DOI:
10.1039/C9TC03110C
(Paper)
J. Mater. Chem. C, 2019,
7, 11118-11125
TiO2 based sensor with butterfly wing configurations for fast acetone detection at room temperature†
Received
10th June 2019
, Accepted 14th August 2019
First published on 16th August 2019
Abstract
The amorphous/nanocrystal hybrid TiO2 based butterfly wing structure (ANH-TiO2-BW) is successfully fabricated via an easily controlled self-deposition sintering method. The hybrid configuration greatly enhances the structural toughness and maintains the original morphology throughout the experiment. The ANH-TiO2-BW configuration is very conductive to gas diffusion, and the calculated value of the diffusion coefficient (Dk) is 0.37 cm2 s−1 at room temperature (27 °C). As the completely reserved structure provides sufficient effective gas diffusion channels and gas molecule reaction sites, the ANH-TiO2-BW based gas sensor demonstrates a remarkable sensitivity to acetone, up to 1.88 at a detection limit of 5 ppm at room temperature and 180.08 for 200 ppm at an optimal operating temperature of 65 °C. Meanwhile, the device exhibits long-term stability, good cycle-to-cycle repeatability and selectivity to trace acetone at room temperature. Moreover, benefiting from the 3D penetrating porous structure, the average response time of the device is only about 1.8 s. This work may open up a new way for the design and application of toxic and harmful gas detection at room temperature.
Introduction
With the rapid development of industrialization, the hazards of dangerous volatile gases to human health and environmental safety have become more and more serious in recent decades. Acetone, as one of the most widely used volatility gases in industry, will cause mild irritation to human skin, eyes, nose, and lung when the concentration reaches 173 ppm.1 More seriously, when the concentration is higher than 2000 ppm, it will damage the central nervous system.2 In order to ensure the safety of millions of acetone-related workers, it is an urgent task to develop gas sensors with high sensitivity and selectivity. The conductometric semiconducting metal oxides have attracted much attention in gas sensor application due to their excellent properties such as high sensitivity, low cost and easy synthesis under atmospheric conditions.3–5 Unfortunately, the optimum response temperature of metal oxides in sensors is usually in the range of 300–450 °C, as the higher temperature can increase the activity of oxygen adsorption.3 However, it also introduces various problems such as the energy waste, device instability and operational risk.6 Therefore, the development of gas sensors operating at room temperature with low concentration detection and high responsivity is of great significance. It is well-known that the sensing process of gas sensors is greatly dependent on the gas diffusion and surface reaction,3 in other words, dramatically influenced by their chemical composition, microstructures and morphologies.7 Therefore, great efforts have been made to improve the gas sensing performance via optimizing chemical components and designing subtle configurations. For chemical component optimization, it usually involves multi-component construction,8 noble metal active site addition,9–11 certain species ion doping,12–14 and metal oxide hetero-structure fabrication.15–17 For subtle configuration design, it involves both numerous artificial structure fabrications and biomorphic structure replication.18 For instance, spheres,19,20 polyhedra,21 irregular shapes22–25 and core–shell26–28 are popular man-made structures, while various organisms are also research objects for morphogenetic materials, such as genetic materials,29–31 bacteria,32 vegetation33–36 and animals.37 Natural genetic engineers are extremely sophisticated, and they can spontaneously generate millions of micro-sized scale structures in a miniature part according to the organism's inner genes so as to form complicated functional structures with 3D nano to sub-micrometer morphologies.18 Such precise functional 3D structures are far more ingenious than artificial structures produced by bottom-up self-organization methods or top-down photolithography, since it can get rid of the limitation of semiconductor technology.
Among various biological structures, butterfly wings have greatly inspired research interests of material scientists. To date, they have been utilized as templates to build a number of novel functional structures and applied to various fields, such as infrared detection,38 high-speed imaging of infrared photons,39 intraocular pressure sensors,40 reverse color diffraction elements,41 photothermal materials,42 photonic crystals43 and optical gas sensors.44 Compared to woods, bamboos and the majority of other porous structures, the micro-structures in butterfly wings are more subtle and complicated. It can be regarded as a 3D porous flat sac shaping as gratings with interlaced horizontal and vertical ribs,45 showing potential advantages in gas sensing applications. On the one hand, compared with the gas-proof constructions, the through hollow regions obtain obvious advantages in unsteady aerodynamic load as well as damping factors,46 providing sufficiently efficient gas diffusion channels. On the other hand, these porous structures could provide adequate surface area to adsorb as many target gas molecules as possible and thus produce remarkable and measurable response, which is crucial for low concentration and low temperature detection.5
However, the configurations made of the replicate biomorphic porous metal oxide materials through traditional soaking and following natural sintering are usually loose and brittle. Extremely sharp interfacial energies regions are formed between neighboring particles of different orientations during the replacing process of the biological template, leading to easy intergranular fracture47 of microstructures. It is difficult to maintain the original morphology for the duplicate structure, which usually turns into powder during the sintering and transfer process. Therefore, the brickle replicates are usually made into pastes and then coated on substrates with patterned electrodes to fabricate gas sensors. Although the specific surface area of the pastes increases relatively, the destroyed porous structures and plugged micro-holes are not conducive to the gas diffusion and the contact of gas molecules with the inner wall. Compared with complete replicas without any structural damage, these configurations cannot fully embrace the advantages of biological structures.
In this work, Princeps paris Linnaeus wings are employed as templates to form the functional sensitive structures via a strictly controlled procedure. The method exhibits the complete replicate 3D porous amorphous/nanocrystal hybrid TiO2 based-butterfly wing structure (ANH-TiO2-BW). The local amorphous areas significantly enhance the toughness of entire hybrid structures, so that the ANH-TiO2-BW entirely conserves the original wing structure as well as the inner hierarchical 3D micro hollow porous configuration. Continuous reaction surface and porous opening structure of ANH-TiO2-BW are beneficial to the flow and adsorption of gas molecules, rather suitable for tracing a target gas at room temperature. In the acetone gas measurement, ANH-TiO2-BW based sensors exhibit a high sensitivity up to 1.88 at a detection limit of 5 ppm at room temperature and 180.08 for 200 ppm at an optimal operating temperature of 65 °C. In addition, in the comparative test of dangerous gases acetone, ammonia, ethylene and CO, the sensors based on ANH-TiO2-BW show excellent selectivity. As comparative experiments, we also fabricate nanocrystal TiO2 based butterfly wing structure powder (N-TiO2-BWP) and the conventional sintered TiO2 powder (TiO2-P) based sensors. The results demonstrate that the acetone sensitivity of sensors based on ANH-TiO2-BW is the best at room temperature. The study on gas sensors based on the complete replicate butterfly wing configuration may pave a new way for sensor design for highly sensitive room temperature gas detection.
Results and discussion
The schematic diagram of the fabrication process of ANH-TiO2-BW and the related sensor is shown in Fig. 1. Fore wings of Princeps paris (family Papilionidae) are chosen as bio-templates, as they contain complex hollow reticular patterns called quasi-honeycomb structures. The ANH-TiO2-BW is fabricated through sol–gel and following sintering method, and the details are depicted in the Experimental section. The X-ray diffraction (XRD) patterns of the structural evolution from ANH-TiO2-BW to N-TiO2-BWP are shown in Fig. S1 (ESI†). With the increase of annealing time, the diffraction peak of the sample transforms from an extremely diffused broad peak to a distinguishable one, indicating that the TiO2 based products obtained by the calcination method are amorphous, and can crystallize gradually when the atoms obtain enough thermal energy. The observed diffraction peaks of N-TiO2-BWP are relatively broad, indicating its tiny grain size. According to the Scherrer equation,48 the average grain size of N-TiO2-BWP is estimated to be approximately 9 nm. There are no notable peaks of impurities, demonstrating that no other phases have been formed.
 |
| | Fig. 1 The schematic diagram of the fabrication process of ANH-TiO2-BW and related sensor: (a) the pretreated wing templates, (b) the templates with coating of metal ions, (c) preparation for the calcination, (d) the fabricated ANH-TiO2-BW sensor, (e) the single scale model of the original butterfly wing, coating with complex metal ions and replicated product after sintering, (f) 2D and 3D porous configurations of the corresponding area in (e). | |
Scanning electron microscopy (SEM) is used here to observe the morphology of sensitive structures, and SEM images of the ANH-TiO2-BW are shown in Fig. 2. The natural Princeps paris wings (Fig. S2a, ESI†) are covered densely with scales around 60 μm wide and 150 μm long (Fig. S2b, ESI†), and the ANH-TiO2-BW completely reproduces the structure of the original butterfly wing and inherited the microstructures of the natural wing and scales (Fig. 2a). In addition, the copied hollow quasi-honeycomb structures are clearly identifiable (Fig. 2b).
 |
| | Fig. 2 SEM images of ANH-TiO2-BW (a and b), the diameter and distribution of nano-holes in ANH-TiO2-BW quasi-honeycomb structures (c), TEM images (d and e) and SAD pattern (f) of ANH-TiO2-BW. | |
The diameter and distribution of nano-holes in ANH-TiO2-BW quasi-honeycomb structures are characterized by the Image-Pro Plus (Fig. 2c). The minimum diameter of these holes is estimated to be 21.2 nm, and the maximum diameter is about 575.8 nm, with an average of about 332.6 nm. These subtle and complicated hollow structures show potential advantages for gas sensing applications. Compared with the gas-proof constructions, the through hollow regions obtain obvious advantages in unsteady aerodynamic load as well as damping factors, providing sufficiently efficient gas diffusion channels. Additionally, these porous structures can provide sufficient surface area to adsorb as many target gas molecules as possible, resulting in remarkable and measurable gas response, which is crucial at low concentration atmosphere and room operating temperature. However, in comparison with ANH-TiO2-BW, the structures of N-TiO2-BWP are fragmentary, as shown in Fig. S2c (ESI†). Meanwhile, the majority of nano-holes are filled and blocked by finer powders (Fig. S2d, ESI†).
To gain deeper insights into the morphology and microstructures, ANH-TiO2-BW samples (Fig. 2d–f) are characterized by using a transmission electron microscope (TEM) and selected area diffraction patterns (SAD) in our work. The TEM image of ANH-TiO2-BW shown in Fig. 2d is observed as a porous framework, and the enlarged portion in Fig. 2e further shows the ANH-TiO2-BW is an amorphous TiO2 and anatase nanocrystal TiO2(110) (circled in blue) composite structure. The local amorphous regions significantly enhance the toughness of the entire structure by connecting the nanocrystals with the amorphous transition regions, thus achieving smooth transitions from ordered to disordered areas. Therefore, large-scale replication of butterfly wings can be completely preserved in the process of conversion from organism to TiO2. The transition regions between amorphous and nanocrystal are clearly demonstrated in the further enlarged image as shown in Fig. S3 (ESI†). However, from the diffuse halo of the diffraction pattern (Fig. 2f), the whole ANH-TiO2-BW still shows an amorphous property owing to the large proportion of amorphous TiO2 and the relatively small amounts of nanocrystals. Although the porous framework of N-TiO2-BWP (Fig. S4a, ESI†) is similar to the ANH-TiO2-BW, the enlarged portion of N-TiO2-BWP in Fig. S4b (ESI†) demonstrates that its structure is fragile polycrystalline, and the rings are indexed as (101), (110), (004), (200), (105), (211) and (204) diffractions (Fig. S4c, ESI†), respectively. We believe that the N-TiO2-BWP sample becomes brittle due to its higher crystallization compared with the ANH-TiO2-BW sample. Because the distance between neighboring crystalline regions is too short, the transition from one type of crystal orientation to another is too violent, which leads to extremely high interfacial energy and eventual fragile property. Therefore, in the process of traditional experiments of preparing gas sensitive structures via a biological template method, the complete biological template replication structure could not be preserved completely. In addition, the TiO2-P samples used for comparative experiments are also characterized by TEM and SAD (Fig. S4d–f, ESI†), demonstrating a typical polycrystalline structure.
TiO2 is used as the base material here because it is cheap, easy to control, and most importantly, it has a d0 electronic configuration and large electronic bandgap of 3.0–3.2 eV,49 which is found to be beneficial for charge transport and surface reaction.50 Generally speaking, the acetone sensing action on the n-type semiconducting TiO2 involves two steps.51–53 The first step is that the TiO2 adsorbs environmental oxygen molecules onto its exposed surface and grain boundaries eqn (1). In this process, these adsorbed oxygen molecules extract electrons from the conduction band, and form oxygen ions by trapping electrons on the surface eqn (2), leading to increased depletion layer thickness and resistance in sensors. The second step is when exposing to the reducing gas acetone, the acetone molecules will react with surface ionic oxygen, and then release extracted electrons back into TiO2 (eqn (3)–(5)), resulting in decreased depletion layer thickness and resistance.
| | | O2(gas) → O2(adsorbed) | (1) |
| | | O2(adsorbed) + e− + O2− | (2) |
| | | CH3COCH3 + O2− → CH3C+O + CH3O− + e− | (3) |
In order to better understand the advantages of the ANH-TiO
2-BW structure in gas sensing applications, the schematic diagrams are given in
Fig. 3. As shown in
Fig. 3a, compared with conventional powders and damaged porous structures, the ANH-TiO
2-BW structure plays a positive role in gas-induced reaction. Specifically, a large number of 3D hollow holes in ANH-TiO
2-BW provide numerous channels for acetone molecules, allowing free and effective airflow into and out of the hollow 3D framework and contact with the inner wall of sensor materials. Furthermore, the longitudinal ridge wall connecting the transverse quasi-honeycomb structure contains a large number of small folds, which again provides numerous chemical reaction sites for the target gas molecules. When exposed to air, the ANH-TiO
2-BW structure adsorbs environmental oxygen molecules onto its exposed surface and grain boundaries. These adsorbed oxygen molecules extract electrons from the conduction band and form oxygen ions by trapping electrons on the surface (
Fig. 3b), leading to increased depletion layer thickness and resistance in sensors. When exposing to the reducing gas acetone, the acetone molecules react with the adsorbed oxygen ions, and the captured electrons are released back to the conduction band (
Fig. 3c), resulting in a sharp increase of either electron mobility or carrier concentration. It has been reported that well-defined pore architectures are very beneficial to ultrafast gas response kinetics.
54,55 The well-organized nano-holes in ANH-TiO
2-BW can greatly promote the response/recovery rates, because they remarkably affect gas diffusion, which can be described by an important factor in the sensing process named Knudsen diffusion. The classic free mean paths of the gas molecules formula is defined as:
| |  | (6) |
where
kB is the Boltzmann constant (J K
−1),
T is temperature (K),
p is pressure (Pa), and
d is the diameter of the gas particles (m). Gas diffusion can be predominantly described by Knudsen diffusion:
| |  | (7) |
where
MA is the molecular mass of gas molecule,
R is the universal gas constant and
T is temperature (K). The ambient temperature during the experiment is 27 °C, therefore the diffusion coefficient
Dk at room temperature can be abbreviated as:
| | Dk = 2.2 × 104·![[r with combining macron]](https://www.rsc.org/images/entities/i_char_0072_0304.gif) | (8) |
which relates to the average pore radius
![[r with combining macron]](https://www.rsc.org/images/entities/i_char_0072_0304.gif)
(cm) linearly. The measured average pore diameter of ANH-TiO
2-BW is about 3.33 × 10
−5 cm (1.66 × 10
−5 cm for radius), and thus
Dk = 0.37 cm
2 s
−1, indicating a very small air resistance. The calculation results demonstrate that well-organized hollow porous structures are very conducive to reduce unsteady aerodynamic loading and low damping coefficients, which is very suitable for improving the gas response ability.
 |
| | Fig. 3 The schematic diagram of the ANH-TiO2-BW structure and the gas flow circulated in any direction (a), the formation of oxygen ions when the ANH-TiO2-BW is exposed to air (b), and acetone molecules react with surface ionic oxygen when the ANH-TiO2-BW is exposed to reducing gas acetone (c). | |
The sensing properties of the three kinds of gas sensors to acetone at room temperature are measured and shown in Fig. 4. In detail, Fig. 4a displays the sensitivity of ANH-TiO2-BW to acetone at room temperature with concentration from 5 ppm to 200 ppm. The sensitivities are 1.88, 6.62, 7.35, 11.12, 23.53, 67.10 and 112.02 corresponding to 5 ppm, 10 ppm, 25 ppm, 50 ppm, 100 ppm, 150 ppm and 200 ppm respectively, showing a growth trend along with the increased concentration. The blue curve in Fig. 4b shows the gas response of the ANH-TiO2-BW based sensor under the optimal operating temperature of 65 °C. The sensitivities are measured up to 3.84, 6.25, 19.17, 32.52, 68.02, 148.61, and 180.08 corresponding to 5 ppm, 10 ppm, 25 ppm, 50 ppm, 100 ppm, 150 ppm and 200 ppm, respectively. For comparison, the gas responses of N-TiO2-BWP (Fig. 4c) and TiO2-P (Fig. 4d) are also measured. Obviously, the sensitivities of sensors based on N-TiO2-BWP (1.28 to 200 ppm) are very low, only about 1% of ANH-TiO2-BW. The worst sensitivity refers to the tTiO2-P based sensor, which has no response at room temperature. The results confirm that the completely reserved hollow porous structure can improve the gas sensing performance greatly. For the N-TiO2-BWP based sensor, as the porous structure is destroyed and the nano-holes are filled and blocked by powders, the inner wall could not effectively contact the target gas molecules, and only the outer surface is retained to participate in the gas sensing reaction, which suppresses the gas sensitivity at room temperature. For the TiO2-P based sensor, the gas sensitivity is limited at room temperature due to having the smallest specific surface area. The gas sensitivity tests are in good agreement with our expectations. The completely 3D hollow porous structure replicated from butterfly wings has great potential in promoting gas sensing performance at room temperature, and structural damage will greatly weaken these advantages due to blockage of gas transmission channels.
 |
| | Fig. 4 The room temperature (a) and 65 °C (b) gas sensing properties of ANH-TiO2-BW based sensors, and the room temperature gas sensing properties of N-TiO2-BWP (c) and TiO2-P (d) based sensors. | |
The long-term stability and cycle-to-cycle repeatability under different acetone concentrations at room temperature are measured and shown in Fig. 5. As shown in Fig. 5a, the ANH-TiO2-BW based sensor is measured again 45 days later, and the sensing curve in green almost coincides with blue curve, indicating that the device has good long-term stability. The cycle-to-cycle stability of the ANH-TiO2-BW based sensor under 5 ppm, 10 ppm and 25 ppm acetone concentration at room temperature is measured and shown in Fig. 5b. Within each of the cycling test cycles, the ANH-TiO2-BW based sensor shows excellent reversible responses and consistent performance. The sensitivity, selectivity and response/recovery time of ANH-TiO2-BW based gas sensors are measured and shown in Fig. 6. The sensitivity of ANH-TiO2-BW to acetone at different temperatures is also measured (Fig. 6a), and the optimal operating temperature is 65 °C. In addition, as observed in Fig. 6c, the average response and recovery times are only about 1.8 s and 25.5 s, respectively. Selectivity is another important parameter of gas sensing performance, which needs to be considered in the actual application environment. As shown in Fig. 6b, the gas sensing properties of three other gases (ammonia, ethylene and carbon monoxide) are measured at room temperature. The concentration of gas ranges from 5 ppm to 200 ppm. It is found that the ANH-TiO2-BW based sensor indicates superior selectivity of acetone, 56, 62.2 and 74.6 times higher than ammonia, carbon monoxide and ethylene at 200 ppm, respectively. Because acetone is one of the most widely used harmful gases and has been deeply researched, the performance comparison of acetone sensors using various metal oxide nanomaterials is exhibited in Table 1.53,56–62 The ANH-TiO2-BW based gas sensors in our work show excellent room temperature detection performance.
 |
| | Fig. 5 The long-time stability (a) and cycle-to-cycle stability (b) under different acetone concentration at room temperature of ANH-TiO2-BW based gas sensors. | |
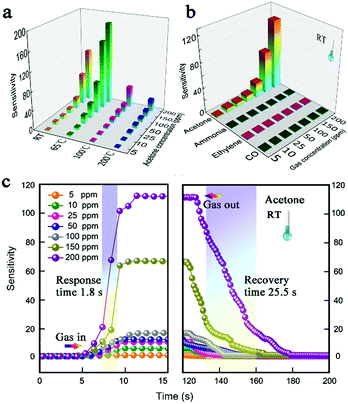 |
| | Fig. 6 The acetone sensitivity at different temperatures (a), and selectivity (b) and response/recovery time (c) of ANH-TiO2-BW based gas sensors. | |
Table 1 The comparison of acetone sensors using various metal oxide nanomaterials
| Response material |
T (°C) |
Concentration (ppm) |
Sensitivity |
Ref. |
| TiO2 nanotubes |
RT |
10 |
1.14 |
53
|
| Ti3AlC2 |
RT |
100 |
1.08 |
56
|
| Pt-doped/SnO2 |
100 |
100 |
11.2 |
57
|
| PdO-doped/Fe2O3 |
225 |
100 |
19.7 |
58
|
| Flower-like ZnFe2O4/ZnO |
250 |
50 |
8.3 |
59
|
| Nano-LaFeO3 |
260 |
5 |
1.94 |
59
|
| ZnO@ZIF-CoZn |
260 |
10 |
27.0 |
60
|
| ZnO nanowire |
260 |
10 |
1.5 |
61
|
| In2O3–WO3 nanofibers |
275 |
200 |
29.0 |
62
|
| ANH-TiO2-BW |
RT |
5/10/50/100 |
1.9/6.6/11.1/23.5 |
This work |
| 65 |
5/10/100/200 |
3.8/6.3/68.0/180.1 |
Conclusions
In summary, a high-performance acetone sensor based on ANH-TiO2-BW is developed via a simple and low-cost self-deposition followed by sintering method. The mixed amorphous and nanocrystalline structure realizes the complete replication of biological templates. The complete replicate hollow 3D porous structure is greatly favorable for gas flow and simultaneously provides sufficient adsorption sites and reaction areas, which is proved by the structural characterization and model analysis. The ANH-TiO2-BW based acetone sensor shows high sensitivity, long-term stability, fast response/recovery time and excellent selectivity at room temperature. The sensitivity is up to 1.88 at a detection limit of 5 ppm at room temperature and 180.08 for 200 ppm at 65 °C, and the average response and recovery times are only about 1.8 s and 25.5 s, respectively. In addition, the device exhibits long-term stability in the 45 day measurement, good cycle-to-cycle repeatability and shows excellent selectivity in acetone, ammonia, ethylene and carbon monoxide gas testing. These findings may offer a new approach to design room temperature gas sensors.
Experimental section
Samples fabrication
Fore wings of Princeps paris (family Papilionidae) were chosen as bio-templates, as they contain complex hollow reticular patterns called quasi-honeycomb structures. The ANH-TiO2-BW was fabricated through sol–gel (Fig. 1a) followed by sintering method by a biological template copying (Fig. 1b) and replacing (Fig. 1c) process. More details were described in the ESI.† In order to carry out room temperature gas sensing comparison tests with ANH-TiO2-BW, the other two samples based on N-TiO2-BWP and TiO2-P were also prepared, respectively. The N-TiO2-BWP was obtained by a long time (48 hours) annealing at 550 °C, and parts of replicate structures encountered naturally brittle fracture and turned into powder during the crystallization process. The TiO2-P was fabricated from a heating decomposed reaction of Ti-colloid at 550 °C for 48 hours.
Characterization
XRD measurements were made on a Philips-X′ Pert Pro instrument operating at a voltage of 3 kV with Cu Kα radiation. A Zeiss Merlin field-emission SEM operating under an accelerating voltage of 5.0 kV was used to investigate the microstructure and morphology of the original wings as well as the final products. Both TEM images and selected area electron diffraction (SAED) graphics were obtained using a FEI Tecnai F30 instrument with an accelerating voltage of 300 kV. TEM samples were prepared by dispersing the outcomes in ethanol, agitating ultrasonically for at least 60 min, then extracting up to carbon thin film copper grids and baking for 30 min by using infrared light.
Gas detection
The ANH-TiO2-BW based sensor (as shown in Fig. 1d) was fabricated by contacting two ends of the ANH-TiO2-BW with the underneath cover glass using silver electrodes. The fabrication process of N-TiO2-BWP and TiO2-P based sensors was in the same manner: first, dispersing the prepared samples in deionized water and forming a paste; second, coating the paste on a plane interdigital alumina electrode with 50 μm in width and 200 μm in distance to form a sensing film. The gas sensing performances of the sensors were measured in an intelligent gas sensing analysis system named CGS-4TPs manufactured by Beijing Elite Tech Co., Ltd. The sensitivity was defined as Ra/Rg, here Ra and Rg were the resistance in air and the target gas respectively. The times taken by the sensors to achieve 90% of the entire resistance change by adsorption and desorption of the test gases were defined as the response and recovery times, respectively. Before the measurements, the ANH-TiO2-BW, N-TiO2-BWP and TiO2-P based sensors were annealed at 300 °C for 2 days to increase stability. The detection gases were acetone, ammonia, ethylene and CO diluted with dry air.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 61805160 and 51602205), the Natural Science Foundation of Guangdong Province (Grant No. 2017A030310325), the Science and Technology Innovation Commission of Shenzhen (Grant No. JCYJ20170818102640668, JCYJ20170818101906654 and JCYJ20180305125423315).
Notes and references
- Q. Q. Jia, H. M. Ji, Y. Zhang, Y. L. Chen, X. H. Sun and Z. G. Jin, J. Hazard. Mater., 2014, 276, 262 CrossRef CAS PubMed.
- J. S. Do and S. H. Wang, Sens. Actuators, B, 2013, 185, 39 CrossRef CAS.
- C. X. Wang, L. W. Yin, L. Y. Zhang, D. Xiang and R. Gao, Sensors, 2010, 10, 2088 CrossRef CAS.
- N. Barsan, D. Koziej and U. Weimar, Sens. Actuators, B, 2007, 121, 18 CrossRef CAS.
- G. F. Fine, L. M. Cavanagh, A. Afonja and R. Binions, Sensors, 2010, 10, 5469 CrossRef CAS.
- S. Park, S. An, Y. Mun and C. Lee, ACS Appl. Mater. Interfaces, 2013, 5, 4285 CrossRef CAS PubMed.
- X. W. Li, X. Zhou, H. Guo, C. Wang, J. Y. Liu, P. Sun, F. M. Liu and G. Y. Lu, ACS Appl. Mater. Interfaces, 2014, 6, 18661 CrossRef CAS PubMed.
- D. Bekermann, A. Gasparotto, D. Barreca, C. Maccato, E. Comini, C. Sada, G. Sberveglieri, A. Devi and R. A. Fischer, ACS Appl. Mater. Interfaces, 2012, 4, 928 CrossRef CAS PubMed.
- M. Shojaee, S. Nasresfahani and M. H. Sheikhi, Sens. Actuators, B, 2018, 254, 457 CrossRef CAS.
- C. S. Lee, Z. F. Dai, D. H. Kim, H. Y. Li, Y. M. Jo, B. Y. Kim, H. G. Byunc, I. Hwangd and J. H. Lee, Sens. Actuators, B, 2018, 273, 1 CrossRef CAS.
- N. M. Li, K. M. Li, S. Wang, K. Q. Yang, L. J. Zhang, Q. Chen and W. M. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 10534 CrossRef CAS PubMed.
- Z. H. Wang, Z. W. Tian, D. M. Han and F. B. Gu, ACS Appl. Mater. Interfaces, 2016, 8, 5466 CrossRef CAS PubMed.
- B. E. Rossinyol, A. Prim, E. Pellicer, J. Arbiol, F. Hernández-Ramírez, F. Peiró, A. Cornet, J. R. Morante, L. A. Solovyov, B. Tian, T. Bo and D. Y. Zhao, Adv. Funct. Mater., 2007, 17, 1801 CrossRef.
- S. K. Sinha and S. Ghosh, Adv. Powder Technol., 2017, 28, 2766 CrossRef CAS.
- K. H. Kim, S. J. Kim, H. J. Cho, N. H. Kim, J. S. Jang, S. J. Choi and I. D. Kim, Sens. Actuators, B, 2017, 241, 1276 CrossRef CAS.
- K. K. Kim, D. Kim, S. H. Kang and S. Park, Sens. Actuators, B, 2017, 248, 43 CrossRef CAS.
- J. Y. Liu, M. J. Dai, T. S. Wang, P. Sun, X. S. Liang, G. Y. Lu, K. Shimanoe and N. Yamazoe, ACS Appl. Mater. Interfaces, 2016, 8, 6669 CrossRef CAS.
-
D. Zhang, Morphology genetic materials templated from nature species, Zhejiang University Press, Hang Zhou and Springer-Verlag, Berlin Heidelberg, 2012 Search PubMed.
- L. P. Zhang, R. Dong, Z. Y. Zhu and S. R. Wang, Sens. Actuators, B, 2017, 245, 112 CrossRef CAS.
- G. X. Zhou, Z. L. Zhang, B. Chen, Y. Wang, Y. Ding, X. L. Zhang and M. Ji, Micro Nano Lett., 2017, 12, 133 CrossRef CAS.
- B. Y. Geng, C. H. Fang, F. M. Zhan and N. Yu, Small, 2008, 4, 1337 CrossRef CAS.
- X. F. Wang, W. Ma, F. Jiang, E. S. Cao, K. M. Sun, L. Cheng and X. Z. Song, Chem. Eng. J., 2018, 338, 504 CrossRef CAS.
- S. Roso, T. Vilic, A. Urakawa and E. Llobet, Pro. Eng., 2016, 168, 247 CrossRef CAS.
- M. Govindhan, B. Sidhureddy and A. C. Chen, ACS Appl. Mater. Interfaces, 2018, 1, 6005 CrossRef CAS.
- R. Zhou, X. P. Lin, D. Y. Xue, F. Y. Zong, J. M. Zhang, X. C. Duan, Q. H. Li and T. H. Wang, Sens. Actuators, B, 2018, 260, 900 CrossRef CAS.
- W. R. Li, H. Y. Xu, T. Zhai, H. Q. Yu, Z. R. Chen, Z. W. Qiu, X. P. Song, J. Q. Wang and B. Q. Cao, Sens. Actuators, B, 2017, 253, 97 CrossRef CAS.
- S. M. Majhi, G. K. Naik, H. J. Lee, H. G. Song, C. R. Lee, I. H. Lee and Y. T. Yu, Sens. Actuators, B, 2018, 268, 223 CrossRef CAS.
- A. Aziz, N. Tiwale, S. A. Hodge, S. J. Attwood, G. Divitini and M. E. Welland, ACS Appl. Mater. Interfaces, 2018, 10, 43817 CrossRef CAS.
- M. N. Al-Hinai, R. Hassanien, N. G. Wright, A. B. Horsfall, A. Houlton and B. R. Horrocks, Faraday Discuss., 2013, 164, 71 RSC.
- T. Prakash, R. Jayaprakash, D. S. Raj, S. Kumar, N. Donato, D. Spadaro and G. Neri, Sens. Actuators, B, 2013, 176, 560 CrossRef CAS.
- Z. H. Ibupoto, A. Tahira, A. B. Mallah, M. Willander and C. Yu, Sens. Lett., 2016, 14, 1 CrossRef.
- Z. H. Li, J. Y. Shi, X. W. Huang, X. B. Zou, X. T. Hu, X. C. Zhou and E. T. Haroon, Anal. Methods, 2018, 10, 138 RSC.
- C. J. Dong, X. X. Xing, N. Chen, X. Liu and Y. D. Wang, Sens. Actuators, B, 2016, 230, 1 CrossRef CAS.
- S. Marchesini, C. M. McGilvery, J. Bailey and C. Petit, ACS Nano, 2017, 11, 10003 CrossRef CAS PubMed.
- M. P. Chen, Y. M. Zhang, J. Zhang, K. J. Li, T. P. Lv, K. Y. Shen, Z. Q. Zhu and Q. J. Liu, J. Mater. Chem. C, 2018, 6, 6138 RSC.
- Z. T. Liu, T. X. Fan, D. Zhang, X. L. Gong and J. Q. Xu, Sens. Actuators, B, 2009, 136, 499 CrossRef CAS.
- S. X. Liu and J. H. He, J. Am. Ceram. Soc., 2005, 88, 3513 CrossRef CAS.
- F. Y. Zhang, Q. C. Shen, X. D. Shi, S. P. Li, W. L. Wang, Z. Luo, G. F. He, P. Zhang, P. Tao, C. Y. Song, W. Zhang, D. Zhang, T. Deng and W. Shang, Adv. Mater., 2015, 27, 1077 CrossRef CAS.
- A. D. Pris, Y. Utturkar, C. Surman, W. G. Morris, A. Vert, S. Zalyubovskiy, T. Deng, H. T. Ghiradella and R. A. Potyrailo, Nat. Photonics, 2012, 6, 195 CrossRef CAS.
- V. Narasimhan, R. H. Siddique, J. O. Lee, S. Kumar, B. Ndjamen, J. Du, N. Hong, D. Sretavan and H. Choo, Nat. Nanotechnol., 2018, 13, 512 CrossRef CAS.
- G. England, M. Kolle, P. Kim, M. Khan, P. Muñoz, E. Mazur and J. Aizenberg, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 15630 CrossRef CAS.
- J. L. Tian, W. Zhang, J. J. Gu, T. Deng and D. Zhang, Nano Energy, 2015, 17, 52 CrossRef CAS.
- W. H. Peng, S. M. Zhu, W. L. Wang, W. Zhang, J. J. Gu, X. B. Hu, D. Zhang and Z. X. Chen, Adv. Funct. Mater., 2012, 22, 2072 CrossRef CAS.
- R. A. Potyrailo, H. Ghiradella, A. Vertiatchikh, K. Dovidenko, J. R. Cournoyer and E. Olson, Nat. Photonics, 2007, 1, 123 CrossRef CAS.
- D. Zhang, W. Zhang, J. J. Gu, T. X. Fan, Q. L. Liu, H. L. Su and S. M. Zhu, Prog. Mater. Sci., 2015, 68, 67 CrossRef.
- I. Kovalev, J. Bionic Eng., 2008, 5, 224 CrossRef.
- N. E. Dowling, Int. J. Fatigue, 1997, 19, 269 CrossRef.
- A. L. Patterson, Phys. Rev., 1939, 56, 978 CrossRef CAS.
- L. Liu and X. B. Chen, Chem. Rev., 2014, 114, 9890 CrossRef CAS PubMed.
- Z. P. Tshabalala, D. E. Motaung, G. H. Mhlongo and O. M. Ntwaeaborwa, Sens. Actuators, B, 2016, 224, 841 CrossRef CAS.
- S. T. Navale, Z. B. Yang, C. S. T. Liu, P. J. Cao, V. B. Patil, N. S. Ramgir, R. S. Mane and F. J. Stadler, Sens. Actuators, B, 2018, 255, 1701 CrossRef CAS.
- N. Chen, Y. X. Li, D. Y. Deng, X. Liu, X. X. Xing, X. C. Xiao and Y. D. Wang, Sens. Actuators, B, 2017, 238, 491 CrossRef CAS.
- B. Bhowmik, A. Hazra, K. Dutta and P. Bhattacharyya, IEEE Trans. Device Mater. Reliab., 2014, 14, 961 CAS.
- J. H. Lee, Sens. Actuators, B, 2009, 140, 319 CrossRef CAS.
- W. Zhang, J. L. Tian, Y. A. Wang, X. T. Fang, Y. Q. Huang, W. X. Chen, Q. L. Liu and D. Zhang, J. Mater. Chem. A, 2014, 2, 4543 RSC.
- E. Lee, A. VahidMohammadi, B. C. Prorok, Y. S. Yoon, M. Beidaghi and D. J. Kim, ACS Appl. Mater. Interfaces, 2017, 9, 37190 Search PubMed.
- L. W. Wang, Y. H. Wang, K. F. Yu, S. P. Wang, Y. Y. Zhang and C. S. Wei, Sens. Actuators, B, 2016, 232, 91 CrossRef CAS.
- P. Sun, B. Q. Wang, L. P. Zhao, H. Y. Gao, T. S. Wang, X. L. Yang, C. Liu and G. Y. Lu, Sens. Actuators, B, 2017, 252, 322 CrossRef CAS.
- C. Liu, B. Q. Wang, T. S. Wang, J. Y. Liu, P. Sun, X. H. Chuai and G. Y. Lu, Sens. Actuators, B, 2017, 248, 902 CrossRef CAS.
- Y. P. Chen, H. W. Qin, X. F. Wang, L. Li and J. F. Hu, Sens. Actuators, B, 2016, 235, 56 CrossRef CAS.
- M. S. Yao, W. X. Tang, G. E. Wang, B. Nath and G. Xu, Adv. Mater., 2016, 26, 5229 CrossRef.
- C. H. Feng, X. Li, J. Ma, Y. F. Sun, C. Wang, P. Sun, J. Zheng and G. Y. Lu, Sens. Actuators, B, 2015, 209, 622 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9tc03110c |
|
| This journal is © The Royal Society of Chemistry 2019 |
Click here to see how this site uses Cookies. View our privacy policy here.  d,
Su-Ting
Han
d,
Su-Ting
Han
 a,
Zongxiang
Xu
a,
Zongxiang
Xu
 e,
Wanlin
Wang
e,
Wanlin
Wang
 *a and
Yan
Yan
*a and
Yan
Yan
 *a
*a



![[r with combining macron]](https://www.rsc.org/images/entities/i_char_0072_0304.gif)
![[r with combining macron]](https://www.rsc.org/images/entities/i_char_0072_0304.gif) (cm) linearly. The measured average pore diameter of ANH-TiO2-BW is about 3.33 × 10−5 cm (1.66 × 10−5 cm for radius), and thus Dk = 0.37 cm2 s−1, indicating a very small air resistance. The calculation results demonstrate that well-organized hollow porous structures are very conducive to reduce unsteady aerodynamic loading and low damping coefficients, which is very suitable for improving the gas response ability.
(cm) linearly. The measured average pore diameter of ANH-TiO2-BW is about 3.33 × 10−5 cm (1.66 × 10−5 cm for radius), and thus Dk = 0.37 cm2 s−1, indicating a very small air resistance. The calculation results demonstrate that well-organized hollow porous structures are very conducive to reduce unsteady aerodynamic loading and low damping coefficients, which is very suitable for improving the gas response ability.





