Retracted Article: Microelectrochemical cell arrays for whole-cell currents recording through ion channel proteins based on trans-electroporation approach†
Tianyang
Zheng
 *ab,
Gerhard
Baaken
cd,
Jan C.
Behrends
c and
Jürgen
Rühe
b
*ab,
Gerhard
Baaken
cd,
Jan C.
Behrends
c and
Jürgen
Rühe
b
aState Key Laboratory of Precision Measurement Technology and Instruments, Department of Precision Instrument, Tsinghua University, 100084, Beijing, China. E-mail: zhengty@mail.tsinghua.edu.cn; Tel: +86-10-62783199
bLaboratory for Chemistry and Physics of Interfaces, Department of Microsystems Engineering (IMTEK), University of Freiburg, Georges-Koehler-Allee 103, D-79110, Freiburg, Germany
cLaboratory for Membrane Physiology and Technology, Institute of Physiology, University of Freiburg, Hermann-Herder-Str. 7, D-79104, Freiburg, Germany
dIonera Technologies GmbH, Hermann-Herder-Str. 7, D-79104, Freiburg, Germany
First published on 8th November 2019
Abstract
High electrostability and long life-time of planar chip technology are crucial for electrophysiological measurements such as ionic current recording through ion channel proteins embedded in biological cell membrane. In this paper, we propose a novel planar microchip integrated with microelectrochemical cell array toward to a feasible solution for ion channel screening with high resolution and long life-time. In order to reduce the interference from the leakage currents, a synthetic lipid bilayer is applied to form a high sealing resistance. The whole-cell electrical access can be constructed via electroporating the lipid bilayer in close proximity to the cell membrane. Parameters of electroporation including amplitude and time scale are firstly optimized by using parallel electroporation to the lipid bilayers. In this approach, individual cells can be trapped to the target positions by applying dielectrophoresis (DEP) manipulation. Poly(ethylene glycol) (PEG) is employed with low concentration to facilitate the closed contact between the cells and the lipid bilayer to increase the efficiency of the whole-cell mode construction. Through this chip based method, stable current recordings through inward rectifier potassium (Kir) ion channels embedded in rat basophilic leukemia (RBL-1) cell membrane are achieved with high electrical sealing resistance (over 1 GΩ). In addition, without need for complex fluidic connections, this method allows for an easy operation and further miniaturization of the measuring system.
Introduction
Ion channels embedded in cell membranes are the fundamental elements underlying cellular excitability and electrical signaling. They play essential roles for many cellular functions in human bodies, such as signal transmission in nervous systems and mass transportation across cell membrane.1 Ion channel dysfunctions are found to be connected to a number of common diseases.2 For instance, cardiac arrhythmia is related to the blocking of the human Ether Related Gene (hERG) potassium channels.3 Screening the influences of novel pharmaceutical compounds onto such ion channel proteins is a mandatory requirement for the approval and introduction of such compounds into medical therapy.4 Therefore, the investigation of ion channels is of utmost importance for medical and physiological researches as well as in drug discovery for the pharmaceutical industry. With the demands of the screening throughput from these application fields, the necessity for a fast and reliable method to investigate ion channel proteins with a high informational yield has been dramatically increased.5Conventional research method on ion channels is based on the patch-clamp technique which has subsequently become the gold standard in electrophysiology for ion channel studies.6 In this technique, a glass pipette with small tip (∅ 3–5 μm) is employed to closely attach the cell membrane. By applying a negative pressure (suction), a small membrane patch is drawn into the interior of the pipette and broken down with further suctions. By this way, the electrical connection between cytosol and the test electrode can be established with the isolation to the extracellular environment by the wall of the glass pipette tip.7 With this method, ionic currents flowing through the entire cell membrane can be recorded with low background noise due to the tight electrical sealing, so-called “gigaseal”. However, this method involves laborious and time-consuming processes which require complex equipment as well as highly trained personnel.8 Consequently, it is not suitable for the screening applications requiring higher throughput.
With the advances of micro-electromechanical systems (MEMS), microfabricated chip-based planar patch-clamp technology is developed to replace the conventional pipette based techniques, which allows a parallel and automated screening with increased throughput. For instance, the devices with “pore in a planar partition” are commonly used to measure the currents through the ion channels. In this devices, a microaperture structure is generated on a flat substrate via microfabrication technologies, such as ion-track etching, photolithography, softlithography, or laser drilling technique,9–11 to connect to a cell. Via applying a negative pressure from the underneath of the microaperture, the cell can be positioned and afterwards form an electrically tight seal between the cell membrane and the micro-opening. Electrical access for whole-cell current recording can be constructed by rapturing the membrane patch in the aperture with brief pressure pulses.12–14 This planar patch-clamp approach eliminates the tedious manipulation of the micropipette and allows parallel recording via combining with microfluidic controls for higher screening throughput.
In addition to the microaperture based planar patch-clamp approach, lateral cell patch-clamp method is another configuration for cell measurement.15,16 In this approach, cell trapping can be performed by the suction through the microchannels laterally connecting to a main channel or main chamber structure. By this way, multiple individual cells can be trapped and fixed simultaneously onto the entrances of several microchannels. In this case, parallel recording becomes available. Nevertheless, the reported sealing resistance is relatively low compared to the planar approaches. Moreover, due to the fluidic connections, the number of recording sites is limited to the number (and the size) of microfluidic channels leading to the main chamber or main channel.
Alternatively, microelectrode is another option to simplify the operation of current recording for single cells.17 For instance, a meta-electrode combining with plasmonic optoacoustic poration is reported, which enables recording of high-quality intracellular signals on high-density complementary metal–oxide semiconductor.18 Another example is Needle-structured micro/nano-electrodes which are also favorable to increase the screening throughput for cell current recording due to the high integrity and easy operation.19,20 In this approach, amount of cells is usually cultured on the micro/nano-needle array which can spontaneously penetrate through the plasma membrane to form an electrical access to the cytosol. In cases where spontaneous penetration of the membrane is not evident, an electroporating pulse can be applied to penetrate the cell membrane.21,22 In other cases, cells can be trapped and penetrated by the microelectrode via DEP process, which allows electrophysiological measurements with a very low cell density.23,24 With these methods, the electrophysiological detections can be performed directly through the microelectrodes, which allows further increasing of the integration density for the measurement systems and overcomes the packaging difficulties due to the necessary fluidic connections in conventional planar patch-clamp methods.25 However, the needle-structure based approach is limited by the relatively low electrical seal between the cell membrane and the matrix.
In our previous work a planar approach with microelectrode array is reported for ionic current recording through single nanopore proteins.26–28 In this approach, a single-layer microcavity structure integrated with Ag/AgCl microelectrode is fabricated and the opening of the cavity is in the same size with the electrode. However, in order to connect with biological cells, the small opening should be remained, which restrains the reaction area of the microelectrode in the case of single layer structure. Moreover, in the current recording through a whole-cell membrane, larger currents will be generated compared to single nanopore proteins.28,29 Consequently, in high current conditions the life-time of this single-layer chip remains intrinsic limitation.
In this article, we present an approach based on a novel microelectrochemical cell array chip for ionic currents recording through the ion channels in biological cell membrane. The microchip consists of a plurality of microcavities containing nanocrystalline Ag/AgCl microelectrodes which construct the microelectrochemical cells and patterned in an array with a 4-by-4 grid. By contrast with the planar single-layer structure reported previously,26 a 3D undercut structure is fabricated with a small aperture on top in order to compliant with the size of the biological cells. Meanwhile, this structure maintains the large size of microcavity associating with sufficient reaction area of Ag/AgCl microelectrode for a highly stable current recording. In this method, a robust electrical seal is formed via painting a synthetic lipid bilayer on the microaperture with a high resistance over tens of GΩ.26–30 Following with adding small volume of cells onto the microchip, positive DEP process is employed to trap individual cells onto the painted lipid bilayer. Afterwards, voltage pulses are applied to facilitate a merge between the lipid bilayer and cell membrane to establish a whole-cell electrical access for ionic current recording through the ion channel proteins in cell membrane, so-called the “trans-electroporation” due to the close-by contact between cell and lipid bilayer.31–33 Compare to conventional patch-clamp methods in which fluidic connections or culture process are usually needed, in this method, whole-cell access can be rapidly established by electrical methods associating with a high sealing resistance, which effectively simplifies the operation process and allows further miniaturization of the measuring system.
In this work, synthetic lipid bilayer as a model is firstly employed to investigate the reactions of lipid membrane to external electric fields and via applying linearly increased voltage, the relation between membrane life-time and breaking voltage is also studied. By this way, the appropriate voltage for trans-electroporation can be attempted to predicted. In order to increase the efficiency of the trans-electroporation, water soluble polymer poly(ethylene glycol) (PEG) is chosen for the purpose to facilitate the contact between the cells and the painted lipid bilayers. Via this approach, ionic currents through Kir ion channels embedded in RBL-1 cell membrane are recorded with high sealing resistance (over 1 GΩ) and low RMS noise (<10 pA). As expected for a Kir channel, the inward current is also abolished by the application of BaCl2. Moreover, the influences from the PEG concentration and electroporation to the obtained sealing resistance are also investigated. Therefore, this method is more suitable in applications of ion channel analysis and drug screening with high electrostability. In addition, the potential advantage in the raising of throughput is also exhibited due to the easy operation and the flexibility in scale up by means of array design.
Materials and methods
Microelectrochemical cell array on a chip
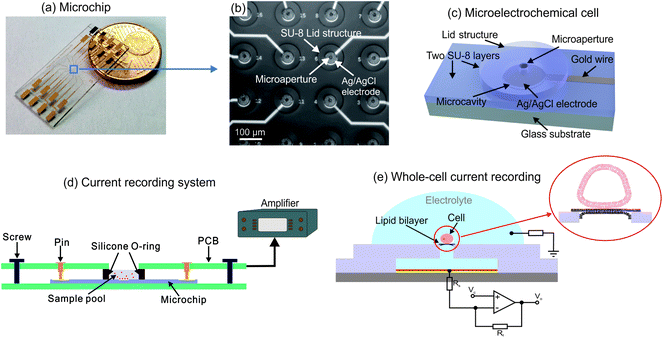
The microchip contains a 4-by-4 microelectrochemical cell array integrated in an area of about 1 mm2 at the center of the chip as shown in Fig. 1(a) and (b). Each cell consists of two SU-8 layers, as Fig. 1(c) indicated, including the a microcavity with the diameter of 60 μm and a lid structure with a microaperture in diameter of 16 μm for biological cell connection. In the microcavity, an Ag/AgCl coated microelectrode is integrated for ionic current recording. Each microelectrode is connected to a contact pad by an individual gold wire protected by covering with SU-8 film. Ionic currents obtained from the electrodes can be recorded by the measurement setups which are connected to the contact pads. The measurement system is shown in Fig. 1(d). The microchip is placed on a holder printed circuit board (PCB). A sample pool formed by a silicone O-ring is held in place on the center of the chip by a second board which contains contact pins on each side and the connections to the patch-clamp amplifier.The fabrication processes of the microchip are described in Fig. S1,† in which the chromium and gold layers are firstly deposited by using electron beam evaporation while the metallic structures are patterned through photolithography process. Afterwards, a layer of SU-8 is coated on the gold structures and the microcavity structures are generated via photolithography process. Ag/AgCl layer is deposited onto the gold electrode through the microcavity by DC electrochemical plating. In order to pattern the lid structure of the microelectrochemical cell, the second SU-8 layer is structured on the other individual substrate after a sacrificial layer (Omnicoat®) is coated for the 3D structure releasing. The obtained two SU-8 layers are bonded together by thermal bonding process at over 100 °C and the microelectrochemical cell array is finally released via stripping the top substrate after solve the Omnicoat® layer.
Cell culture
Rat basophilic leukemia (RBL-1) cells (purchased from ATCC, Germany) are incubated as a monolayer in a 25 cm2 culture flask containing Dulbecco's Modified Eagle Medium (DMEM) with 10% fetal bovine serum (FBS) at 37 °C in a 5% CO2 environment (all obtained from Gibco, Gaithersburg, MD, USA). The cells are harvested through adding 2 mL 0.25% trypsin/EDTA into the flask and then re-suspend in the culture medium at a concentration about 5 × 105 cells per mL.Lipid membrane formation and electroporation
Lipid membranes, which are painted onto the microapertures are generated using 5 mg mL−1 1,2-diphytanoyl-sn-glycero-3-phosphocholine (DPhPC from Avanti Polar Lipids, USA) solvated in octane. The microelectrodes are firstly immersed in an electrolyte (mainly 130 mmol L−1 KCl). A polytetrafluoroethylene (PTFE) stick dipping with lipid solution is manually rubbed over the mciroapertures at the center of the chip. Typically, while painting 2–3 times, the resistivity of the membrane-electrode system will increase from several MΩ to tens of GΩ, indicating the formation of a “Gigaseal” on the microcavities.For lipid membrane electroporation, a linearly increased voltage is applied to the lipid membrane from 0 to 1000 mV with the increasing rate (ΔU/Δt) changing from 10 V s−1 to 1 V s−1. The current responses are recorded by the amplifier connect to the microchip.
Dielectrophoresis cell trapping
Individual cell trapping is performed via positive dielectrophoresis (pDEP) in test medium, mainly containing the mixture of electrophysiological test solution (KCl 130 mmol L−1, NaCl 10 mmol L−1, MgCl2 2 mmol L−1, CaCl2 4 mmol L−1, HEPES 10 mmol L−1; pH = 7.45) and 250 mmol L−1 sucrose with the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. An alternative current (AC) signal with 4 Vpp, 10 MHz is applied between the two groups of electrodes (Electrode No. 1 to No. 8, and Electrode No. 9 to No. 16 shown in Fig. 1(b)). Due to the lower conductivity of the medium than the cytoplasm, cells can be trapped to the regions with maximum electric field intensity by pDEP force and move to the microapertures from the suspension.
5. An alternative current (AC) signal with 4 Vpp, 10 MHz is applied between the two groups of electrodes (Electrode No. 1 to No. 8, and Electrode No. 9 to No. 16 shown in Fig. 1(b)). Due to the lower conductivity of the medium than the cytoplasm, cells can be trapped to the regions with maximum electric field intensity by pDEP force and move to the microapertures from the suspension.
Cell current recording
Firstly, in order to electrically seal the microcavity with high resistivity, DPhPC based lipid bilayer is painted onto a microapertures in 100 μL test medium. An increase of measured resistance from several MΩ to tens of GΩ indicated that the bilayer is formed. Afterwards, 15 mg mL−1 PEG-8000 (Fluka, Germany) solvated in electrophysiological test solution is added to the buffer to facilitate the contact between the cells and the painted lipid bilayers. After addition of 10 μL cell suspension to the cis side of the lipid bilayer, cells fall down to the chip surface in 1 or 2 minutes and DEP process is applied to move individual cells to the microapertures with lipid bilayer, as the indication in Fig. 1(e). Subsequently, a series of short voltage pulses are applied to construct the electrical access for ionic current recording. Finally, a series of voltages from −100 mV to 100 mV with 200 ms duration are applied to record the current responses flowing through the Kir channels.In terms of parallel lipid membrane electroporation, the current responses are recorded by a multichannel patch-clamp amplifier (1 nA saturation, Jet 16, Tecella LLC, USA). The voltage protocol can be controlled by the software TecellaLab (Tecella LLC, USA) with 20 kHz sample frequency. For electrophysiological experiments. Axopatch 200B patch-clamp amplifier (10 nA saturation, Molecular Devices, USA) is employed to generate the voltage signals and record the current responses. The electrical signals applied to the membrane are controlled by an open source software, Gepulse (Michael Pusch, Genova, Italy). The recorded signals are digitized by a NI-PCI 6221 AD converter (National Instruments, USA) and the sample frequency is 200 kHz. In both cases of electroporation tests and electrophysiological current recordings, an Ag/AgCl wire is inserted into the buffer solution on the microchip as a counter electrode.
Results and discussion
Lipid membrane electroporation
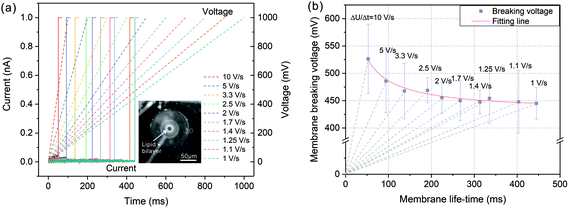
In order to investigate the membrane breaking potential, a linearly increased voltage was applied to the painted lipid membrane and the current responses were recorded during the voltage increasing. By this way, the membrane was exposed to a single voltage protocol instead of a series of incremented rectangular pulses with certain steps, in which lipid bilayer was exposed to voltage stress for many times. As a result, this linearly raised voltage protocol could eliminate the impact of such pretreatment for preserving membrane stability.34 As shown in Fig. 2(a), the applied voltages were linearly increased from 0 to 1000 mV with diverse increasing rates from 10 V s−1 to 1 V s−1 (dashed lines in Fig. 2(a)). The current responses (solid lines in Fig. 2(a)) firstly remained at a very small values due to a tight electrical sealing of the lipid membrane (over 20 GΩ). As the voltage increased, the membranes were disturbed at certain points on the traces of the voltage protocols and the currents increased rapidly to the saturation level of the amplifier (1 nA), which corresponded to the membrane breaking voltage UB and life-time tB.The relationship between the membrane breaking voltage and the measured membrane life-time was shown in Fig. 2(b). The dashed lines indicated the increasing rates of voltage protocol (ΔU/Δt). According to the test results, the breaking voltage was generally lowered when the voltage protocol duration increased. The acquired breaking voltages were fitted by nonlinear regression based on a viscoelastic model of the membrane described in ref. 35 and 36. As a result, with tB tending to infinite, the breaking voltage declined gradually to a stable value referring to the minimum breaking voltage, UBmin = 430 mV. Therefore, the applied voltage potential should be higher than this value to partially break the lipid bilayer for the establishment of electrical access with biological cell.
Whole-cell current recording through Kir ion channel
In whole-cell current recording, RBL-1 cells were employed. The only significant membrane conductance of RBL-1 cells was generated by the K+ selective inward rectifier (Kir channels). Therefore, it was a simple but useful tool to test the whole-cell electrical access on a planar chip due to its characteristic current responses at various voltages.After cell deposition into the sample pool, individual cells were trapped by DEP process from the suspension onto the microapertures which were firstly sealed by painted lipid bilayers. As a result, the possibility to test single-cell was increased compared to adding amount of cells into the sample pool. The DEP cell trapping process was described in detail in Fig. S2† and exhibited by ESI Video 1.† Due to the high frequency of AC signal with relatively low amplitude, the switch between membrane charging and discharging was very fast. As a result, the aggregated energy onto the lipid bilayer was not sufficiently high to interrupt the structure,37,38 while the high sealing resistance was preserved during the DEP process.
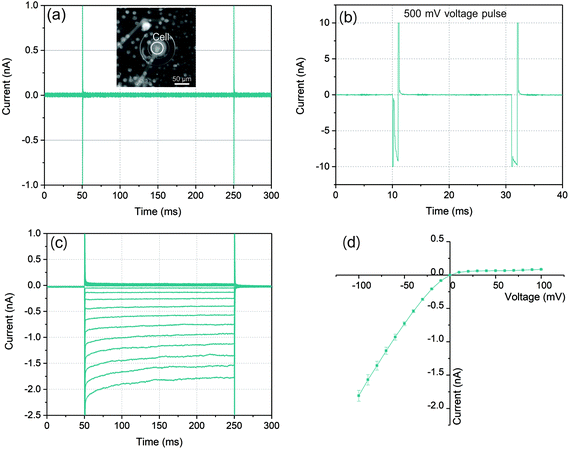
The graphs in Fig. 3(a)–(c) illustrated a typical ionic current recording process with a whole-cell configuration. As a control for the bilayer integrity, voltages from −100 mV to 100 mV with 200 ms duration were applied. As a result of the high sealing resistance the current responses depicted in Fig. 3(a) showed only very small values. After the cell was trapped to the microaperture, a series of voltage pulses increasing from 450 mV with 1 ms duration were applied to partially disturb the cell membrane and the lipid bilayer (Fig. 3(b)) to establish the whole-cell electrical access for the following ionic current recording. When the pulse increased to 500 mV, the current response increased rapidly to nearly 10 nA in a short time after membrane charging. When the followed voltage pulse was applied with the same amplitude, the current response still remained at approximately 10 nA, which indicated that the lipid bilayer-cell system was partially disturbed.
In order to investigate the establishment of whole-cell electrical access, following the electroporation process, the same test voltages from −100 mV to 100 mV with durations of 200 ms were performed. As Fig. 3(c) shown, with a symmetric voltage protocol, the traces of the current response presented an asymmetric distribution around zero which indicated that the construction of whole-cell electrical access due to the observed rectification behavior of Kir channels. In the case of negative voltages, (from −100 mV to 0), the current reached to nearly −2 nA at −100 mV, while it was only less than 0.1 nA at +100 mV. The RMS noise with this high sealing resistance could be lower than 10 pA. Moreover, the exponential time dependence of the negative current response which exhibited especially at relatively high current level was the other evidence of the Kir channel behavior.39,40
At the very first milliseconds after the application of voltages shown in Fig. 3(a) and (c), the transient current responses represented the capacitive responses of the lipid bilayer systems. The magnified views of the transient responses at 100 mV voltage before and after electroporation process were indicated in Fig. S3.† Compared to the lipid bilayer before electroporation, the current response exhibited a longer charging time after the trans-electroporation with voltage pulses referring to a larger capacitance of the lipid bilayer system. This effect could also demonstrate a construction of connection between cell membrane and lipid bilayer with a whole-cell electrical access.
In an ideal whole-cell electrical access with an infinite high sealing resistance, the ionic currents driven by the voltages can only pass through the ion channel proteins embedded in cell membrane. However, in practice, the sealing resistance was not infinite so that the positive current response was often influenced by the sealing resistance between the cell membrane and lipid bilayer. According to the characteristic of the Kir channel in RBL-1 cell membrane, the current response with this channel behaved like a diode in circuit where the negative (inward) current from cis side could pass through the channels with low resistance. In contrast, for positive (outward) current, ions were not permitted entering the channels which presented a high resistance. This diode like behavior of the Kir channel is displayed by an I–V curve in Fig. 3(d). The mean values of the current responses to a particular voltage pulse amplitude are represented by the green symbols in this graph. In the case of negative potentials applied to the trans side of the membrane, as the voltages increased the current raised nearly in linear. Via taking the slope of the I–V curve at the negative potential, the resistance for negative potential was about 55 MΩ. According to the literature, the conductance of single Kir channel in physiological environment (140–150 mM KCl) was about 20–40 pS (50–25 GΩ) which was dependent on the applied voltages.41–43 As a result, the number of Kir channels detected in this experiment could be determined as 900–450 by assuming that each channel had identical conductance since the electrical connection in the circuit model between each channel was in parallel. By contrast, when positive potentials were applied, the currents raised slightly with increased voltages. The sealing resistance was estimated to be approximately 1.1 GΩ according to the calculation of the slope of the I–V curve at positive potential. The reason could be attributed that the Kir channel exhibited ohmic conductance when it was fully activated by hyperpolarization (negative potential).44 However, at depolarization (positive potential), the channels could be blocked by specific intracellular substances, such as magnesium ions (Mg2+) and polyamine at physiological concentrations. Due to the rapid closure of Kir channels, the inward rectification of the resting K+ current was activated with apparently reduced outward currents.
This rectification behavior depended on the electrochemical gradient of K+ over the cell membrane corresponding to the equilibrium potential of K+, EK:45
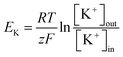 | (1) |
A measurement with multi-times recording was performed with the same electrode to investigate the feasibility of the microelectrode. The mean values of currents at −100 mV were indicated in Fig. S4.† The current responses still maintained high stability with small deviations after multi-times activations at high potential, which exhibited a high electrostability of Ag/AgCl microelectrode. Electrostability of the microelectrode is measured at a constant voltage. Additionally, a long-term electrostability test was also performed as described in ESI and Fig. S5,† the microelectrode could be stable for more than 1 hour at saturation current performed with 150 mM KCl solution. Therefore, it was more suitable to measure the currents through the whole-cell membrane due to the large reaction surface area.
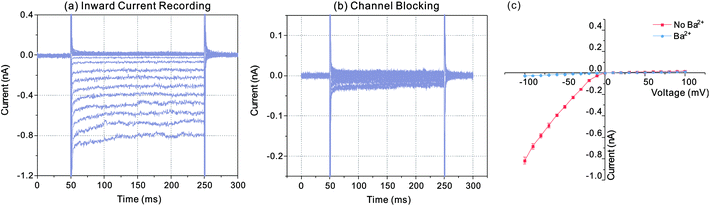
In order to verify the recorded currents caused by the effect of Kir channels, channel blocking experiment was carried out with Ba2+. Fig. 4 indicated the recorded currents in the conditions of inward current flowing and channel blocking. Firstly, the diode like current responses were recorded following with the whole-cell electrical access via trans-electroporation method (Fig. 4(a)). Afterwards 1 μL BaCl2 with the concentration of 1 mmol L−1 was added into the sample pool and the current responses recorded with same voltage protocol were changed as the Fig. 4(b) indicated. According to the I–V curve shown in Fig. 4(c), with the Ba2+ effect, the negative currents were dramatically reduced to the nearly same level to the positive currents, which suggested that the ions were blocked by the Ba2+ ions and the diode like current responses recorded before were the effect of the inward currents flowing through the Kir channels.
According to the literatures and previous work,27,28,46,47 in general, PEG could partially block the pores with very short block events at low concentrations of KCl. Therefore, the influence of PEG to the rectified behavior of Kir channels was also investigated. In terms of PEG absence, trans-electroporation and cell current recording were also carried out as shown in Fig. S6.† Compare to the assistance of PEG molecules, the rectified behavior of Kir channels was still observed in this case but with relatively low sealing resistance (about 600 MΩ) due to the weak adhesion between cell membrane and lipid bilayer. This result indicated that PEG could not influence the rectified behavior although it would reduce the conductivity of single channel in a short time.
Additionally, the current response with lipid bilayer only were also recorded as shown in Fig. S7.† In the case of no cell deposition, the current responses after electroporation were nearly symmetric distribution at negative and positive potentials.
Investigation of sealing resistance with various factors
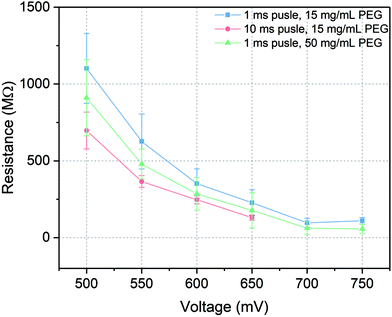
In patch-clamp experiments, the sealing resistance is an important factor for the quality of the experiments since a high electrical sealing resistance allows to perform ionic current recording with low background noise.48 Moreover, high sealing resistance reduces the errors caused by high leakage current in detecting the number of channels and regulates the voltage distribution in the whole system. Usually, the sealing resistance differs from experiment to experiment, from cell to cell and even within a single cell recording. During the current recording with microelectrochemical cell array chip, the sealing resistance was related to the intactness of the painted lipid bilayer and its connection to the cell membrane after trans-electroporation process. To keep that variance as low as possible and to generate a high sealing resistance, several factors which could influence the resistance were investigated, including amplitude and length of the voltage pulses for trans-electroporation as well as the PEG concentration. The sealing resistance was evaluated via taking the slope of the I–V curve of the current recording at positive potential.According to the results indicated in Fig. 5, on the one hand, the sealing resistance decreased with increasing both pulse amplitude and duration at certain PEG concentration (15 mg mL−1) since higher potential or longer pulse duration would reduce the energy barrier for a rupture of the membrane. Therefore, via applying short pulses with appropriated amplitudes, the probability was higher to obtain and maintain an intact membrane per cell seal. For instance, at 500 mV pulse amplitude with 1 ms duration (blue line in Fig. 5), the whole-cell configuration could be established with a relatively high sealing resistance of over 1 GΩ, which was equivalent to the values reported in the literatures.5,49 When the amplitude was increased over 650 mV, relatively large leakage currents were recorded with sealing resistances lower than 200 MΩ. At these amplitudes, the lipid bilayers could be regarded as defective membranes which were not suitable to seal the microcavity. On the other hand, when the length of voltage pulse was extended to 10 ms (red line in Fig. 5), the sealing resistance was generally reduced compared to 1 ms pulses tests. The highest resistance obtained with an extended pulse duration experiment was approximately 700 MΩ. At pulse above 650 mV a stable membrane could not be maintained and no Kir activity could be detected.
Furthermore, as the PEG concentration was raised to 50 mg mL−1, via applying 1 ms pulse duration with different pulse amplitudes (green line in Fig. 5), the sealing resistance was in general reduced but it was still higher than the results obtained at longer voltage pulses. According to the ref. 50 and 51 the yield of cell adhesion was influenced by the concentration of PEG. However, at high concentrations, cell adhesion was restrained due to the high osmotic pressure in the solution triggering cell shrinking. This reduced the area of close contact between the cell membrane and the lipid bilayer. As a result, even though a voltage pulse could trigger a merge between the cell membrane and the lipid bilayer, at high PEG concentrations this construction was not as stable as it at low PEG concentrations. Additionally, at high PEG concentrations, intensive cells aggregation would also impact the efficiency of constructing a whole-cell configuration.
Conclusions
A major cornerstone in the understanding of the nature of electrical reactions in various cells of animal and human organs and tissues have been the discovery of ion channel proteins in cell membranes and the invention of the patch-clamp technique. Since then, electrophysiology as a sub-discipline of physiology started to be realized and individually studied. In this article, a development of novel microelectrochemical cell array chip was proposed for a whole-cell current recording with a high electrostability. In this work, a 3D undercut structure was fabricated to increase the reaction area of the microelectrode while small aperture size could be preserved for good cell connections. DEP cell trapping was applied to move individual cells to the target positions and contact with the lipid bilayers which were painted on the small aperture to form a high sealing resistance. By using the trans-electroporation method associating with PEG assistance for higher cell-lipid bilayer connection, whole-cell electrical access was successfully constructed on the microaperture of the microelectrochemical cell and the typical current responses were recorded through Kir channels with the sealing resistance over 1 GΩ and low RMS noise due to the high electrostability and long life-time of the microelectrode. According to the investigation of the factors influencing sealing resistance such as parameters of electroporation and PEG concentrations, on the one hand, regardless of the voltage duration, high amplitude was disadvantageous to obtain tight seal formation. On the other hand, short voltage pulses were favorable to a robust whole-cell mode construction with high sealing resistance. In addition, appropriately low PEG concentration was helpful to the formation of high sealing resistance. As a result, this microelectrochemical cell array chip combining trans-electroporation method is more suitable for relatively long lasting currents through the whole-cell membrane due to the high electrostability and high sealing resistance. Moreover, based on the simple design and easy operation, the microchip can be easily scaled up, which will further increase the throughput of ion channel screening.Author contributions
All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare the following competing financial interest(s): G. B. is the co-founder of Ionera Technologies GmbH.Acknowledgements
The authors gratefully acknowledge the financial and technical support provided by University of Freiburg, Freiburg im Breisgau, Germany.References
- J. C. Behrends, Chem. Rev., 2012, 112, 6218–6226 CrossRef CAS.
- N. J. Willumsen, M. Bech, S. P. Olesen, B. S. Jensen, M. P. Korsgaard and P. Christophersen, Recept. Channels, 2003, 9, 3–12 CrossRef CAS.
- M. C. Sanguinetti and M. Tristani-Firouzi, Nature, 2006, 440, 463–469 CrossRef CAS.
- J. J. Kasianowicz, Chem. Rev., 2012, 112, 6215–6217 CrossRef CAS.
- A. Brueggemann, M. George, M. Klau, M. Beckler, J. Steindl, J. C. Behrends and N. Fertig, Curr. Drug Discovery Technol., 2004, 1, 91–96 CrossRef CAS.
- E. Neher and B. Sakmann, Nature, 1976, 260, 799–802 CrossRef CAS PubMed.
- B. Matthews and J. W. Judy, J. Microelectromech. Syst., 2006, 15, 214–222 CrossRef.
- J. Xu, X. B. Wang, B. Ensign, M. Li, L. Wu, A. Guia and J. Q. Xu, Drug Discovery Today, 2001, 6, 1278–1287 CrossRef.
- N. Fertig, R. H. Blick and J. C. Behrends, Biophys. J., 2001, 82, 161a–161a Search PubMed.
- N. Fertig, A. Tilke, R. Blick and J. Behrends, Biophys. J., 2000, 78, 266a–266a Search PubMed.
- J. Dunlop, M. Bowlby, R. Peri, D. Vasilyev and R. Arias, Nat. Rev. Drug Discovery, 2008, 7, 358–368 CrossRef CAS.
- A. Bruggemann, M. George, M. Klau, M. Beckler, J. Steindl, J. C. Behrends and N. Fertig, Assay Drug Dev. Technol., 2003, 1, 665–673 CrossRef CAS.
- C. Py, M. W. Denhoff, M. Martina, R. Monette, T. Comas, T. Ahuja, D. Martinez, S. Wingar, J. Caballero, S. Laframboise, J. Mielke, A. Bogdanov, C. Luk, N. Syed and G. Mealing, Biotechnol. Bioeng., 2010, 107, 593–600 CrossRef CAS.
- B. R. Bruhn, H. Y. Liu, S. Schuhladen, A. J. Hunt, A. Mordovanakis and M. Mayer, Lab Chip, 2014, 14, 2410–2417 RSC.
- J. Seo, C. Ionescu-Zanetti, J. Diamond, R. Lal and L. P. Lee, Appl. Phys. Lett., 2004, 84, 1973–1975 CrossRef CAS.
- C. Ionescu-Zanetti, R. M. Shaw, J. Seo, Y. N. Jan, L. Y. Jan and L. P. Lee, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 9112–9117 CrossRef CAS.
- M. E. Spira and A. Hai, Nat. Nanotechnol., 2013, 8, 83–94 CrossRef CAS.
- M. Dipalo, G. Melle, L. Lovato, A. Jacassi, F. Santoro, V. Caprettini, A. Schirato, A. Alabastri, D. Garoli, G. Bruno, F. Tantussi and F. De Angelis, Nat. Nanotechnol., 2018, 13, 972–972 CrossRef CAS.
- J. Abbott, T. Ye, D. Ham and H. Park, Acc. Chem. Res., 2018, 51, 600–608 CrossRef CAS.
- O. Staufer, S. Weber, C. P. Bengtson, H. Bading, A. Rustom and J. P. Spatz, Nano Lett., 2019, 19, 3244–3255 CrossRef CAS.
- J. T. Robinson, M. Jorgolli, A. K. Shalek, M.-H. Yoon, R. S. Gertner and H. Park, Nat. Nanotechnol., 2012, 7, 180 CrossRef CAS.
- J. Abbott, T. Ye, L. Qin, M. Jorgolli, R. S. Gertner, D. Ham and H. Park, Nat. Nanotechnol., 2017, 12, 460 CrossRef CAS.
- P. J. Koester, C. Tautorat, H. Beikirch, J. Gimsa and W. Baumann, Biosens. Bioelectron., 2010, 26, 1731–1735 CrossRef CAS.
- U. Terpitz, V. L. Sukhorukov and D. Zimmermann, Assay Drug Dev. Technol., 2013, 11, 9–16 CrossRef CAS.
- C. Py, D. Salim, R. Monette, T. Comas, J. Fraser, D. Martinez, M. Martina and G. Mealing, Biotechnol. Bioeng., 2011, 108, 1936–1941 CrossRef CAS PubMed.
- G. Baaken, M. Sondermann, C. Schlemmer, J. Ruhe and J. C. Behrends, Lab Chip, 2008, 8, 938–944 RSC.
- G. Baaken, N. Ankri, A. K. Schuler, J. Ruhe and J. C. Behrends, ACS Nano, 2011, 5, 8080–8088 CrossRef CAS PubMed.
- G. Baaken, I. Halimeh, L. Bacri, J. Pelta, A. Oukhaled and J. C. Behrends, ACS Nano, 2015, 9(6), 6443–6449 CrossRef CAS.
- T. Zheng, G. Baaken, M. Vellinger, J. C. Behrends and J. Rühe, Sens. Actuators, B, 2014, 205, 268–275 CrossRef CAS.
- J. M. del Rio Martinez, E. Zaitseva, S. Petersen, G. Baaken and J. C. Behrends, Small, 2014, 119–125, DOI:10.1002/smll.201402016.
- S. K. Dondapati, G. Baaken, J. D. Martinez and J. C. Behrends, Biophys. J., 2012, 102, 502a–502a CrossRef.
- R. Wagner, K. Gall and A. Wirth, USA Patent, US2012214708(A1), 2012 Search PubMed.
- A. V. Samsonov, P. K. Chatterjee, V. I. Razinkov, C. H. Eng, M. Kielian and F. S. Cohen, J. Virol., 2002, 76, 12691–12702 CrossRef CAS.
- P. Kramar, D. Miklavcic and A. M. Lebar, Bioelectrochemistry, 2007, 70, 23–27 CrossRef CAS.
- D. S. Dimitrov, J. Membr. Biol., 1984, 78, 53–60 CrossRef CAS.
- I. Sabotin, A. M. Lebar, D. Miklavcic and P. Kramar, IEEE Trans. Dielectr. Electr. Insul., 2009, 16, 1236–1242 CAS.
- J. C. Weaver, J. Cell. Biochem., 1993, 51, 426–435 CrossRef CAS.
- J. C. Weaver, IEEE Trans. Dielectr. Electr. Insul., 2003, 10, 754–768 CrossRef CAS.
- H. Hibino, A. Inanobe, K. Furutani, S. Murakami, I. Findlay and Y. Kurachi, Physiol. Rev., 2010, 90, 291–366 CrossRef CAS.
- A. N. Lopatin, E. N. Makhina and C. G. Nichols, J. Gen. Physiol., 1995, 106, 923–955 CrossRef CAS.
- I. Josephson and A. Brown, J. Membr. Biol., 1986, 94, 19–35 CrossRef CAS.
- N. A. Burnashev and Y. I. Zilberter, Gen. Physiol. Biophys., 1986, 5, 495–504 CAS.
- G. M. Wahler, Am. J. Physiol., 1992, 262, C1266–C1272 CrossRef CAS.
- H. Matsuda, A. Saigusa and H. Irisawa, Nature, 1987, 325, 156–159 CrossRef CAS.
- S. Hagiwara, S. Miyazaki and N. P. Rosenthal, J. Gen. Physiol., 1976, 67, 621–638 CrossRef CAS.
- T. K. Rostovtseva, E. M. Nestorovich and S. M. Bezrukov, Biophys. J., 2002, 82, 160–169 CrossRef CAS PubMed.
- P. G. Merzlyak, L. N. Yuldasheva, C. G. Rodrigues, C. M. M. Carneiro, O. V. Krasilnikov and S. M. Bezrukov, Biophys. J., 1999, 77, 3023–3033 CrossRef CAS.
- F. J. Sigworth and K. G. Klemic, IEEE Trans. Nanobioscience, 2005, 4, 121–127 CrossRef PubMed.
- A. Stett, C. Burkhardt, U. Weber, P. van Stiphout and T. Knott, Recept. Channels, 2003, 9, 59–66 CAS.
- L. H. Li and S. W. Hui, Biophys. J., 1994, 67, 2361–2366 CrossRef CAS.
- N. G. Stoicheva and S. W. Hui, J. Membr. Biol., 1994, 141, 177–182 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9an01737b |
| This journal is © The Royal Society of Chemistry 2020 |