Study on systemic and reproductive toxicity of acetochlor in male mice†
Xianping
Song‡
 ab,
Feng
Zhang‡
a,
Dongya
Chen
c,
Qian
Bian
c,
Hengdong
Zhang
a,
Xin
Liu
*a and
Baoli
Zhu
*a
ab,
Feng
Zhang‡
a,
Dongya
Chen
c,
Qian
Bian
c,
Hengdong
Zhang
a,
Xin
Liu
*a and
Baoli
Zhu
*a
aInstitute of Occupational Disease Prevention, Jiangsu Provincial Center for Disease Control and Prevention, Nanjing, Jiangsu 210009, China. E-mail: liux@jscdc.cn; zhubl@jscdc.cn
bKunshan Municipal Center for Disease Prevention and Control, Suzhou, Jiangsu 215300, China
cDepartment of Toxicology and Function Assessment, Jiangsu Provincial Center for Disease Control and Prevention, Nanjing, Jiangsu 210009, China
First published on 13th November 2018
Abstract
Acetochlor is one of the three most abundant herbicides used in China, which is a pre-emergence herbicide belonging to chloroacetanilides. It is classified as “likely to be carcinogenic to humans” and suspected as an endocrine disruptor by the US Environmental Protection Agency (USEPA) and the European Union Environmental Protection Agency (EUEPA). Therefore, there is increasing concern about the risk of acetochlor to human health and the environment. However, the current research on acetochlor mainly focuses on the assessment of environmental behaviors, whereas toxicity studies mainly concentrate on non-mammals such as zebrafish. So far, only a few reports of its effects on the male reproductive system of animals have been found. In the present study, C57BL/6 male mice and GC-1 spermatogonia(spgs) were used to analyze in vivo and in vitro toxicities of acetochlor to investigate the relationships among acetochlor-induced oxidative stress, endocrine dysfunction, degenerative diseases of germ cells and apoptosis. The results show that acetochlor can induce systemic and reproductive toxicity in subacutely exposed mice, such as clear reduction in body weight, liver function damage in mice, oxidative stress damage and histopathological degeneration in testicular tissue as the concentration of acetochlor increases. In addition, acetochlor might mediate apoptotic pathways that affect the survival of GC-1 spgs; in detail, cell viability decreases, cell cytotoxicity and apoptosis are elevated, and oxidative stress and expression changes of apoptosis-related proteins are observed compared with that for control in GC-1 spgs. In addition, this study also provides a scientific basis for evaluating the toxicological effects of acetochlor as well as its potential damage to mammals.
1. Introduction
Acetochlor (2-chloro-N-(ethoxymethyl)-N-(2-ethyl-6-methylphenyl)acetamide) is a pre-emergence herbicide that belongs to the class of chloroacetyl acetanilides. It has been extensively used all over the world and has become one of the three most frequently used herbicides in China. In addition, it is also the third most commonly applied herbicide in the agricultural market estimated by the USEPA.1,2 Due to the wide spread use of acetochlor, there exists growing concern about the risk of acetochlor to human health and environment. Moreover, it has been reported that zebrafish and other low-grade vertebrates exposed to acetochlor may trigger DNA damage and neurological abnormalities. However, the long-term toxicity of acetochlor on mammals includes mainly liver and kidney damage; thus, it is not considered as a mutagen and is unlikely to exert genotoxicity and neurotoxicity at related doses on mammals.3,4 Additionally, long-term exposure to acetochlor above the maximum tolerated dose may produce nasal epithelium, thyroid and lung tumors in certain species.5,6 Therefore, the USEPA has classified acetochlor as “possibly carcinogenic to humans” based on the increasing incidence of certain tumors in experimental animals.7Acetochlor and its degradation products have been shown to be potential endocrine disruptors (EDCs) that interact with uterine estrogen receptors ultimately influencing the female reproductive system.8 Meanwhile, USEPA and EUEPA have announced that acetochlor is a suspected EDC among a wide range of vertebrates.9 Pollution of EDCs has become a global hotspot because they are harmful to biological systems and environment. EDCs can induce endocrine disruption, oxidative stress and apoptosis simultaneously.10 It is evident that oxidative stress-induced free radicals can serve as signaling molecules that trigger apoptotic cell death and inflammatory responses having an adverse effect on steroid synthesis.11 Therefore, this study aimed to clarify the effect of subacute exposure to acetochlor on the male reproductive system as well as the possible interactions between oxidative stress, apoptosis and the endocrine system.
C57BL/6 mice are the most popular inbred mouse strains, which are frequently used to research the male reproductive system due to their different fertilities but close genetic distance.12 GC-1 spgs, derived from immortalized mouse spermatogonia cells, are also commonly used for male reproductive toxicity testing. In the proposed study, the toxic effects of acetochlor on male reproduction and the possible molecular mechanisms will be explored based on the use of C57BL/6 mice and GC-1 spgs in vivo and in vitro.
2. Materials and methods
2.1 Characteristics of acetochlor
Acetochlor (97% purity) was purchased from Sigma-Aldrich (St Louis, MO, USA). The stock solution of acetochlor was prepared in absolute ethanol and subsequent dilutions were made in cell culture medium (deprived of serum) for treatment. In animal experiments, acetochlor was first dissolved in corn oil and then diluted to the desired concentrations.2.2 Animals and treatment
In this study, a total of 65 C57BL/6 male mice weighing about 20–25 g were supplied by Vital River Laboratory Animal Technology Co. Ltd (Beijing, China). The certificate number is SYXK(Su)2017-0031. They were kept individually in metabolic cages at temperature of 22 ± 2 °C with 50–60% humidity and 12 h light–dark cycles. They were fed with rodent diets and given drinking water ad libitum for at least 1 week to familiarize the environment. After acclimatization, 40 mice were randomly assigned into four groups (low dose group [250 mg kg−1 day−1], middle dose group [500 mg kg−1 day−1], high dose group [1000 mg per kg bw per day] and the control group [corn oil]). The remaining 25 mice were randomly assigned into five groups for sperm malformation experiments. In addition to the above four concentration groups, cyclophosphamide (40 mg per kg bw per d) was taken as a positive control. Acetochlor was given to the mice consecutively for 30 days by intragastric adminstration (ig). The body weight of the mice was recorded weekly. At the end of 30 days, mice were weighed and starved for 12 h, followed by intraperitoneal injection of sodium pentobarbital (40 mg per kg body weight) for anaesthesia, and subsequently killed by bloodletting. Blood samples were taken from the abdominal aorta and immediately separated by centrifugation at 3000 rpm (835g) for 15 min. The serum samples were analyzed with an Autolab-PM4000 automated biochemical analyzer (AMS Co., Rome, Italy) to test for ALT, AST, BUN, CRE, TP, ALB, GHO, GLU and TG. The internal organs (heart, liver, spleen, lung, kidney, testis and epididymis) of each mouse were excised for organ/body ratios, and only the testis was dissected for histological studies immediately after blood collection. Testis samples were fixed in 10% formalin, processed into 4 mm paraffin sections, and stained with hematoxylin and eosin for histopathological assessment. In addition, the sperm deformity experiment was performed on the 35th day after acetochlor exposure. All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Jiangsu Provincial Center for Disease Control and Prevention and approved by the Animal Ethics Committee of Jiangsu Provincial Center for Disease Control and Prevention.2.3 Cell culture and acetochlor treatment
GC-1 spgs (ATCC®CRL-2053) were kindly gifted by Dr Yi Zhu at Nanjing Medical University. Cells were cultured at 37 °C in 5% CO2 in Dulbecco's Modified Eagle Medium (DMEM) (Gibco, USA) containing 10% fetal bovine serum (FBS) (Gibco, USA), 100 U ml−1 penicillin and 100 mg ml−1 streptomycin (Gibco, USA). The medium was replaced every day. Before treatment to cells, acetochlor were diluted to various concentrations (10−6 M (Mol L−1), 10−5 M, 10−4 M, 2 × 10−4 M, 4 × 10−4 M, 8 × 10−4 M, 10−3 M, 10−2 M). Besides, the exposure time was 12 h.2.4 Determination of oxidative stress status on testis tissue and GC-1 spgs
The oxidative stress enzyme contents of mice testis tissue and GC-1 spgs were detected by different detection kits (Lipid Peroxidation MDA Assay Kit, Total SOD Assay Kit, Total GSH Assay Kit, Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Half of the testis excised from each mouse was homogenized with nine times of ice normal saline in the ratio of weight (g)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) volume (ml) = 1
volume (ml) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, followed by centrifuging at 2500 rpm for 10 minutes. GC-1 cells were harvested and washed twice with PBS. Then, the supernatant was taken to analyze the concentration of total SOD activity, MDA and total GSH. Total SOD activity, MDA and total GSH levels in cell lysates and testicular tissue homogenate were normalized to the total protein.
9, followed by centrifuging at 2500 rpm for 10 minutes. GC-1 cells were harvested and washed twice with PBS. Then, the supernatant was taken to analyze the concentration of total SOD activity, MDA and total GSH. Total SOD activity, MDA and total GSH levels in cell lysates and testicular tissue homogenate were normalized to the total protein.
2.5 Histopathological examination of the testes
A portion of the testes excised from mice in each group was fixed in Bouin's fluid for 48 h and routinely processed for paraffin embedding. Sections of 4 μm thickness were taken serially with a Rotary Microtome and then processed in alcohol-xylene series and stained with hematoxylin and eosin (H&E). Moreover, the prepared slides were examined at ×400 magnification.2.6 Hormone assay
The sera obtained were analyzed to determine the concentration of testosterone using the Enzyme-Linked immunosorbent Assay (ELISA). The ELISA kits were purchased from Biocheck, USA.2.7 Sperm malformation test
Sperm morphology assay was conducted according to Bakare et al.13 Epididymal sperm cells were suspended in normal saline and stained via 1% Eosin Y. The smear was prepared and the slides were air-dried and coded for subsequent microscopic examination at ×1000 magnification. Every 1000 sperm cells were assessed for morphological abnormalities in each mouse.2.8 Cell viability and cytotoxicity assay
Cell viability was analysed using a Cell Counting Kit-8 (CCK-8) (Beyotime Institute of Biotechnology, Nantong, China). Cells were raised at a density of 2.0 × 104 per well in a 96-well plate (Thermo Scientific, USA) and incubated overnight. After exposure to acetochlor at various concentrations for 12 h, 10 μl CCK-8 solution was added to each well and then, the cells were incubated for 1–4 h at 37 °C in 5% CO2. The absorbance was determined at 450 nm. IC50 of acetochlor on GC-1 spgs was calculated by GraphPad Prism 6 (GraphPad Software, USA).Lactate dehydrogenase (LDH) assay was employed to determine the cytotoxicity effect of acetochlor on GC-1 spgs by LDH release kit (Beyotime Institute of Biotechnology, Nantong, China). GC-1 spgs were seeded in 96-well tissue culture plates at the density of 2.0 × 104 cells per well and cultured overnight. After the treatment, the plates were centrifuged at 1000 rpm for 5 min. Also, 60 μl cell-free supernatant was incubated with 30 μl LDH substrate solution for 30 min and subsequently, the absorbance at 490 nm was recorded on a microplate spectrophotometer. The LDH release activity was presented as fold increase over the control group.
2.9 Cell apoptosis assay
Cell apoptosis was measured by flow cytometry using an Annexin V apoptosis detection kit (BD Biosciences Pharmingen, USA). After acetochlor exposure, cells were trypsinised, washed with PBS and centrifuged at 1000 rpm for 5 min. After three times washing, 1 × 105 cells were resuspended in 100 μl of 1× binding buffer and incubated with 5 μl fluorescein isothiocyanate labeled with Annexin V (FITC-Annexin V) and 5 μl propidium iodide (PI) at room temperature avoiding light for 15 min. Afterwards, 400 μl of 1× binding buffer was added and the stained cells were collected for flow cytometry analyses.2.10 Western blot analysis
The total protein of acetochlor-treated GC-1 spgs was extracted with a protein extraction kit (Beyotime Institute of Biotechnology, Nantong, China) supplemented with a protease and phosphatase inhibitor (Roche, Mannhem, Germany) and measured by a BCA assay kit (Beyotime, China). Equal quantities (40 μg) of the protein were subjected to SDS-PAGE (10–12% gel) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore Corp., Billerica, MA, USA). After blocking in 5% skim milk, the membranes were incubated overnight at 4 °C with specific antibodies. All primary antibodies were diluted as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 in the blocking solution and washed with TBST (TBS + 20% Tween). Subsequently, the membranes were incubated with the corresponding secondary antibodies for 1 h at room temperature and developed using a chemiluminescent reagent (Beyotime, China); then, the results were recorded on a gelimaging system (Bio-rad, USA). In addition, relative intensities of specific protein bands were quantified using the ImageJ software (NIH, USA).
1000 in the blocking solution and washed with TBST (TBS + 20% Tween). Subsequently, the membranes were incubated with the corresponding secondary antibodies for 1 h at room temperature and developed using a chemiluminescent reagent (Beyotime, China); then, the results were recorded on a gelimaging system (Bio-rad, USA). In addition, relative intensities of specific protein bands were quantified using the ImageJ software (NIH, USA).
2.11 Statistical analysis
Data obtained were presented as![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif) ± s. Statistical analyses for all measurements were performed using Statistical Package for Social Sciences (SPSS) version 20.0. Besides, the mean, standard error of the mean, analysis of Variance (ANOVA) and Rank sum test were conducted. Post Hoc test was conducted using Student–Newman–Keuls (SNK). P < 0.05 was considered to be statistically significant.
± s. Statistical analyses for all measurements were performed using Statistical Package for Social Sciences (SPSS) version 20.0. Besides, the mean, standard error of the mean, analysis of Variance (ANOVA) and Rank sum test were conducted. Post Hoc test was conducted using Student–Newman–Keuls (SNK). P < 0.05 was considered to be statistically significant.
3. Results
3.1 The systemic toxicity of acetochlor in C57BL/6 male mice
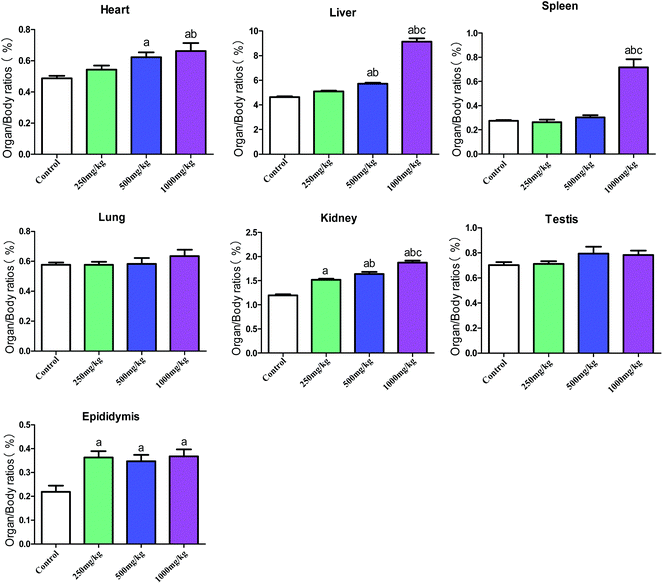
In general, the organ/body ratios showed an increasing trend with the increase in acetochlor concentration. Apart from the organ/body ratios of lung and testis, other organs including heart, liver, spleen, kidney and epididymis presented statistical differences between four dose groups (P < 0.05) (Fig. 2, Table S2†).
3.2 The reproductive toxicity of acetochlor in C57BL/6 male mice
| Groups | n | Rank mean (nmol L−1) |
|---|---|---|
| a: P < 0.05 compared with control. | ||
| Control | 10 | 13.21 |
| 250 mg kg−1 | 10 | 26.33a |
| 500 mg kg−1 | 10 | 29.82a |
| 1000 mg kg−1 | 10 | 28.73a |
| P | 0.013 | |
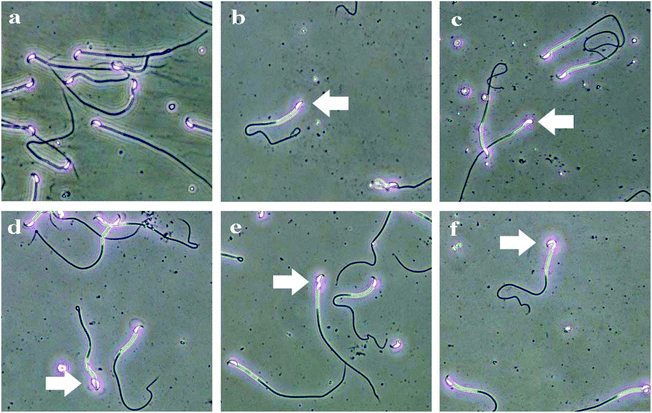 | ||
| Fig. 6 The types of sperm deformities in C57BL/6 male mice induced by Acetochlor. a: Sperm morphology of control; b: lack hook; c: big head; d: two head; e: amorphous; f: banana-like. | ||
| Groups | n | Numbers | Lack hook | Big head | Banana like | Others | Sum | Aberration ratio (%) |
|---|---|---|---|---|---|---|---|---|
| a: P < 0.05 compared with control. | ||||||||
| Control | 5 | 5000 | 22 | 14 | 10 | 38 | 84 | 1.67 ± 0.36 |
| 250 mg kg−1 | 5 | 5000 | 46 | 28 | 15 | 89 | 178 | 3.56 ± 0.43a |
| 500 mg kg−1 | 5 | 5000 | 103 | 59 | 35 | 94 | 291 | 5.81 ± 0.51a |
| 1000 mg kg−1 | 5 | 5000 | 99 | 83 | 56 | 136 | 374 | 7.47 ± 0.64abc |
| Positive control | 5 | 5000 | 83 | 94 | 54 | 160 | 391 | 7.82 ± 0.69a |
| P | 0.001 | |||||||
3.3 Effects of acetochlor on GC-1 spgs
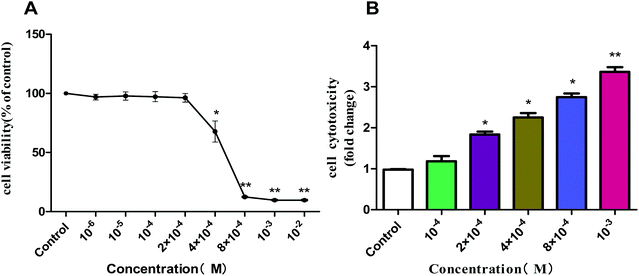 | ||
| Fig. 8 Effect of acetochlor on cell viability (A) and cytotoxicity (B) of GC-1 spg. *P < 0.05, **P < 0.01. | ||
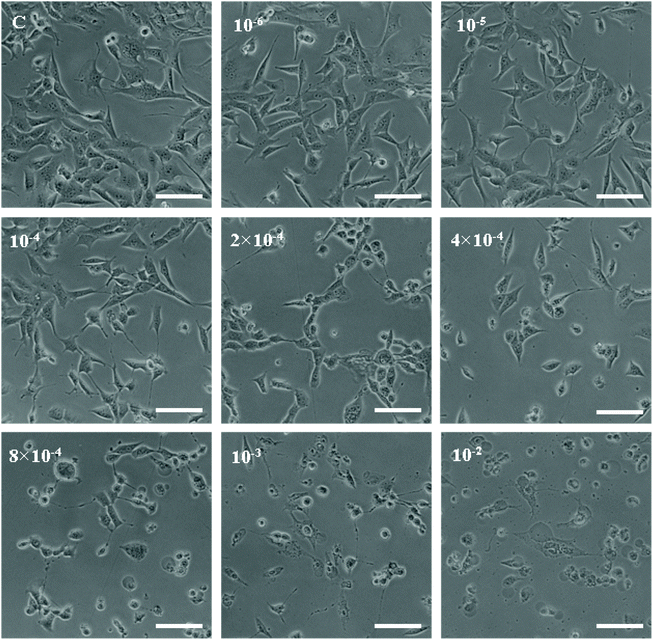
We selected several characteristic exposure concentrations (10−4 M, 2 × 10−4 M, 4 × 10−4 M, 8 × 10−4 M, and 10−3 M) for subsequent experiments according to the results of cell viability. LDH release assay was employed to determine the cytotoxic effect of acetochlor on GC-1 spgs. The cytotoxicity of 2 × 10−4 M, 4 × 10−4 M, 8 × 10−4 M and 10−3 M groups was evidently elevated when compared with that of control (P < 0.05). Besides, the cytotoxicity of 10−3M was approximately 4-fold as much as that of the control, as shown in Fig. 8B.
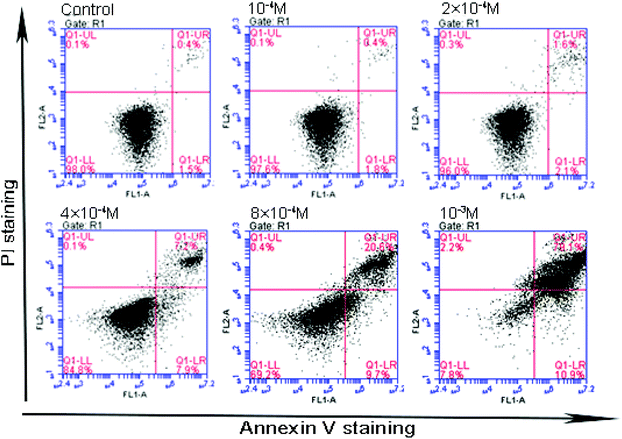
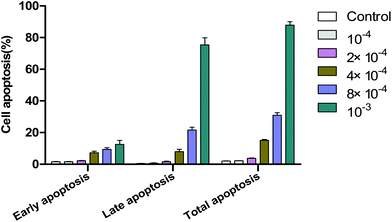
Flow cytometery was utilized to detect cell apoptosis, and the results indicated that acetochor triggered specific cell apoptosis in GC-1 spgs (Fig. 9). The ratio of apoptotic cells clearly increased along with the concentration of acetochor (Fig. 10).
4. Discussion
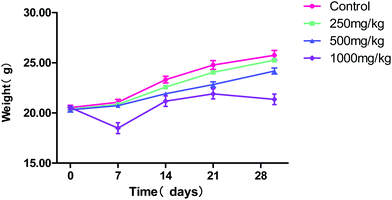
In the proposed study, we employed C57BL/6 mice and GC-1 spgs to explore systemic and reproductive toxicity induced by acetochlor as well as its potential molecular mechanism. The results demonstrated that acetochlor exerts systemic and reproductive toxic effects on mice. Simultaneously, acetochlor caused cytotoxicity and apoptosis in GC-1 spgs. MAPK-ERK and p53-dependent signal pathways could be involved in acetochlor-mediated GC-1 spg apoptosis.Significant changes were observed in the body weights of mice after acetochlor treatment for 30 days, suggesting that the toxicity induced by acetochlor was sufficient to cause observable body weight changes in the mice. The organ/body ratio is a macroscopic index that reflects the development and functional status of organs.14 After acetochlor enters the body, the damage of target organs reaches a certain level, ultimately resulting in alterations in the organ/body ratio. In the current study, except that of the lung and testis, the organ/body ratio of other organs exhibited various degrees of increment, indicating that subacute acetochlor exposure can lead to specific injures of heart, liver, spleen, kidney, and epididymis, in particular formation of edema. During a food-exposed experiment with 42.96 and 107.4 mg per kg body weight per day acetochlor in wistar rats for 24 weeks, there were no definite changes referring to body weight and organ/body ratio.14 In addition, our conclusion also illustrated that 250 mg kg−1, 500 mg kg−1, and 1000 mg kg−1 acetochlor could affect the growth and development of mice.
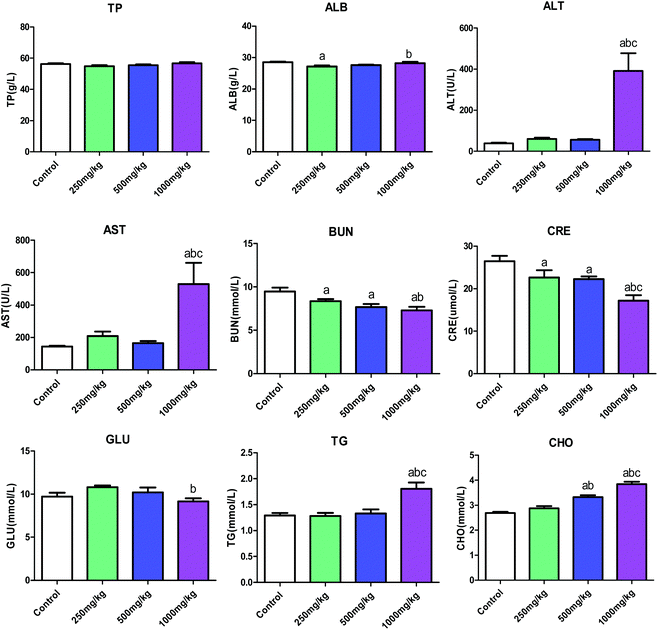
The increase in the levels of ALT and AST is one of the important indicators of liver damage.15 In this study, AST, ALT and the levels of TG and CHO were significantly elevated in the high-dose group compared with that of control. Combined with the increased liver organ/body ratio, this suggested that subacute acetochlor treatment can cause serious liver injury in mice. Consistent with the results reported by Kale et al. on toxicity studies of acetochlor-exposed rat and human liver cells, serious damage to hepatocytes was evident, and liver may be the main target organ.16 On the contrary, there were clearly declined amounts of CRE and BUN in every acetochlor-exposed group compared to that in control when the kidney organ/body ratio was increased, indicating that there existed no functional changes in kidney under acetochlor treatment. In the 24 weeks of acetochlor-exposed test in rats,14 ALT, AST, BUN and CRE levels all increased in a dose-dependent manner. In the meantime, liver and kidney functions exhibited varying extents of damage; liver and kidney may be the most susceptible and more sensitive organs to the toxic effect of acetochlor.
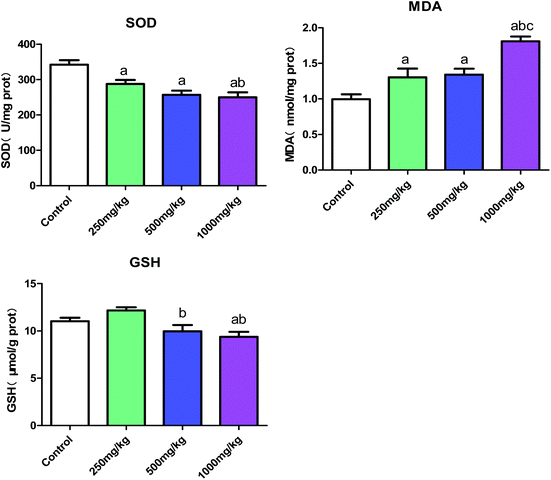
Testis is the most important organ in the male reproductive system; its main role is to secrete and synthesize male hormones and produce sperms.17 The study of Zhang et al.18 has demonstrated that exposure to acetochlor contributes to reproductive toxicity, causing the testis cells to generate excessive reactive oxygen species (ROS), which can lead to intense oxidative stress. In the proposed study, decreased levels of SOD and GSH and increased MDA content were observed in testicular tissues, confirming that acetochlor can result in oxidative stress damage in testes, which might play an important role in the dysfunction of the reproductive system.
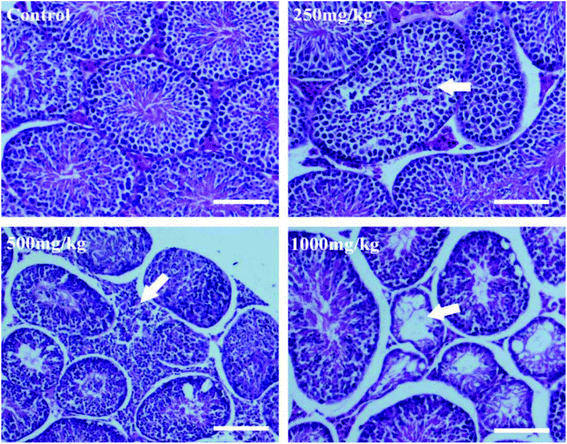
When oxidative stress reaches a certain level, the structure of testis undergoes pathological changes.19 Compared with the observations for control, the histological structure of testis degenerated with increasing acetochlor concentration. The pathological manifestation of testicular tissue is the most direct evidence of acetochlor's toxicity to the reproductive system in mice. Thakur et al. reported that the degeneration of seminiferous tubules caused by chemical substances is an important sign of interference with spermatogenesis.20 Consequently, acetochlor might directly disturb the process of spermatogenesis. In addition, the impaired testicular tissue is also a prerequisite for sperm abnormalities. The results were consistent with the structure disorder and interstitial edema in the testicular tissues of Kunming mice after 90-day treatment of acetochlor.21
The pathological changes in testis can cause a series of problems such as imbalance of sex hormones and elevation of the sperm malformation rate. In our experiment, the levels of testosterone in the serum of each exposed group displayed an upward trend in comparison with that of control (P < 0.05), suggesting that acetochlor subacute exposure can influence the testosterone regulation mechanism in mice. These results were in contrast to those of rats exposed to glyphosate for 12 weeks; however, they were in agreement with the testosterone levels of 90 day acetochlor-exposed mice.22,23 The content of testosterone is mainly dependent on the regulation of the hypothalamus-pituitary-testis (HPT) axis. Increase in testosterone may be due to the abnormal release of hypothalamic gonadotropin-releasing hormone (GnRH). In addition, it can also be explained that the HPT axis negatively stimulates testosterone secretion to promote cell repair after acetochlor-induced pathological injury of testes.
In accordance with results of acetochlor-exposed rats and occupational workers,24,25 the total sperm aberration ratio in each acetochlor-treated group dramatically increased compared with that of control in the proposed study. Spermogenesis is an extremely complicated process regulated by many factors such as hormones and spermatogenic cells. Acetochlor triggered intracellular oxidative stress and ROS excess and thus, spermatogenic and stromal cells were damaged, resulting in dysfunction of blood-testis barrier as well as derangement of spermatozoa. Eventually, the sperm abnormality rate went up.26 Furthermore, the testosterone level also served as a key element in affecting sperm production.
GC-1 spgs were employed to verify the toxic effects of acetochlor on the male reproductive system in vitro. Consistent with the toxic influences on mouse testis, clear cytotoxic effects were exhibited by acetochlor on GC-1 spgs: cell viability decreased, cytotoxicity and apoptosis increased, and generation of overt oxidative stress was observed. Studies have indicated that acetochlor can also decrease the viability of A549, HepG2 and MCF-7 cell lines.27–29 However, the specific mechanism still remains unclear.
Apoptosis is induced by cell apoptotic enzyme caspase activated by extracellular signal or apoptotic enzyme-activating factor released via intracellular mitochondria.30 Detection of the protein expression of related pathway in acetochlor-treated GC-1 spgs demonstrated that p53, pro-apoptosis protein Bax, caspase 3 and caspase 9 were up-regulated. However, the expressions of anti-apoptotic protein Bcl-2 and extracellular signal-regulated kinase p44/42 (ERK1/2) were reduced. ERK1/2 is a critical pathway for signal transduction from surface receptors to the nucleus. Activated ERK1/2 is transferred from cytoplasm to nucleus and then mediates the phosphorylation of cytoplasmic proteins and some nuclear transcription factors such as ATF2, Elk-1 and c-myc, thereby participating in the regulation of cell proliferation and differentiation.31
It was speculated that ERK1/2 activated by acetochlor-induced oxidative stress can lead to decreased expression of Bcl-2 and increased expression of Bax, probably forming a mitochondrial exchange channel of Ca2+ influx and cytochrome C outflow; this results in direct activation of downstream caspase cascade reaction, as reflected by elevated expressions of caspase 3 and caspase 9. Moreover, the results were consistent with Zerin et al.'s findings on the apoptotic mechanisms of acetochlor on alveolar epithelial A549 cells: excessive ROS activated the ERK pathway and promoted the expression of the pro-apoptotic protein Bax, which consequently triggered caspase cascade-induced A549 cell apoptosis.27 Moreover, the increased tendency of p53 indicated DNA damage and repair in GC-1spgs. In brief, acetochlor could regulate the ERK-p53-Bcl-2 cascade response through oxidative stress and mediate apoptotic pathways influencing the survival of GC-1spgs.
5. Conclusions
To conclude, our study demonstrated that acetochlor can induce systemic and reproductive toxicity when subacutely exposed to mice. Acetochlor has remarkable cytotoxic effects on GC-1 spgs. Acetochlor might mediate apoptotic pathways affecting GC-1 spg survival via ERK-p53-Bcl-2 cascade. Moreover, this conclusion also reveals a public health concern considering increased use of acetochlor and the presence of its residues in food and drinking water. In addition, toxic effects of acetochlor on human beings cannot be ignored anymore.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the grants from Jiangsu Province's Outstanding Medical Academic Leader program (CXTDA2017029), Jiangsu Provincial Youth Medical Talent (QNRC2016549) and Jiangsu Provincial Youth Medical Talent program (QNRC2016528).References
- P. Zhao, Global pesticide market overview and development trend in 2015, Pesticide, 2017, 02, 79–85 Search PubMed.
- J. Wang, Y. Li, X. Wang and Y. Wang, The current situation and prospects of pesticide use in China, Outlook Agric., 2017, 02, 56–60 Search PubMed.
- C. Xu, W. Tu, M. Deng, Y. Jin, B. Lu, C. Zhang, C. Lin, Y. Wu and W. Liu, Stereoselective induction of developmental toxicity and immunotoxicity by acetochlor in the early life stage of zebrafish, Chemosphere, 2016, 164, 618–626 CrossRef CAS PubMed.
- X. P. Song, X. Liu and B. L. Zhu, The review of study on toxicity and carcinogenicity of acetochlor, Zhonghua Laodong Weisheng Zhiyebing Zazhi, 2017, 35, 69–71 CAS.
- G. Dirheimer, Commentary on the publication: Evaluation of the potential carcinogenicity and genetic toxicity to humans of the herbicide acetochlor, Hum. Exp. Toxicol., 1997, 16, 188–201 CrossRef CAS PubMed.
- J. Ashby, L. Kier, A. G. Wilson, T. Green, P. A. Lefevre, H. Tinwell, G. A. Willis, W. F. Heydens and M. J. Clapp, Evaluation of the potential carcinogenicity and genetic toxicity to humans of the herbicide acetochlor, Hum. Exp. Toxicol., 1996, 15, 702–735 CrossRef CAS PubMed.
- U.S. Environmental Protection Agency (U.S. EPA), Report of the Food Quality Protection Act (FQPA) Tolerance Reassessment Progress and Risk Management Decision (TRED) for Acetochlor[Z]. March 2006. EPA 738-R-00-009, http://www.epa.gov/oppsrrd1/REDs/acetochlor_tred.pdf.
- E. Rollerova, L. Wsolova and M. Urbancikova, Neonatal exposure to herbicide acetochlor alters pubertal development in female wistar rats, Toxicol. Mech. Methods, 2011, 21, 406–417 CrossRef CAS PubMed.
- T. Frische, J. Bachmann, D. Frein, T. Juffernholz, A. Kehrer, A. Klein, G. Maack, F. Stock, H. C. Stolzenberg, C. Thierbach and S. Walter-Rohde, Identification, assessment and management of “endocrine disruptors” in wildlife in the EU substance legislation–discussion paper from the German Federal Environment Agency (UBA), Toxicol. Lett., 2013, 223, 306–309 CrossRef CAS PubMed.
- M. Mazioti, The potential role of endocrine disrupting chemicals in cellulite, Med. Hypotheses, 2018, 116, 132–135 CrossRef CAS PubMed.
- I. Sidorkiewicz, K. Zareba, S. Wolczynski and J. Czerniecki, Endocrine-disrupting chemicals-Mechanisms of action on male reproductive system, Toxicol. Ind. Health, 2017, 33, 601–609 CrossRef CAS PubMed.
- Y. Fan, Y. Liu, K. Xue, G. Gu, W. Fan, Y. Xu and Z. Ding, Diet-induced obesity in male C57BL/6 mice decreases fertility as a consequence of disrupted blood-testis barrier, PLoS One, 2015, 10, e0120775 CrossRef PubMed.
- A. A. Bakare, A. A. Mosuro and O. Osibanjo, An in vivo evaluation of induction of abnormal sperm morphology in mice by landfill leachates, Mutat. Res., 2005, 582, 28–34 CAS.
- L. Li, M. Wang, S. Chen, W. Zhao, Y. Zhao, X. Wang and Y. Zhang, A urinary metabonomics analysis of long-term effect of acetochlor exposure on rats by ultra-performance liquid chromatography/mass spectrometry, Pestic. Biochem. Physiol., 2016, 128, 82–88 CrossRef CAS PubMed.
- N. Morales-Prieto, J. Ruiz-Laguna, D. Sheehan and N. Abril, Transcriptome signatures of p,p -DDE-induced liver damage in Mus spretus mice, Environ. Pollut., 2018, 238, 150–167 CrossRef CAS PubMed.
- V. M. Kale, S. R. Miranda, M. S. Wilbanks and S. A. Meyer, Comparative cytotoxicity of alachlor, acetochlor, and metolachlor herbicides in isolated rat and cryopreserved human hepatocytes, J. Biochem. Mol. Toxicol., 2008, 22, 41–50 CrossRef CAS PubMed.
- A. L. Forgacs, M. L. D'Souza, I. T. Huhtaniemi, N. A. Rahman and T. R. Zacharewski, Triazine herbicides and their chlorometabolites alter steroidogenesis in BLTK1 murine Leydig cells, Toxicol. Sci., 2013, 134, 155–167 CrossRef CAS PubMed.
- J. Zhang, W. Liang, X. Wu, S. Jiang and Q. Li, Toxic effects of acetochlor on mortality, reproduction and growth of Caenorhabditis elegans and Pristionchus pacificus, Bull. Environ. Contam. Toxicol., 2013, 90, 364–368 CrossRef CAS PubMed.
- F. O. Owagboriaye, G. A. Dedeke, K. O. Ademolu, O. O. Olujimi, J. S. Ashidi and A. A. Adeyinka, Reproductive toxicity of Roundup herbicide exposure in male albino rat, Exp. Toxicol. Pathol., 2017, 69, 461–468 CrossRef CAS PubMed.
- M. Thakur, H. Gupta, D. Singh, I. R. Mohanty, U. Maheswari, G. Vanage and D. S. Joshi, Histopathological and ultra structural effects of nanoparticles on rat testis following 90 days (Chronic study) of repeated oral administration, J. Nanobiotechnol., 2014, 12, 42–46 CrossRef PubMed.
- C. A. Gustin, S. J. Moran, J. D. Fuhrman, M. L. Kurtzweil, J. M. Kronenberg, D. I. Gustafson and M. A. Marshall, Applicator exposure to acetochlor based on biomonitoring, Regul. Toxicol. Pharmacol., 2005, 43, 141–149 CrossRef CAS PubMed.
- F. O. Owagboriaye, G. A. Dedeke, K. O. Ademolu, O. O. Olujimi, J. S. Ashidi and A. A. Adeyinka, Reproductive toxicity of Roundup herbicide exposure in male albino rat, Exp. Toxicol. Pathol., 2017, 69, 461–468 CrossRef CAS PubMed.
- W. J. Hu, Study on the Reproductive Toxicity of Herbicide Acetochlor in Male Mice, Jilin University, 2010 Search PubMed.
- J. Ashby, H. Tinwell, P. A. Lefevre, J. Williams, L. Kier, I. D. Adler and M. J. Clapp, Evaluation of the mutagenicity of acetochlor to male rat germ cells, Mutat. Res., 1997, 393, 263–281 CAS.
- X. Z. Sun, L. F. Dan, Y. N. Li, Q. L. Wang, J. M. Ji, L. S. Chen, J. F. Chen and S. L. Wang, Effects of Occupational Exposure to Acetochlor on Semen Quality of Male Workers, Chin. J. Ind. Med., 2006, 01, 1–3 Search PubMed.
- M. Astiz, D. C. G. Hurtado, M. N. Garcia, S. M. Galletti, A. L. Errecalde, M. J. Alaniz and C. A. Marra, Pesticide-induced decrease in rat testicular steroidogenesis is differentially prevented by lipoate and tocopherol, Ecotoxicol. Environ. Saf., 2013, 91, 129–138 CrossRef CAS PubMed.
- T. Zerin, H. Y. Song and Y. S. Kim, Extracellular signal-regulated kinase pathway play distinct role in acetochlor-mediated toxicity and intrinsic apoptosis in A549 cells, Toxicol. in Vitro, 2015, 29, 85–92 CrossRef CAS PubMed.
- E. Rollerova, L. Wsolova and M. Urbancikova, Herbicide acetochlor interferes with proliferation activity of MCF-7 cells enhanced by estradiol, Endocr. Regul., 2014, 48, 195–200 CrossRef CAS PubMed.
- J. D. Rich, S. M. Gabriel and J. R. Schultz-Norton, In vitro effects of herbicides and insecticides on human breast cells, ISRN Toxicol., 2012, 61, 23–25 Search PubMed.
- A. Agarwal, A. Kasinathan, R. Ganesan, A. Balasubramanian, J. Bhaskaran, S. Suresh, R. Srinivasan, K. B. Aravind and N. Sivalingam, Curcumin induces apoptosis and cell cycle arrest via the activation of reactive oxygen species-independent mitochondrial apoptotic pathway in Smad4 and p53 mutated colon adenocarcinoma HT29 cells, Nutr. Res., 2018, 51, 67–81 CrossRef CAS PubMed.
- L. Li, C. Wang, Y. Wen, Y. Hu, Y. Xie, M. Xu, M. Liang, W. Liu, L. Liu and Y. Wu, ERK1/2 and the Bcl-2 Family Proteins Mcl-1, tBid, and Bim Are Involved in Inhibition of Apoptosis During Persistent Chlamydia psittaci Infection, Inflammation, 2018 Search PubMed, [Pubmed:PMID: 29666982].
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8tx00178b |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |