due to their similar structures and the presence of thiol groups in these amino acids. Here, a novel fluorescence-sensing system is developed for highly specific GSH detection over Cys and Hcy. The sensing system is constructed through facilely mixing dipicolinic acid (DPA) and guanosine 5′-monophosphate (GMP) with cerium acetate at ambient conditions, denoted as DPA-Ce-GMP. The resultant DPA-Ce-GMP possesses fluorescence emission with excellent thermal stability and anti-light bleaching, which can be quenched by copper ions (Cu2+). The GSH, and not Cys or Hcy, can trap Cu2+ from DPA-Ce-GMP, resulting in the restoration of the fluorescence of the sensing system. The limit of detection reaches as low as 7.1 nM. The GSH detection in a real sample of human serum was further explored and exhibits satisfactory recovery. The developed sensing system has the advantages of ease-of-preparation, excellent selectivity and stability, demonstrating its potential application in disease diagnosis in the future.
Induction
Glutathione (GSH), a thiol-containing tripeptide, plays crucial roles in maintaining biological functions, including intracellular redox activities, gene regulation, metabolism and detoxification.1–3 GSH in aberrant levels correlates with numerous diseases, such as heart problems, leucocyte loss, neurodegenerative disorders and even cancer.4–7 Thus, a method for the highly specific detection of GSH is in urgent demand for practical applications in clinical diagnosis. Traditional detection assays for determining GSH include high-performance liquid chromatography (HPLC),8 mass spectrometry,9 and capillary zone electrophoresis.10 It is noted that these methods usually suffer from limitations such as the requirement of expensive instruments, sophisticated operation, or high cost. In recent years, great attention has been focused on constructing biosensors, including colorimetric, electrochemical and fluorescence biosensors due to their simple and easy operation.11–16 Among the biosensors, fluorescence-based systems have attracted increasing attention owing to their real-time, variable luminescence, and photostability properties.17 For example, Yao et al. designed a MnO2 nanomaterial-based sensing platform for the fluorescence sensing of GSH.18 Li et al. reported a dual-emission carbon nanodot-based nanosensor for the ratiometric fluorescence sensing of GSH.19 Though there have been outstanding achievements, it is worth noting that distinguishing GSH from cysteine (Cys) and homocysteine (Hcy), which usually interfere with GSH detection as they have similar thiols, carboxylic, and amino functional groups is still a great challenge.20–22 That limits the potential application of GSH detection in biological analysis and clinical diagnosis.Lanthanides (Ln3+) are considered excellent luminescent centers due to their unique [Xe]4fN electronic configurations and ladder-like energy states.23 Lanthanide-doped nanomaterials (Ln-NMs) are widely recognized for their unique luminescence properties including narrow emission bandwidths, multiple emission bands, long luminescence lifetimes, and large Stokes/anti-Stokes.24–26 Thus, Ln-NMs are usually used as powerful building blocks in constructing fluorescent sensors, optical devices and anti-counterfeiting.27–32 Among Ln-NMs, cerium-based nanomaterials exhibit unique property owing to their mixed-valence (Ce3+ and Ce4+). The reversible switching of Ce3+/Ce4+ can confer mimic-enzyme properties to the cerium compounds,33,34 enabling them as colorimetric probes in bioanalyses.35–37 However, rare cerium-based nanomaterials exhibit strong fluorescence emission features that hinder their application in fluorescence-based bioimaging and sensing.38 In our previous study, a novel Ce-based nanomaterial was synthesized by us via a simple UV irradiation of a mixture of dipicolinic acid (DPA), guanosine 5′-monophosphate (GMP) and cerium acetate under ambient conditions.39 The resultant DPA-Ce-GMP exhibited blue luminescence with a high quantum yield and outstanding stability (Scheme 1A). The fluorescence of DPA-Ce-GMP could be efficiently quenched by copper ions (Cu2+) due to the coordination of Cu2+ with DPA and GMP.40,41 It is interesting to note that GSH and not Cys and Hcy can recover the fluorescence due to the high affinity between Cu2+ and GSH (Scheme 1B).42,43 Thus, a novel sensing system for GSH detection over Cys and Hcy with high sensitivity and specificity is successfully developed. GSH detection in human serum samples is further explored, which might be important for promoting potential applications in disease diagnosis in the future.
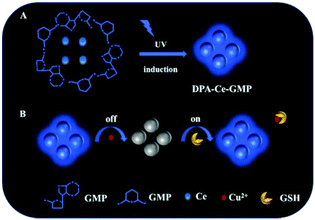 | ||
| Scheme 1 (A) Schematic of the DPA-Ce-GMP synthesis. (B) DPA-Ce-GMP-based fluorescence probe for GSH detection. | ||
Experimental section
Chemicals and materials
Cerium acetate (Ce(CH3COO)3), guanosine 5′-monophosphate disodium salt hydrate (GMP), dipicolinic acid (DPA) were purchased from Aladdin Reagent (Shanghai, China). Metal salts, α-amino acids, GSH, Cys and Hcy were purchased from Sinopharm Chemical Reagent Company (Shanghai, China). All aqueous solutions were prepared with Milli-Q water (18.2 MΩ cm−1).Apparatus and characterization
Fluorescence spectra were recorded on a LUMINA fluorescence spectrometer (Thermo, USA). Absorption spectra were recorded on a UV-2550 UV-vis spectrophotometer (Shimadzu, Japan). Fourier transform infrared (FT-IR) spectra were recorded on a Tensor 27 spectrophotometer (BRUKER, Germany). X-ray photoelectron spectroscopy (XPS) measurement was performed on an Axis Ultra DLD X-ray photoelectron spectrometer (Kratos, England). X-ray powder diffraction (XRD) patterns were recorded on a Bruker D8 ADVANCE (Germany). X-ray diffraction was performed using a diffractometer with Cu Kα radiation. Transmission electron micrographs were recorded on a JEM-3010 transmission electron microscope (TEM) (Hitachi, Japan).Preparation of DPA-Ce-GMP
DPA-Ce-GMP was prepared according to our previous study.39 Briefly, a Ce(CH3COO)3 aqueous solution (10.0 mM, 1 mL) was added into the GMP aqueous solution (10.0 Mm, 1 mL). After stirring for 10 s, the white precipitate was collected via centrifugation (6000 rpm) for 5 min. After being washed three times with deionized water, the precipitate was re-dispersed in 2 mL of deionized water and added to the DPA (10.0 mM, 2 mL) aqueous solution. The whole mixture became clear and transparent with its dropping into DPA. Then, the mixture was subjected to UV irradiation (302 nm) for 20 min. The produced DPA-Ce-GMP was purified via dialysis against ultrapure water for 12 h. The dialysis membrane had a molecular weight cut-off of 10 kDa. The final powder was collected after vacuum freeze-drying.Fluorescence detection of GSH based on DPA-Ce-GMP
GSH with different concentrations (from 0 μM to 50 μM) was added to the Tris-HCl (10 mM, pH = 7.5) buffer containing the DPA-Ce-GMP/Cu2+ sensing system. The concentrations of DPA-Ce-GMP and Cu2+ were 0.1 mg mL−1 and 20 μM, respectively. The measurements were carried out after intensive blending for 0.5 min.Detection of GSH in human blood serum and urine samples
The fresh serum samples were diluted to 100-fold with distilled water. For GSH determination in human urine samples, samples were centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min, and then the supernatant was diluted to 100-fold with distilled water. The GSH solutions (0.2 μM, 8 μM, 20 μM) were then spiked into the diluted samples and further added into the DPA-Ce-GMP/Cu2+ sensing system. The measurements were carried out after intensive blending for 0.5 min. The human serum and urine samples were obtained from healthy individuals at Tianjin University of Science and Technology School Hospital. Serum experiments were performed according to the Guidelines for Ethical Committee of Tianjin University of Science and Technology. All studies were approved by the Ethical Committee of Tianjin University of Science and Technology. Informed consent was obtained from the human participants in this study.
000 rpm for 10 min, and then the supernatant was diluted to 100-fold with distilled water. The GSH solutions (0.2 μM, 8 μM, 20 μM) were then spiked into the diluted samples and further added into the DPA-Ce-GMP/Cu2+ sensing system. The measurements were carried out after intensive blending for 0.5 min. The human serum and urine samples were obtained from healthy individuals at Tianjin University of Science and Technology School Hospital. Serum experiments were performed according to the Guidelines for Ethical Committee of Tianjin University of Science and Technology. All studies were approved by the Ethical Committee of Tianjin University of Science and Technology. Informed consent was obtained from the human participants in this study.
Results and discussion
Characteristics of DPA-Ce-GMP
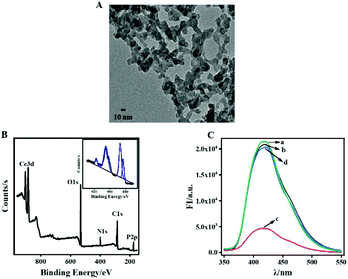
A transmission electron microscope (TEM) was used to investigate the morphology of the as-prepared DPA-Ce-GMP, as shown in Fig. 1A. The TEM image shows a typical network structure due to the coordination among DPA, GMP and Ce, proving the successful synthesis of DPA-Ce-GMP.44 The elemental composition of DPA-Ce-GMP was studied via X-ray photoelectron spectroscopy (XPS). As shown in Fig. 1B, the peaks located at 285 eV, 400 eV, 532 eV and 175 eV are attributed to O 1s, N 1s, C 1s, and P 2p, respectively, originating from DPA and GMP in DPA-Ce-GMP. The features XPS of Ce are resolved into two major peaks at 886 eV and 904 eV, corresponding to the spin–orbit split 3d5/2 and 3d3/2. Moreover, a peak located at 916 eV is ascribed to the characteristic of the Ce4+ state.37,45 Fourier transform infrared spectroscopy and X-ray diffraction experimental results shown in Fig. S1 and S2 in ESI† respectively, further validate the successful preparation of DPA-Ce-GMP.DPA-Ce-GMP exhibits excellent fluorescence properties and is further used for constructing a sensing system for GSH detection. Steady-state fluorescence measurements were further carried out to evaluate the feasibility of the designed strategy. As illustrated in Fig. 1C, DPA-Ce-GMP exhibits bright blue emission with a maximum fluorescence intensity at 415 nm (Fig. 1C, a). The fluorescence of DPA-Ce-GMP cannot be influenced by GSH (Fig. 1C, b) which can be quenched by Cu2+ due to the coordination between DPA and GMP with Cu2+ (Fig. 1C, c). Considering that GSH possesses a tendency to generate Cu–S bonds,46 the extensive trapping of Cu2+ from DPA-Ce-GMP might be achieved by GSH. That recovers the quenched fluorescence (Fig. 1C, d), indicating that the binding capability between Cu2+ and GSH is stronger than that between Cu2+ and DPA-Ce-GMP. The results validate the possibility for GSH detection with Cu2+ as the fluorescence mediator according to the interaction among DPA-Ce-GMP, GSH and Cu2+.
Optimization of experimental parameters
Before GSH detection, optimal detection conditions were obtained by exploring the influence of the Cu2+ concentration, pH of the buffer solution, and incubation time of GSH in the sensing system. Considering the quenching ability of Cu2+ to DPA-Ce-GMP, the amount of Cu2+ used is critical. If the amount of Cu2+ used is too less, GSH detection with a high concentration might not be achieved, thus resulting in a low signal-to-background ratio with a narrow detection range. If the amount of Cu2+ used is superfluous, more GSH is then required to hinder the fluorescence quenching of Cu2+ and that is not in favour of obtaining a low detection limit. Thus, the fluorescence titration experiments were first performed to monitor the quenching ability of different amounts of Cu2+ to DPA-Ce-GMP to find an optimal experimental condition. As illustrated in Fig. 2A, the intensity decreases in the fluorescence spectrum of DPA-Ce-GMP with the increase for the concentration of Cu2+, and the fluorescence response reaches a plateau once the concentration of Cu2+ is higher than 20 μM (Fig. 2B), which is selected for the construction of the DPA-Ce-GMP/Cu2+ sensing system. For pH investigation, the fluorescence of DPA-Ce-GMP increases with the solution pH increasing from 6.0 to 7.5 and then decreases at higher pH values (Fig. 2C). Therefore, pH 7.5 is chosen for the subsequent measurements. The effect of the incubation duration of GSH in the sensing system is also explored, as shown in Fig. 2D. Once the sensor is exposed to GSH, the fluorescence response significantly decreases to a minimum within 0.5 min. After that, no obvious decrease is observed with extended incubation duration, which indicates that the reaction equilibrium is rapidly reached within 0.5 min.Sensitive detection of GSH
Due to the chelation between GSH and Cu2+, more GSH is added, the fewer free Cu2+ ions are left to interact with DPA-Ce-GMP. As a result, the fluorescence of DPA-Ce-GMP is restored with the increase in the GSH concentration (Fig. 3A). To evaluate the repeatability of the constructed sensing system, error bars are calculated by plotting the degree of fluorescence intensity change (ΔFI = FI − FI0) at 415 nm as a function of the concentration of GSH (Fig. 3B), where FI and FI0 are fluorescence intensities in the presence and absence of GSH, respectively. Under the optimal detection condition, two linear relationships of fluorescence intensity change against GSH concentration were found from 0.01 μM to 10 μM with the correlation coefficient (R2) of 0.996 and from 15 μM to 40 μM with correlation coefficient (R2) of 0.997 (Fig. 3B and C). The LOD is found at 7.1 nM by calculating three times of signal to noise, which is much lower than the previous reports (see Table S1 in ESI†), confirming the high sensitivity of the developed assay.Stability of the DPA-Ce-GMP sensing system
The stability of the sensing system is critical in their potential applications to adapt to various environmental conditions. Here, the stability of the developed sensing system is evaluated under different conditions. As illustrated in Fig. 4A, DPA-Ce-GMP can endure continuous high-power UV irradiation (60 W) for 2 h. No obvious fluorescence decay is observed indicating the outstanding anti-light bleaching property. After being stored in the dark for six months, negligible fluorescence attenuation is detected (Fig. 4B), revealing excellent long-term stability. The thermal stability of DPA-Ce-GMP was investigated by monitoring its fluorescence response with increasing temperature. Only a slight change is observed as the temperature goes up from 22 °C to 150 °C (Fig. 4C), suggesting the excellent temperature-quenching resistance. The results indicate that DPA-Ce-GMP can act as excellent building blocks for constructing biosensors used in practical detection. | ||
| Fig. 4 The fluorescent responses of the DPA-Ce-GMP against the UV irradiation time (60 W) (A), long-term reservation up to 180 days (B), and temperature increase from 22 °C to 150 °C. | ||
Determining specificity for GSH detection
The development of a selective sensor in the physiological condition for GSH detection is critically important as a variety of diseases are associated with GSH. Thus, the potential interferences such as amino acids, saccharides, proteins, cations, and ascorbic acid are subsequently evaluated. As illustrated in Fig. 5A, the potential interfering molecules have a negligible effect on the fluorescence response of the constructed sensing system. In particular, no obvious influence is monitored from Cys and Hcy though cysteine and Hcy could also react with Cu2+ due to the presence of the thiol group.47,48 Wang et al. reported that Cys and Hcy tend to form Cu–N bond, the subsequent formation of Cu–S is far less stable than that of the Cu–S bond formed with GSH.46 Hence, the possible reason might be that the Cu–S bond between Cu2+ and Cys/Hcy is far weaker than that between Cu2+ and DPA-Ce-GMP. Further, the effects of GSH, Cys and Hcy on the UV-vis absorption spectra of DPA-Ce-GMP was implemented to further understand the high selectivity of the sensing system towards GSH, Fig. 5B. As learned from the absorption spectra of DPA-Ce-GMP (curve a), two feature peaks at 270 nm and 255 nm are arising from DPA (curve b) and GMP (curve c), respectively. The addition of Cu2+ results in a significant enhancement of the intensities of the two absorption peaks (curve d). The addition of GSH to the DPA-Ce-GMP/Cu2+ sensor restored the absorbance response as that of DPA-Ce-GMP (curve e), that might be ascribed to the stronger coordination interaction between Cu2+ and GSH than that between Cu2+ and DPA-Ce-GMP, which was further validated by first mixing GSH with Cu2+ to form a Cu-GSH complex (curve f). The resultant Cu-GSH was then incubated with DPA-Ce-GMP, and no obvious change was monitored from the respective absorption spectra (curve g), indicating that Cu2+ in Cu-GSH cannot be trapped by DPA-Ce-GMP. Further, as shown in Fig. S3,† a peak at 935 eV was found from the XPS spectrum of DPA-Ce-GMP/Cu2+, which is attributed to the feature of Cu2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 (curve a). After incubating with GSH, the feature peak of Cu2+ shifts to 932.2 eV (curve b), which was caused by the interaction between Cu2+ and GSH.49 The XPS results further validate that the interaction between Cu2+ and GSH is stronger than that between Cu2+ and DPA-Ce-GMP. Different from GSH, the presence of Cys or Hcy has no obvious effect on the absorbance response of the sensing system (curves h and i). A possible reason might be that the coordination interaction between Cys/Hcy and Cu2+ is weaker than that between Cu2+ and DPA-Ce-GMP. The results are consistent with the fluorescence results, further validating the excellent selectivity of the constructed sensing system.
49 (curve a). After incubating with GSH, the feature peak of Cu2+ shifts to 932.2 eV (curve b), which was caused by the interaction between Cu2+ and GSH.49 The XPS results further validate that the interaction between Cu2+ and GSH is stronger than that between Cu2+ and DPA-Ce-GMP. Different from GSH, the presence of Cys or Hcy has no obvious effect on the absorbance response of the sensing system (curves h and i). A possible reason might be that the coordination interaction between Cys/Hcy and Cu2+ is weaker than that between Cu2+ and DPA-Ce-GMP. The results are consistent with the fluorescence results, further validating the excellent selectivity of the constructed sensing system.
Detection of GSH in serum and urine sample
Since GSH plays a crucial role in maintaining biological functions, it is essential to maintain a normal level of glutathione in the serum. Here, human serum was used as a real sample to evaluate the practical feasibility of the developed sensors. GSH concentration in the diluted human serum samples was determined as 0.087 μM by following the same detection procedure as discussed above, while it was not detected in the diluted human urine samples. The standard addition method is further used to explore the practicability of the developed sensing system for GSH detection. The spiked GSH can be accurately measured with good recovery (from 92.5% to 99.9%) and suitable relative standard deviation (RSD < 4.6%) in human serum samples, and good recovery (from 95.2% to 116.0%) and suitable relative standard deviation (RSD < 5.7%) in human urine samples. The results confirm that the developed sensing strategy has potential applications for GSH detection in biological samples (Table 1).| Sample | Found (μM) | Added (μM) | Total found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Human serum | 0.087 | 0.2 | 0.272 ± 0.012 | 92.5 | 4.4 |
| 8 | 8.013 ± 0.131 | 99.1 | 1.6 | ||
| 20 | 20.065 ± 0.936 | 99.9 | 4.6 | ||
| Human | 0.2 | 0.232 ± 0.012 | 116.0 | 5.2 | |
| Urine | — | 8 | 8.135 ± 0.092 | 101.7 | 1.1 |
| 20 | 19.039 ± 1.081 | 95.2 | 5.7 |
Conclusions
We constructed a DPA-Ce-GMP-based fluorescence sensing system for highly specific detection of GSH with Cu2+ as a fluorescence mediator. The fluorescence of DPA-Ce-GMP can be efficiently quenched by Cu2+. The quenched fluorescence of the DPA-Ce-GMP-Cu2+ complex can be restored due to the stronger coordination interaction between GSH and Cu2+. GSH can be specifically determined from Cys and Hcy with high sensitivity. Interestingly, DPA-Ce-GMP can resist high temperature and long light irradiation, presenting outstanding stability, making it excellent building block for constructing the sensing system. The sensing system has the advantages of feasible preparation, simple operation and low cost and has great potential applications in practical GSH detection in real samples.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (no. 21575138 and 21775108) and Tianjin Science and Technology Project (18PTSYJC00130).Notes and references
- S. L. James, Chem. Soc. Rev., 2003, 32, 276–288 RSC
.
- A. Y. Robin and K. M. Fromm, Coord. Chem. Rev., 2006, 250, 2127–2157 CrossRef CAS
.
- S. V. Eliseeva and J. C. G. Bünzli, Chem. Soc. Rev., 2010, 39, 189–227 RSC
.
- A. Gupta, N. C. Verma, S. Khan and C. K. Nandi, Biosens. Bioelectron., 2016, 81, 465–472 CrossRef CAS
.
- Y. Jiao, J. F. Gao, Y. T. Meng, W. J. Lu, Y. Liu, H. Han, S. M. Shuang, C. Li and C. Dong, ACS Appl. Mater. Interfaces, 2019, 11, 16822–16829 CrossRef CAS
.
- Q. B. Wang, C. J. Zhang, Q. Lu, Z. E. Liu, J. S. Yao and X. Zhang, Dyes Pigm., 2020, 176, 108189 CrossRef CAS
.
- R. Jalili, A. Khataee, M. R. Rashidi and R. Luque, Sens. Actuators, B, 2019, 297, 126775 CrossRef CAS
.
- T. R. I. Cataldi and D. Nardiello, J. Chromatogr. A, 2005, 1066, 133–142 CrossRef CAS
.
- S. K. Kailasa, N. Hasan and H. F. Wu, Talanta, 2012, 97, 539–549 CrossRef CAS
.
- P. Kubalczyk and E. Bald, Electrophoresis, 2009, 30, 2280–2283 CrossRef CAS
.
- L. Li, Q. Wang and Z. Chen, Microchim. Acta, 2019, 186, 257 CrossRef
.
- Z. J. Wang, X. J. Ding, Y. Y. Huang, X. J. Yan, B. Ding, Z. Q. Li, C. Z. Xie and J. Y. Xu, Dyes Pigm., 2020, 175, 108156 CrossRef CAS
.
- C. Song, W. Ding, W. W. Zhao, H. B. Liu, J. Wang, Y. W. Yao and C. Yao, Biosens. Bioelectron., 2020, 151, 111983 CrossRef CAS
.
- A. C. Sedgwick, H. H. Han, J. E. Gardiner, S. D. Bull, X. P. He and T. D. James, Chem. Sci., 2018, 9, 3672–3676 RSC
.
- K. M. Xiong, F. J. Huo, J. B. Chao, Y. B. Zhang and C. X. Yin, Anal. Chem., 2019, 91, 1472–1178 CrossRef CAS
.
- Z. X. Wang, P. Han, X. X. Mao, Y. M. Yin and Y. Cao, Sens. Actuators, B, 2017, 238, 325–330 CrossRef CAS
.
- B. Y. Zhang, Q. Q. Duan, Y. Li, Y. X. Zhang, M. X. Che, W. D. Zhang and S. B. Sang, J. Photochem. Photobiol., B, 2019, 197, 111532 CrossRef CAS
.
- C. P. Yao, J. Wang, A. X. Zheng, L. J. Wu, X. L. Zhang and X. L. Liu, Sens. Actuators, B, 2017, 252, 30–36 CrossRef CAS
.
- L. Li, L. H. Shi, J. Jia, O. Eltayeb, W. J. Lu, Y. H. Tang, C. Dong and S. M. Shuang, ACS Appl. Mater. Interfaces, 2020, 12, 18250–18257 CrossRef CAS
.
- J. Ge, R. Cai, X. G. Chen, Q. Wu, L. L. Zhang, Y. Jiang, C. Cui, S. Wan and W. H. Tan, Talanta, 2019, 195, 40–45 CrossRef CAS
.
- J. Liu, L. J. Meng, Z. F. Fei, P. J. Dyson, X. N. Jing and X. Liu, Biosens. Bioelectron., 2017, 90, 69–74 CrossRef CAS
.
- M. J. Huang, H. Wang, D. P. He, P. Jiang and Y. Zhang, Chem. Commun., 2019, 55, 3634–3637 RSC
.
- Q. Q. Ma, J. Wang, Z. H. Li, X. B. Lv, L. Liang and Q. Yuan, Small, 2019, 15, 1804969 CrossRef
.
- X. H. Wang, H. J. Chang, J. Xie, B. Z. Zhao, B. T. Liu, S. L. Xu, W. B. Pei, N. Ren, L. Huang and W. Huang, Coord. Chem. Rev., 2014, 273, 201–212 CrossRef
.
- J. Feng and H. J. Zhang, Chem. Soc. Rev., 2013, 42, 387 RSC
.
- Y. J. Cui, B. L. Chen and G. D. Qian, Coord. Chem. Rev., 2014, 273, 76–86 CrossRef
.
- W. Yan, C. L. Zhang, S. G. Chen, L. J. Han and H. G. Zheng, ACS Appl. Mater. Interfaces, 2017, 9, 1629–1634 CrossRef CAS
.
- T. S. Mahapatra, H. Singh, A. Maity, A. Dey, S. K. Pramanik, E. Suresh and A. Das, J. Mater. Chem. C, 2018, 6, 9756–9766 RSC
.
- S. Schweizer, A. C. Rimbach, M. Mungra, B. Ahrens, F. Steudel and P. W. Nolte, Opt. Mater., 2019, 88, 74–79 CrossRef CAS
.
- D. W. Zhang, W. Zhou, Q. L. Liu and Z. G. Xia, ACS Appl. Mater. Interfaces, 2018, 10, 27875–27884 CrossRef CAS
.
- Y. M. Wang, X. T. Tian, H. Zhang, Z. R. Yang and X. B. Yin, ACS Appl. Mater. Interfaces, 2018, 10, 22445–22452 CrossRef CAS
.
- Z. L. Xue, S. J. Zeng and J. H. Hao, Biomaterials, 2018, 171, 153–163 CrossRef CAS
.
- Y. H. Lin, J. S. Ren and X. G. Qu, Acc. Chem. Res., 2014, 47, 1097–1105 CrossRef CAS
.
- A. Asati, C. Kaittanis, S. Santra and J. M. Perez, Anal. Chem., 2011, 83, 2547–2553 CrossRef CAS
.
- J. D. Wang, X. Xiao, Y. Liu, K. M. Pan, H. Pang and S. Z. Wei, J. Mater. Chem. A, 2019, 7, 17675–17702 RSC
.
- S. X. Zhang, S. F. Xue, J. J. Deng, M. Zhang, G. Y. Shi and T. S. Zhou, Biosens. Bioelectron., 2016, 85, 457–463 CrossRef CAS
.
- H. H. Zeng, W. B. Qiu, L. Zhang, R. P. Liang and J. D. Qiu, Anal. Chem., 2016, 88, 6342–6348 CrossRef CAS
.
- A. Othman, A. Hayat and S. Andreescu, ACS Appl. Nano Mater., 2018, 1, 5722–5735 CrossRef CAS
.
- T. L. Wang, Q. S. Mei, Z. H. Tao, H. T. Wu, Z. M. Yang, S. Wang and Y. Q. Liu, Biosens. Bioelectron., 2020, 148, 111791 CrossRef CAS
.
- J. H. Zhao, S. Wang, S. S. Lu, J. Sun and X. R. Yang, Nanoscale, 2018, 10, 7163–7170 RSC
.
- Y. J. Tong, L. D. Yu, L. L. Wu, S. P. Cao, Y. L. Guo, R. P. Liang and J. D. Qiu, ACS Sustainable Chem. Eng., 2018, 6, 9333–9341 CrossRef CAS
.
- X. Chen, Y. R. Wang, R. Chai, Y. Xu, H. R. Li and B. Y. Liu, ACS Appl. Mater. Interfaces, 2017, 9, 13554–13563 CrossRef CAS
.
- Z. Han, D. Y. Nan, H. Yang, Q. Q. Sun, S. Pan, H. Liu and X. L. Hu, Sens. Actuators, B, 2019, 298, 126842 CrossRef CAS
.
- N. Gao, Y. F. Zhang, P. C. Huang, Z. H. Xiang, F. Y. Wu and L. Q. Mao, Anal. Chem., 2018, 90, 7004–7011 CrossRef CAS
.
- H. H. Zeng, L. Zhang, L. Q. Rong, R. P. Liang and J. D. Qiu, Biosens. Bioelectron., 2017, 89, 721–727 CrossRef CAS
.
- G. G. He, J. Li, Z. Q. Wang, C. X. Liu, X. L. Liu, L. G. Ji, C. Y. Xie and Q. Z. Wang, Tetrahedron, 2017, 73, 272–277 CrossRef CAS
.
- H. S. Jung, J. H. Han, Y. Habata, C. Kang and J. S. Kim, Chem. Commun., 2011, 47, 5142–5144 RSC
.
- T. Masayasu, I. Shohei, O. Akio, H. Itaru and Y. Yukio, J. Am. Chem. Soc., 2010, 132, 5938–5939 CrossRef
.
- J. Sun, B. Wang, X. Zhao, Z. J. Li and X. R. Yang, Anal. Chem., 2016, 88, 1355–1361 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and supplementary figures. See DOI: 10.1039/d0an01740j |
| This journal is © The Royal Society of Chemistry 2021 |