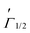Highly-efficient solution-processed green phosphorescent organic light-emitting diodes with reduced efficiency roll-off using ternary blend hosts†
Jianhua
Zhang
 a,
Yuxin
Guan
a,
Jiali
Yang
a,
Wenqiang
Hua
b,
Shuanglong
Wang
a,
Zhitian
Ling
a,
Hong
Lian
a,
Yingjie
Liao
a,
Weixia
Lan
a,
Bin
Wei
a,
Yuxin
Guan
a,
Jiali
Yang
a,
Wenqiang
Hua
b,
Shuanglong
Wang
a,
Zhitian
Ling
a,
Hong
Lian
a,
Yingjie
Liao
a,
Weixia
Lan
a,
Bin
Wei
 *a and
Wai-Yeung
Wong
*a and
Wai-Yeung
Wong
 *c
*c
aKey Laboratory of Advanced Display and System Applications, Ministry of Education, School of Mechatronic Engineering and Automation, Shanghai University, Shanghai, 200072, China. E-mail: bwei@shu.edu.cn
bShanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai, China
cInstitute of Molecular Functional Materials and Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Hung Hom, Kowloon, Hong Kong, China. E-mail: wai-yeung.wong@polyu.edu.hk
First published on 15th July 2019
Abstract
We have investigated the effect of various materials 1,3-bis(carbazol-9-yl)benzene (mCP), 10-(4-(5,5-dimethylbenzofuro[3,2-c]acridin-13(5H)-yl)phenyl)-10-phenylanthracen-9(10H)-one (DpAn-5BzAc), poly(9-vinylcarbazole) and 4,4′,4′′-tris(N-carbazolyl)-triphenylamine (TCTA) as the hosts on the performance of solution-processed green phosphorescent organic light-emitting diodes (PhOLEDs). Compared with the corresponding single and binary host systems, the device with the ternary blend hosts (mCP:DpAn-5BzAc:TCTA) blended with bis(2-phenylpyridine)iridium(III) acetylacetonate as a green dopant is highly efficient with the following performance parameters: a maximum brightness of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 320 cd m−2 and a maximum current efficiency (CE) of 40.9 cd A−1 with the Commission Internationale de L’Eclairage coordinates of (0.30, 0.63). More importantly, the PhOLED device has an extremely low efficiency roll-off: at the brightness values of 1000 and 5000 cd m−2, its CEs are close, being 40.6 and 36.5 cd A−1, with the corresponding efficiency roll-off of only 0.7% and 10.8%, respectively. The superior electroluminescence performance for ternary blend host-based green PhOLEDs was attributed to the enhanced charge carrier balance, improved structural order in the film as verified by using the grazing-incidence small-angle scattering technique, along with excellent multi-component miscibility, which has a dramatic influence on the morphology of the emissive layer and the final device performance. These results demonstrate the great potential of the multi-hosts in solution-processed organic optoelectronic devices.
320 cd m−2 and a maximum current efficiency (CE) of 40.9 cd A−1 with the Commission Internationale de L’Eclairage coordinates of (0.30, 0.63). More importantly, the PhOLED device has an extremely low efficiency roll-off: at the brightness values of 1000 and 5000 cd m−2, its CEs are close, being 40.6 and 36.5 cd A−1, with the corresponding efficiency roll-off of only 0.7% and 10.8%, respectively. The superior electroluminescence performance for ternary blend host-based green PhOLEDs was attributed to the enhanced charge carrier balance, improved structural order in the film as verified by using the grazing-incidence small-angle scattering technique, along with excellent multi-component miscibility, which has a dramatic influence on the morphology of the emissive layer and the final device performance. These results demonstrate the great potential of the multi-hosts in solution-processed organic optoelectronic devices.
1. Introduction
Organic light-emitting diodes (OLEDs) are considered as highly promising candidates for both displays and solid-state lighting sources because of their light-weight, mechanical flexibility and high response speed.1–4 Among the various representative molecular frameworks, phosphorescent materials based on metal complexes have attracted great attention owing to the high theoretical utilization of both singlet and triplet excitons for light emission to realize a maximum internal quantum efficiency up to 100% in principle, as well as high device stability.5–8 Currently, most of the highly efficient phosphorescent OLEDs (PhOLEDs) have been fabricated by evaporation processing under high vacuum to tune the balance of charge carrier injection and transportation in the functional layer with multilayered device structures.9,10 However, the large running cost of fabricating PhOLEDs using the vacuum-deposition method such as the relatively inefficient use of materials, the complex deposition system, high energy consumption and the carefully tuned luminogen doping concentration in multi-components hinders the widespread use for low-cost industrialization of OLEDs.11 Therefore, solution-processing techniques, including spin-coating, spray-coating and inkjet printing are more feasible as a low-cost alternative to manufacture large-area PhOLEDs with the advantage of the facile doping process.12–15In order to reduce the serious bimolecular self-quenching and triplet–triplet annihilation, solution-processed phosphorescent emitters usually adopt appropriate host–guest systems to achieve high efficiency PhOLEDs.16–19 Generally, the majority of the emitting layers of solution-processed PhOLEDs employ polymers as host materials due to their high film-forming ability.20,21 Park et al. employed the soluble polynorbornene bearing 9,9′-(1,10-biphenyl)-4,4′-diyl-bis-9H-carbazole side groups as a host material to afford a green PhOLED device doped with various concentrations of fac-tris(2-phenylpyridine)iridium [Ir(ppy)3] and the external quantum efficiency (EQE) and power efficiency (PE) reaching 7.2% and 11 lm W−1, respectively.22 Li et al. synthesized three carbazole-based host dendritic molecules and achieved a highly efficient soluble green PhOLED based on the Ir(ppy)3 dopant with a current efficiency (CE) of 38.71 cd A−1.23 Nonetheless, the development of novel polymer hosts for solution-processed PhOLEDs remains a great challenge. Finding wide-band-gap, good solution processability and high-triplet-energy conjugated polymers as hosts for phosphorescent dopants still remains difficult which undermines their electroluminescence (EL) performance.24,25 In contrast, small molecules have the merits over polymers in terms of high triplet energy, clearly defined structures, high material purity and better photoluminescence (PL) efficiency and thus they have become popular candidates as hosts in solution-processed PhOLEDs.26–29
Although inspiring achievements have been made in solution-processed devices based on small molecular hosts, there are still some factors limiting the device performance, such as insufficient solubility resulting in poor film quality and easy crystallization with low glass transition temperature.30 Recently, the mixed host system has been proposed as an effective approach to overcome the aforementioned drawbacks of solution-processed PhOLEDs and further improve the device performance.31–33 By changing the composition of the blended host, the charge carrier transporting properties can easily be manipulated.34,35 More importantly, due to the interblending of various host materials, the molecular packing of each host material is hindered by the other host material, which effectively avoids aggregation-caused quenching of small molecules in the emitter.36,37 Up to now, there have been a few reports on high efficiency solution-processed PhOLEDs based on the blended host system. Yao et al. demonstrated a solution-processed PhOLED using 4,4′,4′′-tris[3-methylphenyl(phenyl)amino]triphenylamine mixed with 1,3-bis[(p-tert-butyl)phenyl-1,3,4-oxadiazolyl]benzene as an exciplex co-host and achieved a satisfactory overall EL performance with a peak EQE of 15.5% and a PE of 36.9 lm W−1, respectively.38 A high efficiency solution-processed red PhOLED employing small molecule mixed host system of 4,4′,4′′-tris(N-(2-naphthyl)-N-phenylamino)triphenylamine and 2,2′,2′′-(1,3,5-phenylene)tris(1-phenyl-1H-benzimidazole) has been reported, having a low driving voltage of 5.2 V and maximum CE and PE of 17.8 cd A−1 and 11.3 lm W−1.39 Usually, in such cases, an additional hole-transporting material is normally required to achieve charge-carrier injection balance. However, mixing different hole-transporting small molecular materials as a blended host by solution processing was rarely reported.40
Here, we use 10-(4-(5,5-dimethylbenzofuro[3,2-c]acridin-13(5H)-yl)phenyl)-10-phenylanthracen-9(10H)-one (DpAn-5BzAc) having a high triplet state (ET = 2.97 eV) and bipolar transport capability mixed with 1,3-bis(carbazol-9-yl)benzene (mCP) and a hole transporting material of 4,4′,4′′-tris(N-carbazolyl)-triphenylamine (TCTA) as the ternary blend host to boost the performance of solution-processed green PhOLEDs. The additional host and hole transporting material can be omitted to achieve the enhanced charge-carrier balance, excellent miscibility and improved structural order in the emitting layer as certified by the Grazing Incidence Small Angle X-ray Scattering (GISAXS) measurement. The resulting solution-processed green PhOLEDs afforded an excellent performance with a maximum brightness of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 320 cd m−2, a maximum CE of 40.9 cd A−1 and the corresponding Commission Internationale de L'Eclairage (CIE) coordinates of (0.30, 0.63), which is the best recorded performance among the reported solution processed green PhOLEDs, and exhibit low efficiency roll-off values.
320 cd m−2, a maximum CE of 40.9 cd A−1 and the corresponding Commission Internationale de L'Eclairage (CIE) coordinates of (0.30, 0.63), which is the best recorded performance among the reported solution processed green PhOLEDs, and exhibit low efficiency roll-off values.
2. Experimental section
2.1 Materials
The bipolar and thermally activated delayed fluorescent (TADF) host DpAn-5BzAc was synthesized in our previous work.41 The hole injection material PEDOT:PSS was purchased from Heraeus, Germany. The other organic functional molecules were obtained from e-Ray Optoelectronics Corp. (China). Indium tin oxide (ITO, 15 Ω per sheet, 150 nm)-coated glass substrates were ordered from CSG Holding Co. Ltd (China). All chemicals and reagents in this work were used as received from commercial sources without purification unless otherwise stated.2.2 Device fabrication
Devices were fabricated with a configuration of ITO/PEDOT:PSS (40 nm)/EML (20 nm)/TmPyPB:TPBi (40 nm)/Liq (1 nm)/Al (100 nm), as shown in Fig. 1, where ITO (indium tin oxide) is the anode, poly(3,4-ethylenedioxythiophene)/poly(styrenesulfonate AI-4083) with a conductivity of 2 × 10−4–2 × 10−3 S cm−1 (PEDOT:PSS) is the hole injection layer; 1,3,5-tri(m-pyrid-3-yl-phenyl)benzene (TmPyPB) doped with 1,3,5-tris(1-phenyl-1H-benzimidazol-2-yl)benzene (TPBi) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) functions as the electron transporting layer, and 8-hydroxyquinolinato lithium (Liq) and Al are the electron injection layer and the cathode, respectively. The chemical structures of these materials are depicted in Fig. 1. The prepared ITO glass substrates were first cleaned using detergent, de-ionized water, acetone, and isopropanol treated with a UV–ozone environment for about 20 minutes. Then a 40 nm PEDOT:PSS was spin-coated onto the ITO surface. After being baked at 130 °C for 20 minutes under air conditions, the substrates were transferred into a nitrogen-filled glovebox. mCP, DpAn-5BzAc, poly(9-vinylcarbazole) (PVK), and bis(2-phenylpyridine)iridium(III) acetylacetonate (Ir(ppy)2acac) were all dissolved in chlorobenzene with a concentration of 10 mg ml−1, respectively. Subsequently, an emission layer (EML) of ∼20 nm was spin-coated from a chlorobenzene solution and annealed at 60 °C for 30 minutes to remove the residual solvent. Finally, the upper layers of 40 nm TmPyPB:TPBi, 1 nm Liq and 100 nm Al were evaporated in sequence via a shadow mask to finish the device fabrication in a vacuum chamber at a base pressure less than 4 × 10−6 mbar. The entire organic layers and the Al cathode were deposited without exposure to the atmosphere, in which OLEDs with an active area of 4 mm2 were obtained. The deposition rates for the organic materials and Al were typically 1.0 and 5.0 Å s−1, respectively.
1) functions as the electron transporting layer, and 8-hydroxyquinolinato lithium (Liq) and Al are the electron injection layer and the cathode, respectively. The chemical structures of these materials are depicted in Fig. 1. The prepared ITO glass substrates were first cleaned using detergent, de-ionized water, acetone, and isopropanol treated with a UV–ozone environment for about 20 minutes. Then a 40 nm PEDOT:PSS was spin-coated onto the ITO surface. After being baked at 130 °C for 20 minutes under air conditions, the substrates were transferred into a nitrogen-filled glovebox. mCP, DpAn-5BzAc, poly(9-vinylcarbazole) (PVK), and bis(2-phenylpyridine)iridium(III) acetylacetonate (Ir(ppy)2acac) were all dissolved in chlorobenzene with a concentration of 10 mg ml−1, respectively. Subsequently, an emission layer (EML) of ∼20 nm was spin-coated from a chlorobenzene solution and annealed at 60 °C for 30 minutes to remove the residual solvent. Finally, the upper layers of 40 nm TmPyPB:TPBi, 1 nm Liq and 100 nm Al were evaporated in sequence via a shadow mask to finish the device fabrication in a vacuum chamber at a base pressure less than 4 × 10−6 mbar. The entire organic layers and the Al cathode were deposited without exposure to the atmosphere, in which OLEDs with an active area of 4 mm2 were obtained. The deposition rates for the organic materials and Al were typically 1.0 and 5.0 Å s−1, respectively.
 | ||
| Fig. 1 Chemical structures of the materials investigated in this work and the corresponding relative energy level diagram in the devices. | ||
2.3 Film and device characterization
The UV-vis absorption spectra were recorded on a UV-2501PC spectrophotometer at room temperature. PL spectra and lifetime of these complexes were tested on an Edinburgh Instruments Ltd (FLSP920) fluorescence spectrophotometer in the solid state. Drop shape analysis (Kino optical CA and interface tensionmeter) was used to measure the contact angles of deionized (DI) water and ethylene glycol (EG), which were used to calculate surface energy parameters of the materials. The surface morphological images of the emitting layers were analyzed in air by using AFM (Bruker, Santa Barbara, CA, USA) in a tapping mode. The samples of the emitting layer solutions were spin-coated on the glass substrate for the GISAXS measurement at the Shanghai Synchrotron Radiation Facility. The EL characteristics were measured using a Keithley 2400 source meter and a PR650 Spectra Colorimeter at room temperature. The luminance and spectra of each device were measured in the direction perpendicular to the substrate. All the device characterization steps were carried out at room temperature under ambient laboratory conditions without encapsulation.3. Results and discussion
3.1 Photophysical properties
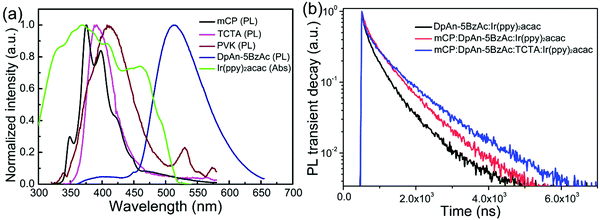
Three fluorescent materials, mCP, PVK and DpAn-5BzAc with different emission peaks, were selected as hosts to evaluate the performance of solution-processed devices employing Ir(ppy)2acac as the green dopant. Here, the hole-transporting small molecule TCTA was also added as a mixed host in the ternary blend host system. Fig. 2(a) shows the UV-vis absorption spectrum of the green phosphor Ir(ppy)2acac and the PL spectra of mCP, PVK, DpAn-5BzAc and TCTA hosts used in this work. The distinct overlap between the PL spectra of these hosts and the absorption of the phosphor was clearly observed which is helpful to realize efficient energy transfer from these hosts to the Ir(ppy)2acac dopant.42The PL transient decay results of the DpAn-5BzAc, mCP:DpAn-5BzAc and mCP:DpAn-5BzAc:TCTA films doped with Ir(ppy)2acac are shown in Fig. 2(b). The corresponding calculated lifetimes were 0.73 μs, 0.81 μs and 1.02 μs, respectively. The decay curves of Ir(ppy)2acac-doped films are long exponential decays, especially for the green phosphor doped ternary host, which showed obviously delayed decay with long exciton lifetime, indicating the low aggregation of the green phosphor. Thus, the mCP:DpAn-5BzAc and third component TCTA are expected to be used as appropriate hosts for green PhOLEDs. Such a host component-dependent exciton decay process indicates the stronger aggregation tendency of Ir(ppy)2acac in the single- and binary-host based films as compared to the mCP:DpAn-5BzAc:TCTA:Ir(ppy)2acac counterparts.
3.2 EL properties of solution-processed single host based PhOLEDs
mCP and PVK are generally used as the hosts in PhOLEDs for their relatively high triplet energy (ET). DpAn-5BzAc with an ET of 2.97 eV and bipolar transporting properties was also reported in our group's previous work by vacuum evaporation.38 Here, we first fabricated a set of solution-processed green PhOLEDs with a single host following the general structure of ITO/PEDOT:PSS (40 nm)/host: 10 wt% Ir(ppy)2acac (20 nm)/TmPyPB:TPBi (40 nm)/Liq (1 nm)/Al (100 nm), where the host is DpAn-5BzAc (Device A-1), mCP (Device A-2) or PVK (Device A-3), respectively. The sources of the materials and device fabrication procedure are given in the Experimental section. The current density–voltage (J–V), luminance–voltage (L–V) characteristics, current efficiency versus luminance (CE–L) and EL spectra of the related devices are shown in Fig. 3. The key parameters are summarized in Table 1, and the external quantum efficiency versus luminance (EQE–L) and power efficiency versus luminance (PE–L) characteristics of the single host based devices are illustrated in Fig. S1 (ESI†).| Devicea | Host structure | Ratiob | V on [V] | L max [cd m−2] | CEe [cd A−1] Max/1000/5000 | ELf [nm] |
|---|---|---|---|---|---|---|
a Device configuration: glass/ITO/PEDOT:PSS (40 nm)/EML (20 nm)/TmPyPB:TPBi (40 nm)/Liq (1 nm)/Al (100 nm).
b The dopant ratio of host materials in emitters.
c The operating voltage at a brightness of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cd cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m−2.
d The maximum luminance.
e CE at the maximum value/at 1000 cd m−2/at 5000 cd m−2.
f The EL emission wavelength at the maximum intensity. m−2.
d The maximum luminance.
e CE at the maximum value/at 1000 cd m−2/at 5000 cd m−2.
f The EL emission wavelength at the maximum intensity.
|
||||||
| A-1 | DpAn-5BzAc | — | 4.7 | 9218 | 21.8/20.6/15.7 | 524 |
| A-2 | mCP | — | 6.2 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 860 860 |
24.0/23.8/22.3 | 520 |
| A-3 | PVK | — | 5.6 | 6891 | 15.3/10.7/5.8 | 524 |
| B-1 | mCP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DpAn-5BzAc DpAn-5BzAc |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4.7 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
31.7/31.1/30.9 | 520 |
| B-2 | PVK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DpAn-5BzAc DpAn-5BzAc |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
4.5 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 940 940 |
28.1/27.9/25.6 | 520 |
| B-3 | PVK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mCP mCP |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6.2 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 410 410 |
19.5/19.2/18.6 | 520 |
| C-1 | mCP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DpAn-5BzAc DpAn-5BzAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TCTA TCTA |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5 4.5 |
4.4 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 320 320 |
40.9/40.6/36.5 | 520 |
| C-2 | PVK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DpAn-5BzAc DpAn-5BzAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TCTA TCTA |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5 4.5 |
4.4 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110 110 |
38.4/38.2/36.6 | 520 |
| C-3 | PVK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mCP mCP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TCTA TCTA |
2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2 2.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5 4.5 |
4.3 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 080 080 |
29.3/28.8/25.0 | 520 |
All devices exhibited bright green emission, and the CIE coordinates were almost the same at (0.30, 0.63), which correspond to the emission of the Ir(ppy)2acac dopant. The bipolar transport characteristic of DpAn-5BzAc has effectively reduced the turn-on voltage (Von) for Device A with the lowest Von of 4.7 V. Device A-2 with the mCP host exhibited the highest efficiency of 24.01 cd A−1 and the maximum luminance of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 860 cd m−2 compared with the DpAn-5BzAc based device (Device A-1) which showed a maximum CE of 21.84 cd A−1. The excellent overlap between the absorption spectrum of the Ir(ppy)2acac dopant and the PL spectrum of the host mCP theoretically results in the most efficient Förster resonance energy transfer and high efficiency. Given a larger overlap spectrum between PVK and Ir(ppy)2acac than that of DpAn-5BzAc and Ir(ppy)2acac, the efficiency of Device A-1 should be the lowest among all the single host based devices. However, when the host mCP or DpAn-5BzAc was replaced by the most widely used polymer host PVK, the Device A-3 showed significantly lower efficiency than Devices A-1 and A-2, with the maximum CE reduced to 15.28 cd A−1 (as shown in Table 1). It can be understood that the bipolar transport and TADF property with the high PL quantum yields (thin film: 76.2%) of DpAn-5BzAc had a positive effect on alleviating the triplet–triplet exciton quenching and afforded more efficient energy transfer properties in the EML. We noticed that the turn-on voltage of 4.7 V in Device A-1 with the DpAn-5BzAc host was much lower than those of mCP and PVK based devices, which could be ascribed to the more efficient hole-injection to reduce the driving voltages in Device A-1.43 As depicted in the energy level diagram of Fig. 1, the highest occupied molecular orbital (HOMO) levels of mCP and PVK are 5.9 eV and 5.8 eV, which were located about 0.3 and 0.2 eV higher than that of DpAn-5BzA, thus the hole-injection barrier from the hole-injection layer of PEDOT:PSS (5.2 eV) to the host in the emissive layer in Device A-1 with the DpAn-5BzAc host was only 0.4 eV, lower than the 0.7 eV and 0.6 eV in mCP and PVK based devices.
860 cd m−2 compared with the DpAn-5BzAc based device (Device A-1) which showed a maximum CE of 21.84 cd A−1. The excellent overlap between the absorption spectrum of the Ir(ppy)2acac dopant and the PL spectrum of the host mCP theoretically results in the most efficient Förster resonance energy transfer and high efficiency. Given a larger overlap spectrum between PVK and Ir(ppy)2acac than that of DpAn-5BzAc and Ir(ppy)2acac, the efficiency of Device A-1 should be the lowest among all the single host based devices. However, when the host mCP or DpAn-5BzAc was replaced by the most widely used polymer host PVK, the Device A-3 showed significantly lower efficiency than Devices A-1 and A-2, with the maximum CE reduced to 15.28 cd A−1 (as shown in Table 1). It can be understood that the bipolar transport and TADF property with the high PL quantum yields (thin film: 76.2%) of DpAn-5BzAc had a positive effect on alleviating the triplet–triplet exciton quenching and afforded more efficient energy transfer properties in the EML. We noticed that the turn-on voltage of 4.7 V in Device A-1 with the DpAn-5BzAc host was much lower than those of mCP and PVK based devices, which could be ascribed to the more efficient hole-injection to reduce the driving voltages in Device A-1.43 As depicted in the energy level diagram of Fig. 1, the highest occupied molecular orbital (HOMO) levels of mCP and PVK are 5.9 eV and 5.8 eV, which were located about 0.3 and 0.2 eV higher than that of DpAn-5BzA, thus the hole-injection barrier from the hole-injection layer of PEDOT:PSS (5.2 eV) to the host in the emissive layer in Device A-1 with the DpAn-5BzAc host was only 0.4 eV, lower than the 0.7 eV and 0.6 eV in mCP and PVK based devices.
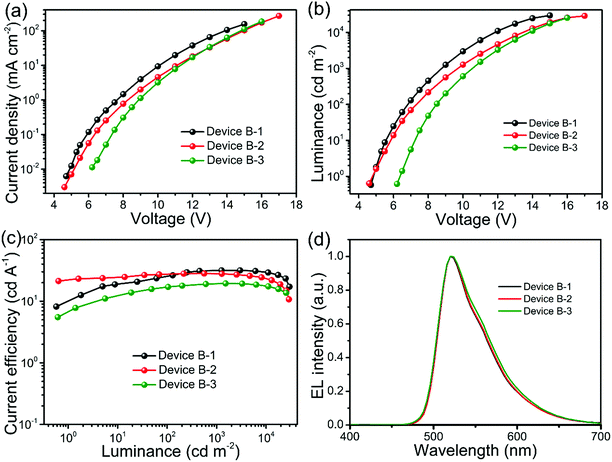
3.3 Performance of solution-processed PhOLEDs based on binary mixed hosts
The performances of solution-processed green PhOLEDs based on binary mixed hosts are also depicted in Fig. 4 for a better comparison. The typical values of binary mixed host devices are summarized in Table 1. The EQE–L and PE–L characteristics of the binary host based devices are illustrated in Fig. S2 (ESI†), and the optimization result of the binary mixed host based devices is illustrated in Fig. S3 (ESI†). It is observed that Device B-1 employing the mCP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DpAn-5BzAc (2
DpAn-5BzAc (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) binary co-host demonstrates superior EL performance compared to Devices B-2 and B-3 employing the PVK
1, w/w) binary co-host demonstrates superior EL performance compared to Devices B-2 and B-3 employing the PVK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DpAn-5BzAc (1
DpAn-5BzAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, w/w) and PVK
2, w/w) and PVK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mCP (1
mCP (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) hosts. As it is shown, the EL performance of Device B-1 demonstrates simultaneously the highest luminance of 29
1, w/w) hosts. As it is shown, the EL performance of Device B-1 demonstrates simultaneously the highest luminance of 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 cd m−2, a high CE of 31.7 cd A−1, along with a green emission peak at 520 nm, and also displays a low turn-on voltage at 4.7 V. These results indicate that Device B-1 is favorable for achieving efficient charge injection and transport, which is analogous to that of the PhOLED counterpart based on the evaporated bulk binary co-host.44 In contrast, Device B-2 suffers from poor diode behavior although its turn-on voltage is as low as 4.5 V compared with the single host devices. In particular, the driving voltage of Device B-3 is shifted to as high as 6.2 V along with distinctly high driving voltages for a given practical luminance (e.g. 8.4 V at 100 cd m−2 and 10.5 V at 1000 cd m−2), suggesting the severely hampered charge injection and/or transport in the device.45
700 cd m−2, a high CE of 31.7 cd A−1, along with a green emission peak at 520 nm, and also displays a low turn-on voltage at 4.7 V. These results indicate that Device B-1 is favorable for achieving efficient charge injection and transport, which is analogous to that of the PhOLED counterpart based on the evaporated bulk binary co-host.44 In contrast, Device B-2 suffers from poor diode behavior although its turn-on voltage is as low as 4.5 V compared with the single host devices. In particular, the driving voltage of Device B-3 is shifted to as high as 6.2 V along with distinctly high driving voltages for a given practical luminance (e.g. 8.4 V at 100 cd m−2 and 10.5 V at 1000 cd m−2), suggesting the severely hampered charge injection and/or transport in the device.45
 | ||
| Fig. 4 Comparison of (a) J–V, (b) L–V, (c) CE–L and (d) the corresponding EL spectra of binary host based devices. | ||
It is clearly seen that the device containing the PVK host (Devices B-2 and B-3) shows distinctly low current density and efficiency as compared to its counterpart device without the PVK host (Devices B-1). In order to determine the reason for these drastic differences which depend on the various binary host based emitting layers, thermodynamic analysis was first performed to investigate the intrinsic miscibility between PVK and mCP or DpAn-5BzAc. The relative surface free energies of these three materials, i.e., PVK, mCP and DpAn-5BzAc, were calculated with the contact angle according to the Young equation and Owens equation and are listed in Table 2. The contact angles (CAs) of the various hosts deposited on a glass substrate are shown in Table S1 (ESI†). As reported, the interfacial energy, γ1/2, between materials 1 and 2 was calculated by using the following formalism:46
3.4 Performance of solution-processed PhOLEDs based on ternary blend hosts
Based on these results, we also fabricated the ternary blend host based devices with a configuration of ITO/PEDOT:PSS (40 nm)/ternary blend host: 10 wt% Ir(ppy)2acac (20 nm)/TmPyPB:TPBi (40 nm)/Liq (1 nm)/Al, where the optimized ternary blend hosts are mCP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DpAn-5BzAc
DpAn-5BzAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TCTA (3
TCTA (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5, w/w/w) (Device C-1), PVK
4.5, w/w/w) (Device C-1), PVK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DpAn-5BzAc
DpAn-5BzAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TCTA (1.5
TCTA (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5, w/w/w) (Device C-2) and PVK
4.5, w/w/w) (Device C-2) and PVK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mCP
mCP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TCTA (2.3
TCTA (2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2
2.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
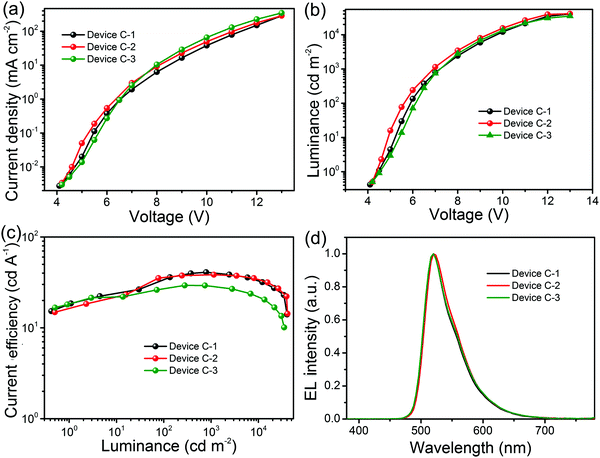
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5, w/w/w) (Device C-3), respectively. The key EL data of the ternary blended host based devices are shown in Table 1. The corresponding characteristic curves are displayed in Fig. 5. The EQE–L and PE–L characteristics of the ternary blend host based devices are illustrated in Fig. S5 (ESI†), and the optimization result of the ternary mixed host based devices is illustrated in Fig. S6 (ESI†).
4.5, w/w/w) (Device C-3), respectively. The key EL data of the ternary blended host based devices are shown in Table 1. The corresponding characteristic curves are displayed in Fig. 5. The EQE–L and PE–L characteristics of the ternary blend host based devices are illustrated in Fig. S5 (ESI†), and the optimization result of the ternary mixed host based devices is illustrated in Fig. S6 (ESI†).
The ET of TCTA is 2.79 eV, higher than that of Ir(ppy)2acac and the concentration of TCTA was kept at 45 wt% in the emitters with a concentration of 10 mg ml−1. All the ternary mixed host based devices show low turn-on voltages of 4.3–4.4 V compared to the single and binary host based devices with the same green light emission peak at 520 nm. Device C-1 with the EML of mCP:DpAn-5BzAc:TCTA:Ir(ppy)2acac shows the best EL performance with maximum luminance and CE values of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 320 cd m−2 and 40.88 cd A−1, respectively, which are comparable to those of the evaporated devices. Furthermore, the efficiency roll-off of Device C-1 is considerably small with a roll-off value of only 0.7% and 10.8% at the brightness of 1000 and 5000 cd m−2, respectively. The HOMO level of TCTA, which is 0.2 eV and 0.1 eV lower than that of mCP and PVK, may improve the hole injection into the EML and reduce the voltage. More importantly, the glass transition temperature of TCTA is 151 °C, which is helpful to improve the quantum efficiency by stabilizing the morphology of the EML.48 After adding TCTA as the third component in the emission layer, similarly enhanced EL performances can also be observed in Devices C-2 and C-3. The RMS roughness value is 1.67 nm for mCP:DpAn-5BzAc:TCTA:Ir(ppy)2acac films (Fig. S4d, ESI†). The increased RMS value after adding the third component TCTA is mainly attributed to the large interfacial energy between TCTA and mCP or DpAn-5BzAc, (γTCTA/mCP and γTCTA/DpAn-5BzAc were calculated to be 48.95 mJ m−2 and 26.56 mJ m−2, respectively). To explain the enhanced efficiency in the ternary system containing TCTA as the third host, we used the GISAXS technique to probe nanostructures formed in the mCP:DpAn-5BzAc:TCTA:Ir(ppy)2acac EML and compared them with that in the binary blend films. Fig. 6 presents the two-dimensional (2D) GISAXS images of the mixed thin films and also line-cut profiles along the in-plane direction, and also the corresponding 2D GISAXS image is shown in Fig. S7 (ESI†). For these binary and ternary mixed films, their profiles present a similar line shape, and the first-order scattering peak is about ±1.4 Å, and the domain spacing is about 4.49 Å. When TCTA was blended into the mCP:DpAn-5BzAc:Ir(ppy)2acac based EML, two high order peaks appear at about ±2.5 Å, indicating that nano-organized domains were formed with the help of TCTA. This could contribute to the increase in the device efficiency using a ternary system.
320 cd m−2 and 40.88 cd A−1, respectively, which are comparable to those of the evaporated devices. Furthermore, the efficiency roll-off of Device C-1 is considerably small with a roll-off value of only 0.7% and 10.8% at the brightness of 1000 and 5000 cd m−2, respectively. The HOMO level of TCTA, which is 0.2 eV and 0.1 eV lower than that of mCP and PVK, may improve the hole injection into the EML and reduce the voltage. More importantly, the glass transition temperature of TCTA is 151 °C, which is helpful to improve the quantum efficiency by stabilizing the morphology of the EML.48 After adding TCTA as the third component in the emission layer, similarly enhanced EL performances can also be observed in Devices C-2 and C-3. The RMS roughness value is 1.67 nm for mCP:DpAn-5BzAc:TCTA:Ir(ppy)2acac films (Fig. S4d, ESI†). The increased RMS value after adding the third component TCTA is mainly attributed to the large interfacial energy between TCTA and mCP or DpAn-5BzAc, (γTCTA/mCP and γTCTA/DpAn-5BzAc were calculated to be 48.95 mJ m−2 and 26.56 mJ m−2, respectively). To explain the enhanced efficiency in the ternary system containing TCTA as the third host, we used the GISAXS technique to probe nanostructures formed in the mCP:DpAn-5BzAc:TCTA:Ir(ppy)2acac EML and compared them with that in the binary blend films. Fig. 6 presents the two-dimensional (2D) GISAXS images of the mixed thin films and also line-cut profiles along the in-plane direction, and also the corresponding 2D GISAXS image is shown in Fig. S7 (ESI†). For these binary and ternary mixed films, their profiles present a similar line shape, and the first-order scattering peak is about ±1.4 Å, and the domain spacing is about 4.49 Å. When TCTA was blended into the mCP:DpAn-5BzAc:Ir(ppy)2acac based EML, two high order peaks appear at about ±2.5 Å, indicating that nano-organized domains were formed with the help of TCTA. This could contribute to the increase in the device efficiency using a ternary system.
 | ||
| Fig. 6 In-plane GISAXS profiles of the binary and ternary mixed thin films deposited on the glass substrate. | ||
4. Conclusion
In summary, we use the mixed host small molecular materials mCP and DpAn-5BzAc blended with TCTA to improve the hole-injection ability, along with Ir(ppy)2acac as a green dopant to constitute the emission layer for the fabrication of solution-processed green PhOLEDs. The device gives high efficiency with a maximum luminance of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 320 cd m−2, a peak CE of 40.88 cd A−1 and low efficiency roll-offs of 40.6 and 36.5 cd A−1 at 1000 and 5000 cd m−2, with the corresponding efficiency roll-off of only 0.7% and 10.8%, respectively, which is the best recorded performance among the reported solution-processed green PhOLEDs. The improved overall performance of the mixed host device is realized by fine-tuning the miscibility in the solution-processed EML, enhancing the charge carrier balance, and improving the structural order in the film. This work demonstrates a simple way by using the mixed host structure for the fabrication of high-performance solution-processed PhOLEDs.
320 cd m−2, a peak CE of 40.88 cd A−1 and low efficiency roll-offs of 40.6 and 36.5 cd A−1 at 1000 and 5000 cd m−2, with the corresponding efficiency roll-off of only 0.7% and 10.8%, respectively, which is the best recorded performance among the reported solution-processed green PhOLEDs. The improved overall performance of the mixed host device is realized by fine-tuning the miscibility in the solution-processed EML, enhancing the charge carrier balance, and improving the structural order in the film. This work demonstrates a simple way by using the mixed host structure for the fabrication of high-performance solution-processed PhOLEDs.
Author contributions
J. Z. and Y. G. performed device optimization as well as measurements with assistance from J. Y., Z. L., H. L., and W. L., W. H. assisted with the GISAXS measurements, and Y. L. acquired and analyzed in situ GISAXS. W.-Y. W. and B. W. supervised this project and prepared the manuscript with assistance from co-author S. W. All authors have approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Project of the National Science Foundation of China (grant numbers 51725505 and 61775130). The authors acknowledge beamline BL16B1 at the Shanghai Synchrotron Radiation Facility for providing beam time. W.-Y. W. thanks the Areas of Excellence Scheme of the University Grants Committee of HKSAR (AoE/P-03/08), the Hong Kong Polytechnic University (1-ZE1C) and Ms Clarea Au (847S) for financial support.References
- Y. Q. Miao, K. X. Wang, B. Zhao, L. Gao, P. Tao, X. G. Liu, Y. Y. Hao, H. Wang, B. S. Xu and F. R. Zhu, Nanophotonics, 2018, 7, 295–304 CAS.
- S. L. Wang, M. Y. Qiao, Z. H. Ye, D. H. Dou, M. Y. Chen, Y. Peng, Y. Shi, X. Y. Yang, L. Cui, J. Y. Li, B. Wei and W. Y. Wong, iScience, 2018, 9, 532–541 CrossRef CAS PubMed.
- C. M. Han, Z. Zhang, D. X. Ding and H. Xu, Chem, 2018, 4, 2154–2167 CAS.
- S. L. Wang, J. L. Yang, T. Xu, D. H. Dou, Z. Y. Tang, Z. X. Gao, M. Y. Chen, K. P. Guo, J. S. Yu, J. Plaind, R. Bachelot, J. H. Zhang and B. Wei, Org. Electron., 2019, 64, 146–153 CrossRef CAS.
- X. Liu, J. Rao, X. Li, S. Wang, J. Ding and L. Wang, iScience, 2019, 15, 147–155 CrossRef CAS PubMed.
- M. Sarma, W. L. Tsai, W. K. Lee, Y. Chi, C. C. Wu, S. H. Liu, P. T. Chou, K. T. Wong and K. T. Wong, Chem, 2017, 3, 461–476 CAS.
- K. O. Kirlikovali and A. M. Spokoyny, Chem, 2017, 3, 385–387 CAS.
- S. M. Wang, L. Zhao, B. H. Zhang, J. Q. Ding, Z. Y. Xie, L. X. Wang and W. Y. Wong, iScience, 2018, 6, 128–137 CrossRef CAS PubMed.
- D. L. Zhao, C. C. Huang, X. Y. Liu, B. Song, L. Ding, M. K. Fung and J. Fan, Org. Electron., 2018, 62, 542–547 CrossRef CAS.
- B. N. Patil, J. J. Lade, K. S. Vadagaonkar, P. Chetti and A. C. Chaskar, ChemistrySelect, 2018, 3, 10010–10018 CrossRef CAS.
- S. W. Liu, Y. T. Chang, C. C. Lee, C. H. Yuan, L. A. Liu, Y. S. Chen, C. F. Lin, C. I. Wu and C. T. Chen, Jpn. J. Appl. Phys., 2012, 52, 012101 CrossRef.
- N. L. Liu, N. Ai, D. G. Hu, S. F. Yu, J. B. Peng, Y. Cao and J. Wang, Acta Phys. Sin., 2011, 60, 087805 Search PubMed.
- S. Olivier, E. Ishow, S. M. Della-Gatta and T. Maindron, Org. Electron., 2017, 49, 24–32 CrossRef CAS.
- K. Kim, G. Kim, B. R. Lee, S. Ji, S. Y. Kim, B. W. An, M. H. Song and J. U. Park, Nanoscale, 2015, 7, 13410–13415 RSC.
- M. Zhu, T. Ye, X. He, X. Cao, C. Zhong, D. Ma, J. Qiu and C. Yang, J. Mater. Chem., 2011, 21, 9326–9331 RSC.
- S. H. Liu, M. S. Lin, L. Y. Chen, Y. H. Hong, C. H. Tsai, C. C. Wu, A. Poloek, Y. Chi, C. A. Chen, S. H. Chen and H. F. Hsu, Org. Electron., 2011, 12, 15–21 CrossRef CAS.
- T. Zhu and T. V. Voorhis, J. Phys. Chem. C, 2016, 120, 19987–19994 CrossRef CAS.
- T. Basel, D. Sun, B. Gautam and Z. V. Vardeny, J. Lumin., 2014, 155, 89–94 CrossRef CAS.
- W. Jiang, J. Tang, X. Ban, Y. Sun, L. Duan and Y. Qiu, Org. Lett., 2014, 16, 5346–5349 CrossRef CAS PubMed.
- C. H. Yang, S. H. Yang and C. S. Hsu, Nanotechnology, 2009, 20, 315601 CrossRef PubMed.
- I. Venegoni, F. Carniato, F. Olivero, C. Bisio, N. L. Pira, V. G. Lambertini and L. Marchese, Nanotechnology, 2012, 23, 435702 CrossRef PubMed.
- J. H. Park, C. H. Yun, T. W. Koh, Y. Do, S. Yoo and M. H. Lee, J. Mater. Chem., 2011, 21, 5422–5429 RSC.
- J. Y. Li, T. Zhang, Y. J. Liang and R. X. Yang, Adv. Funct. Mater., 2013, 23, 619–628 CrossRef CAS.
- C. Mitsui, H. Tanaka, H. Tsuji and E. Nakamura, Chem. – Asian J., 2011, 6, 2296–2300 CrossRef CAS PubMed.
- S. Reineke, K. Walzer and K. Leo, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 125328 CrossRef.
- Z. Liu, Z. F. Tang, A. Liu, G. Y. Meng, X. R. Yuan, H. J. Tang, D. H. Hu, Z. Q. Xie, J. B. Wang and L. T. Hou, J. Lumin., 2018, 195, 31–39 CrossRef CAS.
- S. M. Wang, X. D. Wang, B. Yao, B. H. Zhang, J. Q. Ding, Z. Y. Xie and L. X. Wang, Sci. Rep., 2015, 5, 12487 CrossRef CAS PubMed.
- Y. J. Doh, J. S. Park, W. S. Jeon, R. Pode and J. H. Kwon, Org. Electron., 2012, 13, 586–592 CrossRef CAS.
- N. Aizawa, Y. J. Pu, M. Watanabe, T. Chiba, K. Ideta, N. Toyota, M. Igarashi, Y. Suzuri, H. Sasabe and J. Kido, Nat. Commun., 2014, 5, 5756 CrossRef PubMed.
- D. M. Sun, X. K. Zhou, J. T. Liu, X. L. Sun, H. H. Li, Z. J. Ren, D. G. Ma, M. R. Bryce and S. K. Yan, ACS Appl. Mater. Interfaces, 2015, 7, 27989–27998 CrossRef CAS PubMed.
- Y. F. Chang, C. H. Yu, S. C. Yang, I. H. Hong, S. C. Jiang, H. F. Meng, H. Huang, H. W. Zan and S. F. Horng, Org. Electron., 2017, 42, 75–86 CrossRef CAS.
- S. W. Liu, Y. T. Chang, C. C. Lee, C. H. Yuan, L. A. Liu, Y. S. Chen, C. F. Lin, C. I. Wu and C. T. Chen, Jpn. J. Appl. Phys., 2012, 52, 012101 CrossRef.
- C. C. Lee, C. H. Yuan, S. W. Liu, L. A. Liu and Y. S. Chen, J. Disp. Technol., 2011, 7, 636–639 CAS.
- Y. J. Doh, J. S. Park, W. S. Jeon, R. Pode and J. H. Kwon, Org. Electron., 2012, 13, 586–592 CrossRef CAS.
- Y. Tao, X. Guo, L. Hao, R. F. Chen, H. H. Li, Y. H. Chen, X. W. Zhang, W. Y. Lai and W. Huang, Adv. Mater., 2015, 27, 6939–6944 CrossRef CAS PubMed.
- Y. Chen, X. Wei, J. Cao, J. H. Huang, L. Gao, J. H. Zhang, J. H. Su and H. Tian, ACS Appl. Mater. Interfaces, 2017, 9, 14112–14119 CrossRef CAS PubMed.
- H. Ye, H. Y. Wu, L. Y. Chen, S. H. Ma, K. F. Zhou, G. B. Yan, J. Z. Shen, D. C. Chen and S. J. Su, Electron. Mater. Lett., 2018, 14, 89–100 CrossRef CAS.
- B. Yao, X. D. Lin, B. H. Zhang, H. L. Wang, X. J. Liu and Z. Y. Xie, J. Mater. Chem. C, 2018, 6, 4409–4417 RSC.
- K. H. Kim, J. Y. Lee, T. J. Park, W. S. Jeon, G. P. Kennedy and J. H. Kwon, Synth. Met., 2010, 160, 631–635 CrossRef CAS.
- D. Dong, J. C. Xia, S. Yang, X. K. Wu, B. W. Wei, L. Lian, D. X. Feng, Y. X. Zheng and G. F. He, Org. Electron., 2016, 38, 29–34 CrossRef CAS.
- Z. H. Ye, Z. T. Ling, M. Y. Chen, J. L. Yang, S. L. Wang, Y. Q. Zheng, B. Wei, C. Li, G. Chen and Y. Shi, RSC Adv., 2019, 9, 6881–6889 RSC.
- R. J. Holmes, S. R. Forrest, Y. J. Tung, R. C. Kwong, J. J. Brown, S. Garon and M. E. Thompson, Appl. Phys. Lett., 2003, 82, 2422–2424 CrossRef CAS.
- M. Vasilopoulou, G. Papadimitropoulos, L. C. Palilis, D. G. Georgiadou, P. Argitis, S. Kennou, I. Kostis, N. Vourdasa and D. Davazoglou, Org. Electron., 2012, 13, 796–806 CrossRef CAS.
- J. H. Lee and J. J. Kim, Phys. Status Solidi A, 2012, 209, 1399–1413 CrossRef CAS.
- S. M. Wang, X. D. Wang, B. Yao, B. H. Zhang, J. Q. Ding, Z. Y. Xie and L. X. Wang, Sci. Rep., 2015, 5, 12487 CrossRef CAS PubMed.
- S. Wu, J. Adhes., 1973, 5, 39–55 CrossRef CAS.
- L. Liu, B. Zhang, Z. Xie, J. Ding and L. Wang, Org. Electron., 2013, 14, 55–61 CrossRef CAS.
- Z. L. Jiang, W. Tian, Z. Q. Kou, S. Cheng and Y. H. Li, Opt. Commun., 2016, 372, 49–52 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9tc02701g |
| This journal is © The Royal Society of Chemistry 2019 |