Biocompatibility studies on cerium oxide nanoparticles – combined study for local effects, systemic toxicity and genotoxicity via implantation route
V.
Kalyanaraman†
a,
Sangeetha Vasudevaraj
Naveen†
a,
N.
Mohana
a,
R. M.
Balaje
a,
K. R.
Navaneethakrishnan
a,
B.
Brabu
 b,
S. S.
Murugan
ac and
T. S.
Kumaravel
b,
S. S.
Murugan
ac and
T. S.
Kumaravel
 *ac
*ac
aGLR Laboratories Private Limited, 444 Gokulam Street, Mathur, Chennai 600068, India. E-mail: kumaravelts@glrlabs.com
bNanoregulatory Platform, Pharma Chemistry, Drug Discovery and Development, Instituto Italiano di Tecnologia (IIT), Genova 16163, Italy
cGLR Laboratories (Europe) Private Limited, No 4, The Exchange, Colworth Science Park, Sharnbrook MK44 1LZ, UK
First published on 18th October 2018
Abstract
An implantation study of cerium oxide nanoparticles (CeO2-NP) combined with 28-day systemic toxicity and genotoxicity studies aligned to current regulatory standards was conducted. The results suggested that local tissue reactions caused by CeO2-NP was minimal (implantation irritation index of less than 3) and was better tolerated than most other implant materials tested in our laboratory. Furthermore, CeO2-NP showed virtually no systemic toxicity or in vivo micronucleus induction in bone marrow via implantation route. Chemical analysis showed that CeO2-NP migrated from the implant sites (250 mg per site) in low levels and was deposited predominantly in liver (191.8 ± 35.1 ng g−1 of tissue; P < 0.01), lungs (263.4 ± 30.9 ng g−1 of tissue; P < 0.001), spleen (211.2 ± 6.5 ng g−1 of tissue; P < 0.001) and kidneys (272.8 ± 20.4 ng g−1 of tissue; P < 0.001). These observations provide a base line biocompatibility and toxicity data on CeO2-NP. The current findings will also be useful in defining standards for nanoparticle containing biomaterials and devices.
1. Introduction
There is an increasing interest in the use of nanomaterials in medical devices to exert therapeutic effects, e.g., antimicrobial effects, blockage of inflammatory responses, inhibition of oxidative stress, reduction of cell proliferation, among others.1–3 Layers of nanoparticles can be deposited on implantable devices (e.g., stents, pacing leads, balloons, vascular closing devices, etc.), which can either be permanently bound to the device to exert local antimicrobial effects or designed to release a flux of appropriately-sized nanoparticles to a patient after implantation in the body. The later type is usually designed to reduce inflammation, inhibit oxidative stress and reduce cell proliferation, that otherwise would lead to extensive fibrosis. These nanoparticles are attached to the medical devices by using a degradable inorganic material. Examples of such nanoparticles that are currently being investigated for sustained release effects are cerium, yttrium, titanium, iron, aluminium, tantalum, or gold, or a mixture of at least two thereof. Of the above nanoparticles, cerium and iron nanoparticles are preferred because of their super-paramagnetic properties.Cerium is a member of the lanthanide series of metals and is the most abundant of the rare-earth elements in the earth's crust and it is separated out from the other rare-earth elements through oxidation in the form of Cerium oxide (CeO2).4,5 Cerium oxide nanoparticles (CeO2-NP or Nanoceria) have recently received attention from the scientific community due to their unique free radicals scavenging property in biological system, both in vitro and in vivo.6 It decreases hepatic reactive oxygen species (ROS) levels linked to the progression of diabetes,7 inhibits myocardial oxidative stress8 and thus proving to be an effective antioxidant agent.9 It is also proposed to have regenerative antioxidant and hepatoprotective potentials against multiple oxidative stress-induced pathologies.10 CeO2-NP is also well suited for applications in nanobiology and regenerative medicine for its promising efficacy against endometriosis related pathogenesis,11 diseases associated with chronic oxidative stress and inflammation,12 in neurodegenerative disorders13 and the delay it brings in the progression of retinal degeneration and as anti-angiogenic agent in rodent models.14 Studies have shown CeO2-NP to be toxic to cancer cells, inhibit invasion, and sensitize cancer cells to radiation therapy.15 Despite these advantages of nanoceria over other therapeutic strategies, there are toxicity issues to be considered.16 CeO2-NP have unique surface chemistry allowing catalyst-like antioxidant properties and are being investigated for several disease indications in medicine. Zirconium coated CeO2-NPs showed benefit in terms of biodistribution compared to uncoated CeO2-NPs, while retaining the intrinsic antioxidant properties. Coated CeO2-NPs demonstrated excellent candidacy for clinical implementation due to their enhanced renal clearance and low reticuloendothelial system uptake.17 Nanoceria ameliorates doxorubicin induced cardiotoxicity, possibly by mitigation via reduction of oxidative stress and inflammation.18 Cerium oxide NP has been patented for its use in enhancing cell survivability and therefore has applications in surgical dressings, implants with the nanoparticles, and applications for wound healing, treating arthritis and joint diseases, anti-aging and the treating of inflammation.19
As described above, CeO2-NP has potential therapeutic uses in medicine, mainly as a regenerative antioxidant with antimicrobial properties. Because of these properties, it offers an explicit advantage in implant devices. It is speculated that medical devices coated (or made with) with CeO2-NP will have low inflammatory reaction and better infection control after surgery. Presently many publications on CeO2-NP are directed towards mechanistic studies and potential therapeutic uses. Only minimal toxicity or biocompatibility information is available on nanoceria to support its safety.
International Organization for Standardization (ISO) ISO 10993 standard, Parts 1 to 20 and 22, gives broad principles for assessing biocompatibility of medical devices depending on site and duration of contact.20 These standards give only general guidelines on testing the biocompatibility of materials in the nanoscale range. Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR)21 gave opinion on assessing the potential health effects of nanomaterials used in the manufacture of medical devices. We therefore adopted these wide principles and combined them with common techniques used in nanotoxicology to assess the biocompatibility of nanoceria.
There are several toxicity endpoints to assess to establish biocompatibility for implant devices. These are cytotoxicity, irritation (intracutaneous test), sensitization, acute systemic toxicity, hemolysis, genotoxicity, implantation and subacute toxicity test. We used standard methodologies described in various parts of ISO10993 with inputs from the SCENIHR opinion to evaluate the toxicity of CeO2-NP. Moreover, our results will be supportive in evaluating the safety of medical device technologies using CeO2-NP as a raw material. Increasing interest in the biocompatibility of nanomaterials22–24 necessitated the need to undertake this work to scale down the research gaps that exist with reference to toxicity and health risk assessment of CeO2-NP employed in medical practice.
Our group has already published the results of cytotoxicity induced by CeO2-NP.25 In this project, we conducted a combined implantation and 28-day systemic toxicity and genotoxicity study. By combining these two biocompatibility studies, we reduced the use of animals in biocompatibility testing.26 Implantation route of administration for systemic toxicity studies is an accepted method for implant devices.27 After implantation, the animals were subjected to the usual systemic toxicity assessment. The results of the implantation study will indicate the local reactions caused by CeO2-NP. These results will be compared with local reactions caused by other implant materials, for which regulatory implantation studies were carried out in our laboratory over the last two years.
Implantation route is commonly used to study systemic toxicity of implant devices and is described in ISO10993, Part 11. However, the standard is lacking on how much material is to be implanted for a proper and acceptable systemic toxicity risk assessment. It should be appreciated that if small amounts of material are implanted, then systemic toxicity will not be elicited and would give a false sense of safety. For a proper risk assessment, systemic toxicity should be investigated after implanting an appropriate amount of material. Appropriate amount of material should be calculated based on clinical use, surface area of the device and should be allometrically scaled to test species used for implantation.
2. Materials and methods
2.1 Characterization of cerium oxide(IV) nanoparticles (CeO2-NP)
CeO2-NP were obtained from Strem Chemicals Inc, USA. The certificate of analysis provided from the manufacturer specified the purity as 99.99%. The impurities listed were in negligible amounts and therefore unlikely to affect the results obtained. To confirm that the taken test material is in nanosize, the morphology of the sample was inspected using Scanning Electron Microscopy (SEM) with an accelerating voltage of 20 kV (Quanta 200 FEG). The chemical composition of the sample was determined by Energy Dispersive X-ray Spectroscopic analysis. The crystal structure of the CeO2-NP was characterized by X-ray Diffraction (XRD) studies with Cu Kα radiation at a scanning rate of 0.02 s−1 using PANalytical (X′pert PRO) X-Ray Diffractrogram.2.2 Experimental design
This study was designed to investigate both local effects and systemic toxicity following subcutaneous implantation of CeO2-NP. The experimental design and quantities of CeO2-NP implanted is shown in Table 1. Nineteen male and 19 female Wistar rats, aged 8–10 weeks and weighing 90–115 g, were obtained from the National Institute of Nutrition, Hyderabad, India. The animals were acclimatized, fed with a standard laboratory pellet diet with ad libitum water and maintained under standard conditions of humidity (40–70%), temperature (22 ± 3 °C) and light (12 h light/12 h dark cycles). Animals were randomly assigned to different groups. All animal procedures were performed in accordance with the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines, Government of India, for care and use of laboratory animals; and approved by the institutional animal ethics committee (IAEC) of GLR Laboratories Pvt Ltd.| No of animals/group | Sex | Number of implantation sites per animal | Implantation material | |
|---|---|---|---|---|
| CeO2-NP, Cerium oxide nanoparticles; HDPE, High density polyethylene. | ||||
| Group 1 | 2 | Male | N/A | Untreated |
| Group 2 | 2 | Male | 2 | Control (1 × 10 mm HDPE) |
| Group 3 | 5 | Male | 2 | CeO2-NP (250 mg per implantation site) |
| Group 4 | 5 | Male | 2 | CeO2-NP (500 mg per implantation site) |
| Group 5 | 5 | Male | 2 | CeO2-NP (1000 mg per implantation site) |
| Group 6 | 2 | Female | N/A | Untreated |
| Group 7 | 2 | Female | 2 | Control (1 × 10 mm HDPE) |
| Group 8 | 5 | Female | 2 | CeO2-NP (250 mg per implantation site) |
| Group 9 | 5 | Female | 2 | CeO2-NP (500 mg per implantation site) |
| Group 10 | 5 | Female | 2 | CeO2-NP (1000 mg per implantation site) |
2.3 Subcutaneous implantation
Implantation study was carried out based on the procedures detailed in ISO10993, Part 6. Prior to implantation, the fur over the dorsal surface of all the animals was carefully clipped off on both sides of the vertebral column without making any skin abrasions. Just prior to the surgery, the animals were weighed and anesthetized by intramuscular injection of ketamine (40 mg kg−1; Themis Medicare Limited, India) and xylazine (5 mg kg−1; Indian Immunological Limited, India). The surgical site was scrubbed with povidone-iodine and the skin was wiped dried with sterile cotton gauze. Two small pockets (measuring approximately 1 × 10 mm) were surgically created on the dorsum of each animal, 2 on each side of the vertebral column. Each pocket was created by making a small surgical incision in the skin and inserting a blunt trocar in the subcutaneous space. Appropriate weights (as given in Table 1) of CeO2-NP were inserted in the pockets by using a wide bore needle and trocar. Test items stayed in place and did not leak after insertions. Therefore, the incisions were not secured by sutures after insertion of test items. Post-operative antibiotics and analgesics were administered to all animals for three days following surgery, which included topical application of povidone iodine, administration of anti-inflammatory (meloxicam 1 mg kg−1; Intas pharmaceutical ltd, India) and antibiotics (enrofloxacin 10 mg kg−1; Vetoquinol India animal health private ltd, India). The animals had free access to feed and water, and were observed for local, systemic and behavioural abnormalities during the observation period of 28 days.2.4 Clinical observations
All animals were observed for a 28-days period (on day-to-day basis) following the implantation surgery. The observations included inspection of surgical site, general health check, clinical observations for signs of toxicity and morbidity. Body weight was taken weekly.2.5 Haematological and biochemical analysis
At the end of 28 days, just prior to sacrifice, blood samples were collected from sinus orbital in two tubes, one in ethylene diamine tetra acetic acid (EDTA) vacutainer for haematological analyses, and the other in clot activator tube without anticoagulant, for biochemical analyses. Samples without anticoagulant were allowed to clot and centrifuged at 3000 rpm for 10 minutes, and serum collected.Hemoglobin (Hb; %), haematocrit (%), mean corpuscular volume (MCV; fL), mean corpuscular haemoglobin (MCH; pg), mean corpuscular haemoglobin concentration (MCHC; g dL−1), RBC count (×106 μL−1), WBC count (×103 μL−1), lymphocytes (%), monocytes (%), granulocytes (%) and platelets (×103 μL−1) were measured by routine automated procedures (Mindray Bio-Medical Electronics Co., Ltd). Serum alanine transaminase (ALT; U L−1), aspartate transaminase (AST; U L−1), alkaline phosphatase (ALP; U L−1), total bilirubin (TB; mg dL−1), albumin (ALB; mg dL−1), total proteins (TP; mg dL−1), albumin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) globulin ratio (A
globulin ratio (A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G), cholesterol (CHO; mg dL−1), glucose (GLU; mg dL−1), urea (UR; mg dL−1), creatinine (CR; mg dL−1) were analysed using commercially available kits in a biochemistry analyser (Mindray Bio-Medical Electronics Co., Ltd). Serum electrolytes – calcium (mg dL−1), inorganic phosphorus (mMol L−1), sodium (mMol L−1), potassium (mMol L−1) and chloride (mMol L−1) were also measured.
G), cholesterol (CHO; mg dL−1), glucose (GLU; mg dL−1), urea (UR; mg dL−1), creatinine (CR; mg dL−1) were analysed using commercially available kits in a biochemistry analyser (Mindray Bio-Medical Electronics Co., Ltd). Serum electrolytes – calcium (mg dL−1), inorganic phosphorus (mMol L−1), sodium (mMol L−1), potassium (mMol L−1) and chloride (mMol L−1) were also measured.
2.6 Gross pathology
Gross pathology of the implanted site was carried out as a part of implantation study. The implants were excised along with the surrounding tissues and examined for gross pathology. Each implanted site was examined for alterations of normal structure, which included assessment of regional draining lymph nodes and haematoma, oedema, encapsulation and/or additional gross findings. After gross pathology examination, the incised implants along with the surrounding tissues were preserved for histopathology.All organ systems were also examined for gross pathology as a part of systemic toxicity study and stored for histopathology. Key organs (tier 1) – heart, liver, adrenal, kidney, thymus, spleen, brain, testis, ovaries, lungs, thyroid, pituitary were weighed. Five hundred milligrams of heart, lungs, liver, kidney and brain were used for analysis of CeO/Ce levels from the top dose treated and untreated control group of animals. Furthermore, femur and tibia were flushed with fetal calf serum and bone marrow was collected for micronucleus assay.
2.7 Histological evaluation
Tier 1 organs and implant site tissues were fixed in 10% neutral buffered formalin for 48 hours and subjected to routine processing for histological analysis. Serial 4 μm thick sections were made and stained with hematoxylin and eosin. The specimens were examined under light microscope (Olympus CX21i LED, Japan) at 4, 10, 40, and 100× magnifications.Implanted site was subjected to detailed histopathology and scored semi-quantitatively as described in ISO 10993, Part 6: 2016.28 In this semiquantitative scoring scheme, inflammatory cell infiltrates and necrosis are scored using the scoring scheme in Table 2. Neo-vascularization, fibrosis, and fatty infiltration are scored using the scoring scheme in Table 2. Due to the greater importance of inflammatory cell infiltrates and necrosis, these parameters (see Table 2) are multiplied by a factor of two to provide a weighted value as compared to neovascularization, fibrosis, and fatty infiltration parameters (see Table 2). The values are totalled, and then an average score for test and control treatments is calculated. The average score for the control treatment is subtracted from the test treatment average to determine a reactivity grade.
| Cell type/response | Score | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Scoring for ellular response | |||||
| Polymorphonuclear cells | 0 | Rare, 1–5 phf−1 | 5–10 phf−1 | Heavy infiltrate | Packed |
| Lymphocytes | 0 | Rare, 1–5 phf−1 | 5–10 phf−1 | Heavy infiltrate | Packed |
| Plasma cells | 0 | Rare, 1–5 phf−1 | 5–10 phf−1 | Heavy infiltrate | Packed |
| Macrophages | 0 | Rare, 1–5 phf−1 | 5–10 phf−1 | Heavy infiltrate | Packed |
| Giant cells | 0 | Rare, 1–2 phf−1 | 3–5 phf−1 | Heavy infiltrate | Sheets |
| Necrosis | 0 | Minimal | Mild | Moderate | Severe |
| Tissue response | Score | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Phf = per high powered field (400×). | |||||
| Scoring for tissue response | |||||
| Neovascularisation | 0 | Minimal capillary proliferation, focal, 1–3 buds | Groups of 4–7 capillaries with supporting fibroblastic structures | Broad band of capillaries with supporting structures | Extensive band of capillaries with supporting fibroblastic structures |
| Fibrosis | 0 | Narrow band | Moderately thick band | Thick band | Extensive band |
| Fatty infiltrate | 0 | Minimal amount of fat associated with fibrosis | Several layers of fat and fibrosis | Elongated and broad accumulation of fat cells about the implant site | Extensive fat completely surrounding the implant |
Tier 1 organs were evaluated histologically and scored for lesions, if any, then the remaining organs were to be scored to assess the systemic toxicity of CeO2-NP.
2.8 Data evaluation
Local effects after implantation of CeO2-NP were analysed by semi-quantitative methods as described above. Furthermore, the local tissue reaction of CeO2-NP was compared with few other implantation studies conducted in our laboratory on different implant materials for regulatory purposes. It should be noted that these studies were conducted using animals from the same source and lineage as those in the CeO2-NP studies. These studies are listed in Table 3.| Implant material | Size of implant | Number of studies | Number of animals | |
|---|---|---|---|---|
| a data collected from 1 previous study conducted in our laboratory the last year. b data collected from last 5 studies conducted in our laboratory. | ||||
| 1 | Cerium oxide coated hydroxyapatitea | (1 × 10 mm) | 1 | 3 |
| 2 | Cerium oxide coated stainless steela | (1 × 10 mm) | 1 | 3 |
| 3 | Stainless steelb | (1 × 10 mm) | 5 | 15 |
| 4 | Hydroxyapatiteb | (1 × 10 mm) | 5 | 15 |
| 5 | Ti6Al4V alloyb | (1 × 10 mm) | 5 | 15 |
| 6 | Cobalt chromiumb | (1 × 10 mm) | 5 | 15 |
Systemic toxicity effects produced by CeO2-NP treated animals were compared with those of concurrent untreated control animals and negative control animals.
2.9 Micronucleus assay
Bone marrow collected were centrifuged and the pellet was resuspended in 500 μL of fetal calf serum. A drop of this suspension was gently applied to a glass slide and slowly spread to form a smear. The smear was then dried and fixed with ice cold methanol. This was stained with 0.25% acridine orange and scored under fluorescent microscope at appropriate magnification. Polychromatic (PCE) and normochromatic (NCE) cell were counted (totally 500 cells) and the results expressed as PCE/NCE ratio. Two thousand PCEs were also scored for the presence of micronuclei and results were expressed as percentage micronucleated PCEs (% MNPCEs).29,302.10 ICP-MS analysis
Five hundred milligrams of heart, lungs, liver, kidney, spleen and brain were used for analysis of CeO/Ce levels from the top dose treated and control group of animals of both sexes. Each organ from the five animals were grouped and digested with nitric acid. The digested samples were then diluted with 100 mL of distilled water and sent for commercial ICP-MS analysis (Model Number-ICPE9800; manufactured by Shimadzu Corporation Ltd, Japan). The detection level for ICP-MS for CeO/Ce was 0.05 ppm.2.11 Statistical analysis
Statistical analysis was performed to compare the CeO2-NP implanted groups with that of the negative and untreated controls. Changes in body weight (paired T-test to compare Day 0 with Day 28; unpaired T-test to compare between groups), biochemistry (unpaired T-test), hematology (unpaired T-test), organ weights (unpaired T-test) and CeO levels in various tissues (unpaired T-test) were statistically analysed.3. Results
3.1 Clinical observation
All animals were observed for daily clinical signs and symptoms after implantation surgery. All operated animals (Groups 2–5 & 7–10) withstood the implantation surgery well. Post-operative period was uneventful in all animals. The implanted site healed well with no unexpected local reactions. No mortality or unexpected morbidity was observed in any animal. No clinical signs of ill health or overt toxicity were observed in any of the animals.3.2 Body weight changes
All animals showed statistically significant increase (P < 0.001) in body weights at the end of 28-day observation period (see Table 4). However, the animals that underwent surgery showed a decreasing trend (though not statistically significant) in body weight gain in the first week after surgery but regained the weights at the end of 28 days. No significant weight changes were observed in any of the treatment groups compared to negative control or untreated controls.| Group | Sex | Body weight (g) | ||||
|---|---|---|---|---|---|---|
| Day 0 | Day 71 | Day 14 | Day 21 | Day 282,3 | ||
| 1 Statistical analysis (unpaired T-test) was performed to compare the body weights between control group and CeO-NP implanted groups. No significant changes were observed. 2 Statistical analysis was performed to compare the body weights between Day-0 and Day-28, for all groups using paired T-test. This showed significant increase (#, P < 0.05; ##, P < 0.01; ###, P < 0.001) in body weights in all groups. 3 Statistical analysis (unpaired T-test) was performed to compare the body weights between untreated/control group and CeO-NP implanted groups. No significant changes were observed. | ||||||
| Untreated | Male | 110.0 ± 2.83 | 120.5 ± 2.12 | 131.5 ± 4.95 | 141.0 ± 4.24 | 150.0## ± 2.83 |
| Control (1 × 10 mm HDPE) | Male | 112.5 ± 2.12 | 114.5 ± 0.71 | 137.5 ± 3.54 | 147.0 ± 4.24 | 156.5# ± 4.95 |
| CeO2-NP (250 mg per implantation site) | Male | 105.4 ± 6.73 | 110.2$ ± 6.30 | 121.8 ± 4.71 | 133.2 ± 3.27 | 149.0### ± 1.87 |
| CeO2-NP (500 mg per implantation site) | Male | 107.2 ± 8.90 | 112.2 ± 8.53 | 123.2 ± 10.99 | 134.6 ± 12.78 | 149.4### ± 11.89 |
| CeO2-NP (1000 mg per implantation site) | Male | 106.4 ± 3.91 | 112.0$ ± 3.94 | 125.8 ± 5.36 | 137.2 ± 5.93 | 149.6### ± 7.54 |
| Untreated | Female | 97.5 ± 4.95 | 116.0 ± 4.95 | 122.0 ± 4.24 | 138.0 ± 2.83 | 150.0## ± 5.66 |
| Control (1 × 10 mm HDPE) | Female | 101.5 ± 10.61 | 113.0 ± 9.90 | 126.5 ± 9.19 | 140.0 ± 14.14 | 152.0# ± 11.31 |
| CeO2-NP (250 mg per implantation site) | Female | 106.0 ± 3.81 | 111.0 ± 4.36 | 125.6 ± 6.15 | 138.4 ± 5.68 | 152.6### ± 7.20 |
| CeO2-NP (500 mg per implantation site) | Female | 98.4 ± 6.84 | 103.8 ± 6.38 | 117.6 ± 8.38 | 131.2 ± 10.06 | 143.2### ± 9.65 |
| CeO2-NP (1000 mg per implantation site) | Female | 103.4 ± 2.30 | 108.6 ± 2.70 | 121.4 ± 5.59 | 133.6 ± 3.78 | 147.6### ± 6.43 |
3.3 Clinical biochemistry, hematology and serum electrolytes
No biologically relevant changes in biochemistry, hematology and serum electrolytes were observed in any of the groups compared to negative control and untreated control animals (see Tables 5–7). Small changes (P < 0.05) in WBC (decrease) and platelet (increase) counts were seen in high dose male group; and decrease in monocyte (P < 0.05) in high dose female group. However, these values fell within the laboratory's normal reference ranges and therefore not considered biologically relevant.| Groups | ALT | AST | ALP | TB | ALB | TP | A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G G |
CHO | GLU | UR | CR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| U L−1 | U L−1 | U L−1 | mg dL−1 | g dL−1 | g dL−1 | mg dL−1 | mg dL−1 | mg dL−1 | mg dL−1 | ||
Serum alanine transaminase (ALT), Aspartate transaminase (AST), Alkaline Phosphatase (ALP), Total Bilirubin (TB), Albumin (ALB), Total Proteins (TP), Albumin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Globulin Ratio (A Globulin Ratio (A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G), Cholesterol (CHO), Glucose (GLU), Urea (UR), Creatinine (CR); all values are represented as mean ± SD; unpaired T-test was used to compare the statistical significance between untreated and control as well as between control and CeO-NP implanted groups. No significant changes were observed. G), Cholesterol (CHO), Glucose (GLU), Urea (UR), Creatinine (CR); all values are represented as mean ± SD; unpaired T-test was used to compare the statistical significance between untreated and control as well as between control and CeO-NP implanted groups. No significant changes were observed. |
|||||||||||
| Male animals | |||||||||||
| Untreated | 32.5 ± 2.12 | 134.5 ± 12.02 | 391 ± 49.5 | 0.125 ± 0.02 | 4.3 ± 0.28 | 6.25 ± 0.49 | 2.35 ± 0.07 | 60 ± 19.8 | 93 ± 2.83 | 36.5 ± 3.54 | 0.405 ± 0.04 |
| Control (1 × 10 mm HDPE) | 23 ± 1.41 | 147 ± 8.49 | 372 ± 43.84 | 0.06 ± 0.01 | 3.95 ± 0.35 | 6.2 ± 0.14 | 1.85 ± 0.78 | 61 ± 2.83 | 84.5 ± 21.92 | 29.5 ± 7.78 | 0.435 ± 0.04 |
| CeO2-NP (250 mg per implantation site) | 27.2 ± 5.36 | 130 ± 13.19 | 371.8 ± 44.1 | 0.112 ± 0.03 | 3.76 ± 0.46 | 6 ± 0.47 | 1.52 ± 0.62 | 62 ± 13.77 | 82.2 ± 12.6 | 32.4 ± 9.07 | 0.452 ± 0.05 |
| CeO2-NP (500 mg per implantation site) | 24.6 ± 5.5 | 135.4 ± 14.48 | 403 ± 27.16 | 0.09 ± 0.01 | 4.12 ± 0.49 | 6.24 ± 0.47 | 2 ± 1.15 | 66.2 ± 15.32 | 85 ± 11.22 | 35.6 ± 4.67 | 0.436 ± 0.04 |
| CeO2-NP (1000 mg per implantation site) | 29.6 ± 5.5 | 131.8 ± 9.01 | 392.4 ± 30.46 | 0.108 ± 0.04 | 3.86 ± 0.47 | 5.92 ± 0.41 | 1.9 ± 0.91 | 68.4 ± 12.14 | 85.6 ± 12.48 | 31.6 ± 7.16 | 0.482 ± 0.03 |
| Female animals | |||||||||||
| Untreated | 28 ± 5.66 | 124.5 ± 13.44 | 384.5 ± 23.33 | 0.13 ± 0.03 | 4.1 ± 0.42 | 6.2 ± 0.71 | 2 ± 0.14 | 58 ± 19.8 | 80 ± 7.07 | 30.5 ± 12.02 | 0.46 ± 0.07 |
| Control (1 × 10 mm HDPE) | 23 ± 5.66 | 134 ± 12.73 | 417 ± 28.28 | 0.09 ± 0.04 | 3.9 ± 0.57 | 6.15 ± 0.35 | 1.65 ± 1.48 | 61.5 ± 12.02 | 83.5 ± 23.33 | 38 ± 4.24 | 0.41 ± 0.03 |
| CeO2-NP (250 mg per implantation site) | 27 ± 5.1 | 130.2 ± 14.86 | 383.2 ± 29.29 | 0.14 ± 0.02 | 4.2 ± 0.42 | 6.22 ± 0.47 | 2.46 ± 1.15 | 61.6 ± 15.98 | 90.6 ± 8.17 | 28 ± 6 | 0.512 ± 0.03 |
| CeO2-NP (500 mg per implantation site) | 26 ± 4.69 | 138.4 ± 6.88 | 402.4 ± 50.63 | 0.124 ± 0.04 | 3.98 ± 0.28 | 6.2 ± 0.44 | 1.88 ± 0.29 | 59 ± 12.88 | 80.6 ± 15.44 | 34.6 ± 9.37 | 0.46 ± 0.07 |
| CeO2-NP (1000 mg per implantation site) | 25.8 ± 4.76 | 146.6 ± 11.15 | 383.4 ± 39.43 | 0.128 ± 0.04 | 4.2 ± 0.19 | 6.24 ± 0.44 | 2.16 ± 0.63 | 70 ± 7.62 | 89.6 ± 14.05 | 31.6 ± 8.08 | 0.47 0.05 |
| Groups | Hb | hematocrit | MCV | MCH | MCHC | RBC | WBC | Lymph | Mono | Granul | Platelets |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg dL−1 | % | fL | pg | g dL−1 | ×106 μL−1 | ×103 μL−1 | % | % | % | ×103 μL−1 | |
| Hb, Hemoglobin; MCV, mean corpuscular volume; MCH mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; RBC, red blood cell count; WBC, white blood cell count; Lymph, % of lymphocytes; Mono, % of monocytes (%); Granul, % of granulocytes; all values are represented as mean ± SD; unpaired T-test was used to compare the statistical significance between untreated and control as well as between control and CeO-NP implanted groups. Small statistical decrease (*, P < 0.05) was seen in monocyte counts in the intermediate dose, but this decrease was not dose related and therefore not considered biologically relevant. | |||||||||||
| Male animals | |||||||||||
| Untreated | 14.35 ± 0.07 | 43.3 ± 0.57 | 55.9 ± 1.84 | 20.4 ± 0.99 | 36.45 ± 0.78 | 7.855 ± 0.21 | 8.65 ± 0.64 | 78 ± 1.41 | 3.5 ± 0.71 | 18.5 ± 0.71 | 1063 ± 89 |
| Control (1 × 10 mm HDPE) | 14.35 ± 0.21 | 44 ± 0.57 | 53.5 ± 1.84 | 22 ± 0.42 | 37.25 ± 2.19 | 7.775 ± 0.08 | 11.5 ± 0.42* | 77 ± 7.07 | 3.5 ± 2.12 | 19.5 ± 9.19 | 1002.5 ± 19 |
| CeO2-NP (250 mg per implantation site) | 14.36 ± 0.28 | 42.6 ± 0.99 | 56.34 ± 3.38 | 20.98 ± 0.88 | 37.08 ± 1.08 | 7.61 ± 0.44 | 9.76 ± 1.6 | 76.2 ± 4.32 | 3.8 ± 1.1 | 20 ± 4.42 | 1083.4 ± 70 |
| CeO2-NP (500 mg per implantation site) | 14.44 ± 0.29 | 43.42 ± 0.82 | 56.22 ± 3.03 | 20.94 ± 0.63 | 37.14 ± 0.96 | 7.97 ± 0.27 | 9.66 ± 1.49 | 76.2 ± 5.54 | 2.6 ± 0.55 | 21.2 ± 5.54 | 1093.2 ± 50* |
| CeO2-NP (1000 mg per implantation site) | 14.44 ± 0.21 | 44.28 ± 1.76 | 56.52 ± 2.95 | 20.94 ± 0.71 | 37.22 ± 1.32 | 7.896 ± 0.48 | 9.86 ± 1.15* | 74.8 ± 2.49 | 2.4 ± 1.14 | 22.8 ± 2.28 | 1095.8 ± 41* |
| Female animals | |||||||||||
| Untreated | 14.5 ± 0.14 | 44.3 ± 2.83 | 55.95 ± 2.62 | 21.85 ± 0.49 | 37.05 ± 0.35 | 8.2 ± 0.21 | 11.9 ± 0.14 | 76.5 ± 3.54 | 3 ± 1.41 | 20.5 ± 2.12 | 1106 ± 83 |
| Control (1 × 10 mm HDPE) | 14.2 ± 0.14 | 43.9 ± 2.26 | 56.45 ± 5.59 | 21.75 ± 1.06 | 37.2 ± 0.42 | 7.815 ± 1.05 | 11.8 ± 0.14 | 73.5 ± 0.71 | 5 ± 0 | 21.5 ± 0.71 | 1092 ± 56 |
| CeO2-NP (250 mg per implantation site) | 14.7 ± 0.12 | 44.36 ± 1.45 | 56.56 ± 3.14 | 20.78 ± 1.34 | 37.12 ± 0.64 | 7.78 ± 0.47 | 10.94 ± 1.96 | 78 ± 4.53 | 3.4 ± 1.34 | 18.6 ± 3.65 | 1102.4 ± 48 |
| CeO2-NP (500 mg per implantation site) | 14.5 ± 0.25 | 44.2 ± 1.61 | 58.06 ± 2.69 | 21.18 ± 1.51 | 37.3 ± 1.07 | 7.748 ± 0.68 | 10.64 ± 2.19 | 76.2 ± 4.44 | 1.6 ± 0.89* | 22.2 ± 5.26 | 1117.6 ± 50 |
| CeO2-NP (1000 mg per implantation site) | 14.48 ± 0.16 | 42.38 ± 1.96 | 56.94 ± 3.36 | 21.38 ± 1.31 | 37.92 ± 0.56 | 7.816 ± 0.36 | 10.44 ± 1.32 | 75.2 ± 2.77 | 3.6 ± 1.14 | 21.2 ± 3.03 | 1073.2 ± 60 |
| Groups | Calcium | Inorganic phosphorus | Sodium | Potassium | Chloride |
|---|---|---|---|---|---|
| mg dL−1 | mMol L−1 | mMol L−1 | mMol L−1 | mMol L−1 | |
| All values are represented as mean ± SD; unpaired T-test was used to compare the statistical significance between untreated and control as well as between control and CeO-NP implanted groups. Small statistical decreases (*, P < 0.05; **, P < 0.01) were seen in calcium and chloride levels, but these values fell within the normal reference ranges for our laboratory are not considered biologically relevant. No statistical differences were observed. | |||||
| Male animals | |||||
| Untreated | 10.35 ± 0.21 | 10.05 ± 0.35 | 142.8 ± 0.57 | 5.41 ± 0.21 | 102.5 ± 3.54 |
| Control (1 × 10 mm HDPE) | 11.15 ± 0.07 | 10.1 ± 0.57 | 143.25 ± 1.34 | 5.39 ± 0.62 | 102.5 ± 0.71 |
| CeO2-NP (250 mg per implantation site) | 11.22 ± 0.13 | 10.24 ± 0.36 | 143.08 ± 1.24 | 5.138 ± 0.44 | 100.6 ± 3.05 |
| CeO2-NP (500 mg per implantation site) | 10.92 ± 0.54 | 9.98 ± 0.34 | 143.62 ± 1.28 | 5.5 ± 0.3 | 101 ± 2.65 |
| CeO2-NP (1000 mg per implantation site) | 10.18 ± 0.4** | 10.36 ± 0.38 | 143.16 ± 1.32 | 5.152 ± 0.28 | 99.2 ± 1.48* |
| Female animals | |||||
| Untreated | 11.25 ± 0.49 | 10 ± 0.42 | 142.35 ± 0.07 | 5.535 ± 0.47 | 101.5 ± 0.71 |
| Control (1 × 10 mm HDPE) | 11 ± 0.85 | 10.6 ± 0.28 | 143.35 ± 0.21 | 5.41 ± 0.41 | 99 ± 1.41 |
| CeO2-NP (250 mg per implantation site) | 10.86 ± 0.42 | 10.24 ± 0.5 | 142.82 ± 1.33 | 5.138 ± 0.34 | 99.2 ± 3.27 |
| CeO2-NP (500 mg per implantation site) | 10.8 ± 0.74 | 10.14 ± 0.4 | 142.78 ± 1.09 | 5.21 ± 0.17 | 100.8 ± 2.28 |
| CeO2-NP (1000 mg per implantation site) | 11.42 ± 0.31 | 9.7 ± 0.17 | 144.24 ± 0.77 | 5.18 ± 0.36 | 97.8 ± 1.1 |
3.4 Gross pathology
Each implant site was examined qualitatively for alterations of the normal structure including the assessment of the axillary and inguinal lymph nodes. The implant was well defined with a thin fibrous capsule, which was not adhering with the adjoining tissues. No unusual vascularization was seen on the capsule or adjoining tissue. No necrosis or hematoma was observed in the implant capsule or adjoining tissues. The capsule was gently excised, and the interior of the implant capsule was examined. The contents of the capsule were intact. No hematoma or other fluid collection was observed within the capsule. Some fibrous infiltration was seen extending inside the capsule content. The draining lymph nodes appeared normal. Representative photographs of 1000 μg implant site is given in Fig. 1a and b.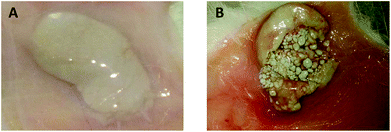 | ||
| Fig. 1 Gross pathology appearance of cerium oxide nanoparticles implanted sites after 28 days of subcutaneous implantation. A. Unopened capsule. B. Incised capsule. | ||
Gross pathology of all organs did not reveal any abnormality. The organ weights of key organs did not show any difference between the various groups (see Table 8).
| Group | Sex | Heart, g | Liver, g | Adrenal, mg | Kidney, g | Thymus, g | Spleen, g | Brain, g | Testis, g | Ovaries, g | Lungs, g | Thyroid, mg | Pituitary, mg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All values are represented as mean ± SD; unpaired T-test was used to compare the statistical significance between untreated and control as well as between control and CeO-NP implanted groups. No statistical differences were observed. | |||||||||||||
| Untreated | Male | 1.61 ± 0.13 | 15.52 ± 3.92 | 67.5 ± 0.71 | 3.70 ± 0.11 | 0.60 ± 0.11 | 1.07 ± 0.01 | 2.04 ± 0.23 | 4.82 ± 0.06 | n/a | 2.09 ± 0.02 | 13.5 ± 3.54 | 15 ± 8.49 |
| Control (1 × 10 mm HDPE) | Male | 1.49 ± 0.12 | 15.55 ± 3.72 | 55 ± 12.73 | 3.18 ± 0.11 | 0.66 ± 0.01 | 1.04 ± 0.22 | 2.17 ± 0.02 | 4.92 ± 0.22 | n/a | 1.71 ± 0.00 | 15 ± 1.41 | 22.5 ± 7.78 |
| CeO2-NP (250 mg per implantation site) | Male | 1.47 ± 0.22 | 17.85 ± 2.76 | 60.40 ± 9.50 | 2.97 ± 0.24 | 0.61 ± 0.10 | 1.42 ± 0.43 | 2.11 ± 0.08 | 4.62 ± 0.29 | n/a | 1.72 ± 0.16 | 20.40 ± 3.58 | 22.00 ± 8.69 |
| CeO2-NP (500 mg per implantation site) | Male | 1.52 ± 0.16 | 17.40 ± 5.43 | 54.80 ± 10.96 | 3.33 ± 0.50 | 0.57 ± 0.09 | 1.42 ± 0.35 | 2.07 ± 0.16 | 4.47 ± 0.34 | n/a | 1.84 ± 0.17 | 25.60 ± 6.99 | 16.60 ± 6.62 |
| CeO2-NP (1000 mg per implantation site) | Male | 1.38 ± 0.18 | 19.08 ± 3.72 | 51.20 ± 3.90 | 3.29 ± 0.40 | 0.74 ± 0.12 | 1.44 ± 0.21 | 1.98 ± 0.16 | 4.67 ± 0.31 | n/a | 1.92 ± 0.09 | 20.60 ± 6.50 | 21.80 ± 7.33 |
| Untreated | Female | 1.37 ± 0.10 | 18.53 ± 2.35 | 59.5 ± 6.36 | 3.24 ± 0.62 | 0.59 ± 0.12 | 1.63 ± 0.18 | 2.28 ± 0.04 | n/a | 1.28 ± 0.15 | 1.91 ± 0.13 | 20 ± 12.73 | 20.5 ± 4.95 |
| Control (1 × 10 mm HDPE) | Female | 1.49 ± 0.09 | 18.54 ± 1.24 | 55.5 ± 14.85 | 3.04 ± 0.54 | 0.63 ± 0.16 | 1.71 ± 0.07 | 2.17 ± 0.16 | n/a | 1.09 ± 0.02 | 1.87 ± 0.17 | 15 ± 5.66 | 27 ± 2.83 |
| CeO2-NP (250 mg per implantation site) | Female | 1.44 ± 0.14 | 18.58 ± 2.40 | 53.80 ± 9.04 | 3.20 ± 0.47 | 0.70 ± 0.09 | 1.32 ± 0.35 | 2.00 ± 0.15 | n/a | 1.24 ± 0.25 | 1.70 ± 0.10 | 18.20 ± 6.91 | 19.40 ± 7.70 |
| CeO2-NP (500 mg per implantation site) | Female | 1.44 ± 0.10 | 19.78 ± 3.55 | 57.00 ± 10.84 | 3.20 ± 0.47 | 0.65 ± 0.15 | 1.43 ± 0.39 | 1.92 ± 0.06 | n/a | 1.14 ± 0.18 | 1.89 ± 0.19 | 24.00 ± 6.04 | 18.60 ± 5.73 |
| CeO2-NP (1000 mg per implantation site) | Female | 1.33 ± 0.17 | 16.02 ± 3.18 | 58.20 ± 4.38 | 3.05 ± 0.47 | 0.70 ± 0.10 | 1.26 ± 0.44 | 2.00 ± 0.20 | n/a | 1.38 ± 0.17 | 1.91 ± 0.21 | 22.40 ± 7.77 | 16.80 ± 4.09 |
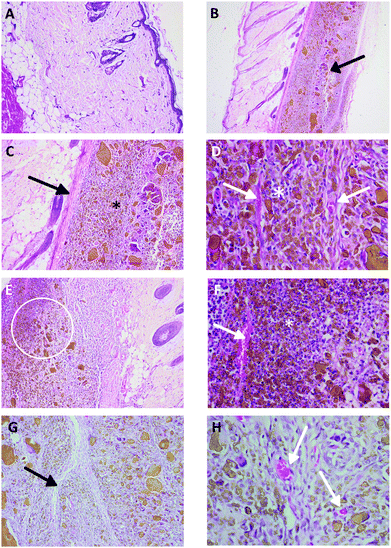
3.5 Histopathology
The results of semiquantitative analysis of histopathology of various implants are given in Table 9. Implantation irritation index for all groups ranged between 0 and 2.0 for males and 0.0 for females. Key histopathology findings in all implanted tissue were similar. There were some polymorphonuclear cell infiltration in all implants suggesting minimal inflammation reaction (please see Fig. 2). Few lymphocytes were also present in all examined implant tissue. The fibrous tissue capsule was thin with some neovascularization.| Implantation material | No of animals/group | Sex | Total number of implantation sites | Implantation Group Score | Implantation irritation index | Key histopathology findingsb | Conclusiona | |
|---|---|---|---|---|---|---|---|---|
| a 0.0–2.9: minimal or no reaction; 3.0–8.9: slight reaction; 9.0–15: moderate reaction; >15.1: severe reaction. b P, Polymorphonuclear cells; L, Lymphocytes; N, neovascularization; F, Fibrosis. | ||||||||
| Group 1 | Untreated | 2 | Male | N/A | — | — | ||
| Group 2 | Control (1 × 10 mm HDPE) | 2 | Male | 4 | 10 | — | P, L, N, F | |
| Group 3 | CeO2-NP (250 mg per implantation site) | 5 | Male | 10 | 9.5 | 0 | P, L, N, F | Minimal or no reaction |
| Group 4 | CeO2-NP (500 mg per implantation site) | 5 | Male | 10 | 10.5 | 0.5 | P, L, N, F | Minimal or no reaction |
| Group 5 | CeO2-NP (1000 mg per implantation site) | 5 | Male | 10 | 12.0 | 2.0 | P, L, N, F | Minimal or no reaction |
| Group 6 | Untreated | 2 | Female | N/A | — | — | ||
| Group 7 | Control (1 × 10 mm HDPE) | 2 | Female | 4 | 8.5 | — | P, L, N, F | |
| Group 8 | CeO2-NP (250 mg per implantation site) | 5 | Female | 10 | 7.5 | 0 | P, L, N, F | Minimal or no reaction |
| Group 9 | CeO2-NP (500 mg per implantation site) | 5 | Female | 10 | 8.0 | 0 | P, L, N, F | Minimal or no reaction |
| Group 10 | CeO2-NP (1000 mg per implantation site) | 5 | Female | 10 | 8.0 | 0 | P, L, N, F | Minimal or no reaction |
Implantation scores obtained with CeO2-NP was compared with other implant materials tested in our laboratory (see Table 10).
| Implant material | Animal species | Site of implantation | Implantation score for control implant | Implantation score for test implant | Implantation irritation index | Key histopathology findings | Implantation reaction |
|---|---|---|---|---|---|---|---|
| SC, subcutaneous; P, Polymorphonuclear cells; F, fibrosis; L, lymphocytes; D, debris; N, neovascularization; Pl, plasma cells; Fi, fatty infiltrate.a Data collected from 1 previous study conducted in our laboratory the last year.b Data collected from last 5 studies conducted in our laboratory. –, minimal or no reaction (0 to 2.9); +, slight reaction (3.0 to 8.9); ++, moderate reaction (9.0 to 15.0); +++, severe reaction (>15.1). | |||||||
| Cerium oxide coated hydroxyapatitea | Rats | SC | 8.8 | 12.6 | 3.8 | P, F, L, D | + |
| Cerium oxide coated stainless steela | Rabbit | SC | 10.3 | 16.2 | 5.9 | P, F, L, N | + |
| Stainless steelb | Rats | SC | 10.7 | 15.9 | 5.2 | P, F, L, N, Pl | + |
| Stainless steelb | Rats | SC | 9.8 | 14.4 | 4.6 | P, F, L, N | + |
| Stainless steelb | Rabbits | SC | 11.6 | 20 | 8.4 | P, F, L, N | + |
| Stainless steelb | Rabbits | SC | 9.1 | 18.3 | 9.2 | P, F, L, N | ++ |
| Stainless steelb | Rats | SC | 9.1 | 13.3 | 4.2 | P, F, L, N | + |
| Hydroxyapatiteb | Rats | SC | 11.2 | 20.6 | 9.4 | P, F, L, N, Pl, Fi | ++ |
| Hydroxyapatiteb | Rabbits | SC | 8.2 | 17.3 | 9.1 | P, F, L, N, Pl, Fi | ++ |
| Hydroxyapatiteb | Rats | SC | 10.9 | 17.5 | 6.6 | P, F, L, N, Pl, Fi | + |
| Hydroxyapatiteb | Rats | SC | 10.6 | 17 | 6.4 | P, F, L, N, Pl, Fi | + |
| Hydroxyapatiteb | Rats | SC | 11.3 | 18.2 | 6.9 | P, F, L, N, Pl, Fi | + |
| Ti6Al4V alloyb | Rabbits | IM | 9.6 | 14.2 | 4.6 | P, F | + |
| Ti6Al4V alloyb | Rabbits | SC | 8.6 | 11.6 | 3 | P, F | + |
| Ti6Al4V alloyb | Rats | SC | 9.9 | 15 | 5.1 | P, F | + |
| Ti6Al4V alloyb | Rats | SC | 9 | 11.9 | 2.9 | P, F | − |
| Ti6Al4V alloyb | Rats | SC | 8.7 | 12.4 | 3.7 | P, F | + |
| Cobalt chromiumb | Rats | SC | 11.2 | 19.7 | 8.5 | P, F, L, N, Pl, Fi | + |
| Cobalt chromiumb | Rats | SC | 9.2 | 16 | 6.8 | P, F, L, N, Pl, Fi | + |
| Cobalt chromiumb | Rats | SC | 11.6 | 17.1 | 5.5 | P, F, L, N, Pl, Fi | + |
| Cobalt chromiumb | Rats | SC | 10.7 | 19.3 | 8.6 | P, F, L, N, Pl, Fi | + |
| Cobalt chromiumb | Rats | SC | 9.2 | 16.6 | 7.4 | P, F, L, N, Pl, Fi | + |
Since, no significant treatment related histopathology changes were observed in tier 1 organs, other organs were not examined.
3.6 ICP-MS analysis
ICP-MS analysis showed that the levels of CeO2-NP accumulated in spleen, lungs, liver and kidneys (please see Table 11). No significant difference between the organs or sexes were found. The levels of this material in brain and heart were similar to control animal levels (please see Table 11).| Group | Ce levels (ng g−1 of tissue) | ||||||
|---|---|---|---|---|---|---|---|
| Lungs | Spleen | Heart | Liver | Brain | Kidneys | ||
| Unpaired T-test was used to compare the levels of Ce/CeO in control group with that of CeO-NP implanted groups. The levels of Ce/CeO are significantly higher (*, P < 0.05; **, P < 0.01; ***, P < 0.001) in lungs, spleen, liver and kidneys. | |||||||
| Untreated | Male | 39.5 ± 16.3 | 40 ± 11.3 | 35.5 ± 9.2 | 52 ± 9.9 | 41 ± 25.5 | 35 ± 9.9 |
| Control (1 × 10 mm HDPE) | Male | 45 ± 17.0 | 41 ± 21.2 | 39.5 ± 21.9 | 23.5 ± 2.1 | 43 ± 8.5 | 45.5 ± 13.4 |
| CeO2-NP (250 mg per implantation site) | Male | 241.8 ± 20.7** | 191 ± 26.0** | 37.6 ± 16.6 | 195.8 ± 25.0*** | 44.2 ± 18.5 | 257.4 ± 17.4** |
| CeO2-NP (500 mg per implantation site) | Male | 225.8 ± 22.2** | 189.4 ± 30.8** | 41.6 ± 12.0 | 199.8 ± 28.7*** | 42.4 ± 11.4 | 242.8 ± 30.8*** |
| CeO2-NP (1000 mg per implantation site) | Male | 255.6 ± 40.5*** | 205.2 ± 23.5* | 39.8 ± 17.4 | 216.8 ± 28.5*** | 44.2 ± 9.1 | 270.2 ± 24.3*** |
| Untreated | Female | 45.5 ± 9.2 | 40 ± 24 | 33.5 ± 0.7 | 51 ± 0.0 | 38.5 ± 19.1 | 41 ± 5.7 |
| Control (1 × 10 mm HDPE) | Female | 36 ± 5.7 | 50 ± 9.9 | 42.5 ± 4.9 | 35.5 ± 21.9 | 45.5 ± 0.7 | 35 ± 4.2 |
| CeO2-NP (250 mg per implantation site) | Female | 263.4 ± 30.9*** | 211.2 ± 6.5* | 37.8 ± 10.1 | 191.8 ± 35.1** | 38.8 ± 12.2 | 272.8 ± 20.4*** |
| CeO2-NP (500 mg per implantation site) | Female | 271 ± 26*** | 183.8 ± 25.7*** | 35.8 ± 11.6 | 199.6 ± 34** | 41.4 ± 14.5 | 254.4 ± 33.3*** |
| CeO2-NP (1000 mg per implantation site) | Female | 263 ± 39.5*** | 214 ± 37.5*** | 40.2 ± 15.4 | 178 ± 18.5* | 38.2 ± 11.7 | 234.2 ± 13.3*** |
3.7 Micronuclei analysis
% MNPCE of all implanted and control animals were within the laboratory historical negative control value of 0.6%. Unmarked positive control slides were scored as clear positive micronuclei response. Therefore CeO2-NP was not considered to induce micronuclei via implantation route.4. Discussion
We have conducted an implantation study of CeO2-NP, combined with 28-day systemic toxicity and genotoxicity studies aligned to current regulatory standards. The results of implantation study highlight the local toxic effects caused by implants and is mandated by ISO-10993, Part 1 for all implant devices. Systemic toxicity studies give an indication of the toxic effects caused by chemicals leaching out of the implants and their effects on organs, distant from the site of implantation. In vivo micronucleus test is also a regulatory requirement to demonstrate lack of genotoxicity potential of long term implants. By combining these three studies, we used lesser animals in line with 3Rs of animal testing. The data generated from this combined study can be safely used to perform safety assessment of medical devices. It should be noted that unlike the OECD guidelines for testing of chemicals, the ISO10993 standards are designed for medical device risk assessment rather than hazard identification. Also, there are still some ambiguity in ISO 10993, Parts 3, 11 and 33, in terms of scientific robustness of bolt on systemic and genotoxicity studies.27,29,30 These are discussed later in the discussion.We have used a combined multiple endpoint study to generate hazard data for cerium oxide nanoparticles. Also, the use of implantation route for generating systemic toxicity data is also unique. Systemic toxicity data is usually generated by giving repeated exposure of same dose of chemicals or extracts. Risk assessment using data generated can be over conservative for implant medical devices. Using implantation route to generate systemic toxicity data is clinically more relevant. There will be an initial burst of chemicals leaching out from the implanted devices resulting in a high exposure. Subsequently the exposure decreases as a function of time until it completely disappears from the system or enters a low-level steady state exposure, depending on the nature of the device material.
ISO10993, Part 11 mentions the use of implantation route to generate systemic toxicity data, but no guidance is given on the use of dose (size or quantity) to be implanted. For a robust systemic toxicity risk assessment, sufficiently high top-dose should be used. For chemicals, there is clear recommendation in OECD guidelines on the how to select the top and lower doses. Whereas, for medical device biocompatibility no such recommendation exists. The current practice takes inspiration from ISO10993, Part 6 (local effects after implantation) and use the sizes (5–10 numbers of 1 × 10 mm implants) defined therein to generate systemic toxicity data. Clearly this is not correct. We feel that human equivalent dose (HED) or higher (worst case), should be used for generating systemic toxicity data for biocompatibility, as with pharmaceuticals. In this study we used such high doses to generate systemic toxicity data on cerium oxide nanoparticles.
The local effect after implantation is mainly driven by inflammatory response pathway. Various doses of CeO2-NPs in both males and females elicited minimal or no local implantation reaction compared to negative control (HDPE) implants. Local reaction scores for CeO2-NP was also generally less compared to other materials (Cerium oxide coated hydroxyapatite, Cerium oxide coated stainless steel, Stainless steel, Hydroxyapatite, Ti6Al4V alloy, Cobalt chromium) tested in our laboratory. There were generally less inflammatory cells and the fibrous capsule was thinner with CeO2-NP compared other materials. Plasma cells seen with other materials were predominantly absent with CeO2-NP implants. These findings suggest that CeO2-NP was tolerated better than other materials, most probably due to its anti-inflammatory31,32 properties and/or lesser immunological reactions.33 It is also speculated that nanoparticles can modulate tissue remodelling via extra cellular matrix.34 Nelson et al. has reviewed CeO2-NP for its antioxidant ability via reduction in ROS and NO species in invitro and in vivo systems.35 It has been shown that CeO2-NP may have potential anti-inflammatory31,32 and wound healing36 properties. Inhibition of lipid peroxidation and a decrease in oxidative stress markers were observed in CeO2-NP treated mice and the effects were comparable to mice treated with the widely used antioxidant, N-acetyl cysteine (NAC).32 CeO2-NP applied locally to incised rats enhanced wound healing activity with regard to hydroxyproline content, wound tensile strength, and wound closure time relative to treatment with the well-known antiseptic, povidone-iodine.36 These enhancements were attributed to the ROS-scavenging abilities of the CeO2-NP at the site of injury and protection of the native tissue by increased production of collagen and hydroxyproline. Our findings are therefore in line with these biological properties of CeO2-NP and further strengthens these relationships.
Following implantation of a biomaterial, the host initiates an inflammatory reaction that fails to turn off in the presence of the foreign (nonhost) material. This response is termed the foreign body response and is characterized by persistent inflammation, macrophage infiltration and fusion to form foreign body giant cells, and fibrotic capsule formation.37,38 Extensive fibrosis and encapsulation can result in biomaterial malfunction, extrusion, infection, thrombosis, and soft-tissue contracture.39,40 Following injury, normal wound healing proceeds through overlapping phases of inflammation, proliferation, and tissue remodeling, resulting in granulation tissue deposition and scar formation. The typical host reaction to a biomaterial is similar in many aspects to the early stages of wound healing, but the presence of an implanted material significantly alters progression through the subsequent phases of repair. The magnitude of this response is modulated by numerous factors, including whether the biomaterial is synthetic or bioactive (contains ligands or receptors that modulate living systems) and its biodegradability.41 Presence of nanoparticles in implants is expected to modulate this tissue repair response due to their different surface properties. Local effects after CeO2-NP showed typical inflammatory and foreign body reactions seen with all implants. However, these reactions were diminished compared to other materials as described above. Injury to vascularized tissue during implantation leads to the deposition of blood proteins and thrombotic agents onto the biomaterial surface in a process known as “biofouling”, resulting in the formation of a provisional matrix (composed of fibrinogen, chemokines, activated platelets, inflammatory cells and endothelial cells) around the biomaterial.42 It is possible that CeO2-NP could some how inhibit the process of biofouling; or possibly degrade the provisional matrix. Fibrinogen plays an important role in recruiting inflammatory cells to the site of injury and the formation of a layer of phagocytes around the implanted biomaterial. Our initial studies on hemocompatibility suggested that CeO2-NP modulated the levels of fibrinopeptide A in human serum ex vivo.
Regulatory systemic toxicity study of CeO2-NP via implanted route showed little or no toxicity. This route was selected based on potential use of CeO2-NP in surgical implants. No test item related changes in clinical, biochemistry, hematology or histopathology was seen. It should be noted that in surgical implants, the CeO2-NP will be firmly adhered to the device, where as in this study we used nanoparticles as powder. Therefore, systemic bioavailability would be significantly higher in this study compared to a surgical implant, and therefore represents a worst-case scenario for biological safety assessments. ICP-MS analysis for CeO/Ce showed extremely small quantities of the material in liver and kidneys, but in brain, lungs and heart were below the detection levels from 1000 mg per implanted animals groups. This suggest that the nanoparticles actually migrated from the implant sites and deposited in liver, lungs, spleen and kidneys. This distribution is similar to those observed by Hirst et al., who found similar distribution with a top dose of 0.5 mg kg−1 following intravenous, intraperitoneal or oral distribution.32 Our study fulfils the regulatory biocompatibility requirements of ISO10993, Parts 3, 6 and 11, and indicates that CeO2-NP is relatively non-toxic when used as implants. Furthermore, bone marrow harvested at the end of 28 days did not show any increase in MNPCEs.
It should be noted that the guidance from the current ISO standards for implantation and systemic toxicity does not fully address the requirements for combined implantation-systemic toxicity studies. We would therefore like to list out few requirements to be followed while conducting a combined implantation and systemic toxicity study, in order to make these studies scientifically robust. It is recommended that the test implants and negative control implants be used on separate animals. For systemic toxicity studies, it is preferable to use subcutaneous route for implantation, rather than intramuscular (IM) route, because the thick fibrous capsule associated with IM route will prevent leaching of materials to the systemic circulation. Also, it is recommended to use animal equivalent dose of human clinical exposure or more for a scientifically robust systemic toxicity study.
In conclusion, CeO2-NP is a highly biocompatible implant material, with no systemic or genotoxicity, which also reduces local inflammatory reactions when used as implants. These observations provide a base line biocompatibility and toxicity data on CeO2-NP. The current findings will also be useful in defining standards for nanoparticle containing biomaterials and medical devices.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.Conflicts of interest
There are no conflicts to declare.References
- S. A. Brennan, C. Ní Fhoghlú, B. M. Devitt, F. J. O'Mahony and D. Brabazon, A Walsh, Silver nanoparticles and their Orthopaedic applications, Bone Joint J., 2015, 97-B(5), 582–589 CrossRef CAS PubMed.
- E. H. Abdulkareem, K. Memarzadeh, R. P. Allaker, J. Huang, J. Pratten and D. Spratt, Anti-biofilm activity of zinc oxide and hydroxyapatite nanoparticles as dental implant coating materials, J. Dent., 2015, 43(12), 1462–1469 CrossRef CAS PubMed.
- V. H. Matsubara, F. Igai, R. Tamaki, P. Tortamano Neto, A. E. Nakamae and M. Mori, Use of Silver Nanoparticles Reduces Internal Contamination of External Hexagon Implants by Candida albicans, Braz. Dent. J., 2015, 26(5), 458–462 CrossRef PubMed.
- K. Reinhardt and H. Winkler, Cerium mischmetal, cerium alloys, and cerium compounds, in: Ullmann's encyclopedia of industrial chemistry, Wiley-VCH, Weinheim, Germany, 2002, vol. 7, pp. 285–300 Search PubMed.
- J. B. Hedrick, Rare earths. Minerals yearbook. Vol. I. Metals and minerals. U.S. Geological Survey, U.S. Department of the Interior, Reston, VA, 2004 Search PubMed.
- C. Walkey, S. Das, S. Seal, J. Erlichman, K. Heckman, L. Ghibelli, E. Traversa, J. F. McGinnis and W. T. Self, Catalytic Properties and Biomedical Applications of Cerium Oxide Nanoparticles, Environ. Sci.: Nano, 2015, 2(1), 33–53 RSC.
- N. Pourkhalili, A. Hosseini, A. Nili-Ahmadabadi, S. Hassani, M. Pakzad, M. Baeeri, A. Mohammadirad and M. Abdollahi, Biochemical and cellular evidence of the benefit of a combination of cerium oxide nanoparticles and selenium to diabetic rats, World J. Diabetes, 2011, 152(11), 204–210 CrossRef PubMed.
- J. Niu, A. Azfer, L. M. Rogers, X. Wang and P. E. Kolattukudy, Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy, Cardiovasc. Res., 2007, 73(3), 549–559 CrossRef CAS PubMed.
- I. Celardo, M. De Nicola, C. Mandoli, J. Z. Pedersen, E. Traversa and L. Ghibelli, Ce3+ ions determine redox-dependent anti-apoptotic effect of cerium oxide nanoparticles, ACS Nano, 2011, 285(6), 4537–4549 CrossRef PubMed.
- H. G. Ibrahim, N. Attia, F. E. Z. A. Hashem and M. A. R. El Heneidy, Cerium oxide nanoparticles: In pursuit of liver protection against doxorubicin-induced injury in rats, Biomed. Pharmacother., 2018, 103, 773–781 CrossRef CAS PubMed.
- K. Chaudhury, K. N. Babu, A. K. Singh, S. Das, A. Kumar and S. Seal, Mitigation of endometriosis using regenerative cerium oxide nanoparticles, Nanomedicine, 2013, 9(3), 439–448 CrossRef CAS PubMed.
- S. Das, J. M. Dowding, K. E. Klump, J. F. McGinnis, W. Self and S. Seal, Cerium oxide nanoparticles: applications and prospects in nanomedicine, Nanomedicine, 2013, 8(9), 1483–1508 CrossRef CAS PubMed.
- A. Y. Estevez, S. Pritchard, K. Harper, J. W. Aston, A. Lynch, J. J. Lucky, J. S. Ludington, P. Chatani, W. P. Mosenthal, J. C. Leiter, S. Andreescu and J. S. Erlichman, Neuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemia, Free Radical Biol. Med., 2011, 51(6), 1155–1163 CrossRef CAS PubMed.
- L. L. Wong and J. F. McGinnis, Nanoceria as bona fide catalytic antioxidants in medicine: what we know and what we want to know, Adv. Exp. Med. Biol., 2014, 801, 821–828 CrossRef PubMed.
- M. S. Wason and J. Zhao, Cerium oxide nanoparticles: potential applications for cancer and other diseases, Am. J. Transl. Res., 2013, 5(2), 126–131 CAS.
- A. Y. Estevez and J. S. Erlichman, The potential of cerium oxide nanoparticles (nanoceria) for neurodegenerative disease therapy, Nanomedicine, 2014, 9(10), 1437–1440 CrossRef CAS PubMed.
- P. R. McDonagh, G. Sundaresan, L. Yang, M. Sun, R. Mikkelsen and J. Zweit, Biodistribution and PET imaging of 89-zirconium labeled cerium oxide nanoparticles synthesized with several surface coatings, Nanomedicine, 2018, 14(4), 1429–1440 CrossRef CAS PubMed.
- S. Sangomla, M. A. Saifi, A. Khurana and C. Godugu, Nanoceria ameliorates doxorubicin induced cardiotoxicity: Possible mitigation via reduction of oxidative stress and inflammation, J. Trace Elem. Med. Biol., 2018, 47, 53–62 CrossRef CAS PubMed.
- B. A. Rzigalinski, S. Seal and D. Bailey, Patent application: Cerium oxide nanoparticles and use in enhancing cell survivability, US7534453B1, Swanand Patil University of Central Florida Research Foundation Inc (UCFRF), 2009 Search PubMed.
- Biological Evaluation of Medical Devices - Part 1, Evaluation and Testing within a Risk Management Process, ISO 10993-1:2018 (E).
- SCENIHR (Scientific Committee on Emerging and Newly Identified Health Risks), Final Opinion on the Guidance on the Determination of Potential Health Effects of Nanomaterials Used in Medical Devices, January 2015.
- X. Zhang, S. Wang, M. Liu, J. Hui, B. Yang, L. Tao and Y. Wei, Surfactant-dispersed nanodiamond: biocompatibility evaluation and drug delivery applications, Toxicol, Res., 2013, 2, 335–342 CAS.
- X. Zhang, M. Liu, X. Zhang, F. Deng, C. Zhou, J. Hui, W. Liu and Y. Wei, Interaction of tannic acid with carbon nanotubes: enhancement of dispersibility and biocompatibility, Toxicol. Res., 2015, 4, 160–168 RSC.
- J. H. Liu, T. Wang, H. Wang, Y. Gu, Y. Xu, H. Tang, G. Jia and Y. Liu, Biocompatibility of graphene oxide intravenously administrated in mice-effects of dose, size and exposure protocols, Toxicol, Res., 2015, 4, 83–91 Search PubMed.
- V. Kalyanaraman, T. S. Kumaravel and S. S. Murugan, In Vitro Cytotoxicity Studies of Cerium Oxide Nanoparticles, Indian J. Nanosci., 2016, 4(1), 1–5 Search PubMed.
- Biological Evaluation of Medical Devices - Part 2, Animal Welfare Requirements, ISO 10993-2:2006(E).
- Biological Evaluation of Medical Devices - Part 11, Tests for Systemic Toxicity, ISO 10993-11:2017(E).
- Biological Evaluation of Medical Devices - Part 6, Tests for Local Effects after Implantation, ISO 10993-6:2016(E).
- Biological Evaluation of Medical Devices - Part 3: Tests for genotoxicity, carcinogenicity and reproductive toxicity, ISO 10993-3:2014(E).
- Biological Evaluation of Medical Devices - Part 33: Guidance on tests to evaluate genotoxicity - Supplement to ISO 10993-3, ISO 10993-33:2015(E).
- S. M. Hirst, A. S. Karakoti, R. D. Tyler, N. Sriranganathan, S. Seal and C. M. Reilly, Anti-inflammatory properties of cerium oxide nanoparticles, Small, 2009, 5, 2848–2856 CrossRef CAS PubMed.
- S. M. Hirst, A. Karakoti, S. Singh, W. Self, R. Tyler, S. Seal and C. M. Reilly, Bio-distribution and in vivo antioxidant effects of cerium oxide nanoparticles in mice, Environ, Toxicol., 2013, 28, 107–118 CAS.
- R. Simón-Vázquez, T. Lozano-Fernández, A. Dávila-Grana and A. González-Fernández, Metal oxide nanoparticles interact with immune cells and activate different cellular responses, Int. J. Nanomed., 2016, 11, 4657–4668 CrossRef PubMed.
- A. B. Engin, D. Nikitovic, M. Neagu, P. Henrich-Noack, A. O. Docea, M. I. Shtilman, K. Golokhvast and A. M. Tsatsakis, Mechanistic understanding of nanoparticles’ interactions with extracellular matrix: the cell and immune system, Part. Fibre Toxicol., 2017, 14, 22 CrossRef PubMed.
- B. C. Nelson, M. E. Johnson, M. L. Walker, K. R. Riley and C. M. Sims, Antioxidant Cerium Oxide Nanoparticles in Biology and Medicine, Antioxidants, 2016, 5(2), 15 CrossRef PubMed.
- R. Davan, R. G. S. V. Prasad, V. S. Jakka, R. S. L. Aparna, A. R. Phani, B. Jacob, P. C. Salins and D. B. Raju, Cerium oxide nanoparticles promotes wound healing activity in in vivo animal model, J. Bionanosci., 2012, 6, 78–83 CrossRef CAS.
- J. D. Bryers, C. M. Giachelli and B. D. Ratner, Engineering biomaterials to integrate and heal: The biocompatibility paradigm shifts, Biotechnol. Bioeng., 2012, 109, 1898–1911 CrossRef CAS PubMed.
- M. R. Major, V. W. Wong, E. R. Nelson, M. T. Longaker and G. C. Gurtner, The foreign body response: at the interface of surgery and bioengineering, Plast. Reconstr. Surg., 2015, 135(5), 1489–1498 CrossRef CAS PubMed.
- K. Hwang, H. B. Sim, F. Huan and D. J. Kim, Myofibroblasts and capsular tissue tension in breast capsular contracture, Aesthetic Plast. Surg., 2010, 34, 716–721 CrossRef PubMed.
- R. C. Rennert, K. Rustad, K. Levi, M. Harwood, M. Sorkin, V. W. Wong, A. Al-Ahmad, P. Zei, H. Hsia, R. E. Beygui, L. Norton, P. Wang and G. C. Gurtner, A histological and mechanical analysis of the cardiac lead-tissue interface: Implications for lead extraction, Acta Biomater., 2014, 10, 2200–2208 CrossRef PubMed.
- P. X. Ma, Biomimetic materials for tissue engineering, Adv. Drug Delivery Rev., 2008, 60, 184–198 CrossRef CAS PubMed.
- J. M. Anderson, A. Rodriguez and J. M. Chang, Foreign body reaction to biomaterials, Semin. Immunol., 2008, 20, 86–100 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to be the first author. |
| This journal is © The Royal Society of Chemistry 2019 |