Classification of MAOX phases and semiconductor screening for next-generation energy conversion ceramic materials†
Zhenyu
Wang‡
,
Xin
Chen‡
 * and
Chunming
Niu
*
* and
Chunming
Niu
*
Center of Nanomaterials for Renewable Energy (CNRE), State Key Lab of Electrical Insulation and Power Equipment, School of Electrical Engineering, Xi’an Jiaotong University, 99 Yanxiang Road, Xi’an 710054, China. E-mail: xin.chen.nj@xjtu.edu.cn; cniu@xjtu.edu.cn
First published on 13th April 2019
Abstract
MAOX semiconductors, a family of layered oxycarbides, have recently been discovered [Z. Wang, X. Chen and C. Niu, J. Phys. Chem. C, 2018, 122, 14240–14247]. Spectroscopic limited maximum efficiency (SLME) calculations show that MAOX semiconductors can have fantastic theoretical efficiencies. In particular, four MAOX semiconductors, La2CdS2C, La2HgS2C, Cr2MgO2C, and Cr2ZnO2C, are identified to be excellent solar absorbers with maximum efficiencies of 30% and above. To classify 2121 MAOX phases and screen semiconductors, a computational screening scheme is proposed. The SLME, a/c ratio, electron affinity (EA) and ionization potential (IP)/work function (WF) are found to be good descriptors for the design of MAOX absorber layers. Linear relationships are found between the a/c ratios and fundamental bandgaps with fixed M and O. The electronic properties of MAOX can be easily tuned with different metal cations and chalcogen anions. Additionally, the band alignment of MAOX is determined by A's EA and WF.
1. Introduction
The search for novel energy materials is critically important to a renewable energy future. Currently, identifying novel functional energy materials computationally will transform the materials industry and define the renewable/clean energy future.1–4 Ceramic materials have recently found a broad range of applications in energy conversion, storage, and distribution systems.5–7 Interest in multifunctional ceramics has been renewed in recent years.6,8,9 Oxycarbide/oxynitride materials exhibit superior properties10–12 and find a wide range of applications.13,14 We recently have discovered a new family of layered hexagonal oxycarbides and oxynitrides, MAOX, which are excellent emerging semiconductors.15 These materials have high electricity-to-light efficiencies and exhibit flexibly tunable electronic properties. To our best knowledge, such outstanding PV performance has never been discovered among the oxycarbides and oxynitrides. However, there are more MAOX semiconductors beyond the five materials reported15 to be explored.Traditionally, the discovery of novel materials in most cases was achieved by enormous trial-and-error experiments.16–18 However, unlike the highly costly and time-consuming synthesis-based approach, discovering materials computationally based on density functional theory (DFT) is much cost-effective to perform high-throughput (HT) screening among a tremendous amount of possible element combinations, structures, and configurations.19–21 The rapid development of supercomputers and theoretical methods, such as machine learning and big data mining, can further improve the screening process.1,3,22–26 Identifying good descriptors correlated with targeted properties is key to implementing effective HT screening.3
As a quaternary system, MAOX phases provide more flexibility for tuning in comparison to binary and ternary materials. MAOX phases have representative hexagonal close-packed structures; therefore, what we learn in MAOX can be transferred to the study of other materials with hexagonal structures. Also, MAOX phases can be metals, semi-metals, and semiconductors.
2. Screening procedure
In this paper, ninety 2121 MAOX phases15 are used to learn the correlations between the descriptors and target properties as solar absorbers. Fifty-three new MAOX semiconductors are identified and labeled with the screening scheme in Fig. 1 based on hybrid density functional theory in consideration of stabilities, electronic properties, and optical properties. The details are provided in Section S1 (ESI†). Oxycarbide and sulcarbide MAOX phases are generated according to the layered hexagonal structures with cations and anions from the allowed elements in the periodic table.15 M is chosen from early transition metals, and A from alkaline earth metals in Group IIA or late transition metals in Groups IB and IIB. O is either O or S and X is either C or N. To our knowledge, the MAX phases and MXenes (parents of MAOX) containing N are all metallic. Our calculations show all the representative nitride MAOX phases are metallic. As a result, N will not be considered. A detailed discussion can be found in Section S2 (ESI†), and the corresponding band structures are presented in Fig. S1 (ESI†). Additionally, our previous study15 shows that MAOX with M = Sc, Y, La, Cr, and Mo are likely to be semiconducting. To make the screening more effective, the M component is limited to Sc, Y, La, Cr, and Mo, and N is excluded from the procedure. In the following sections, the ninety 2121 MAOX phases are grouped into ten categories from the Sc, Y, La, Cr, and Mo-based (five) series of oxycarbide MAOX and the same five series of sulcarbide MAOX.The thermal stability is evaluated in terms of the formation of energy with respect to a set of competing phases. The competing phases are chosen from the possible precursors for the fabrication of MAOX. The competing phases for the calculations of formation energies are listed in Table S1 (ESI†). The hybrid HSE06 functional is used to obtain an accurate description of the electronic properties. The absorption spectrum and SLME are obtained to screen whether compounds can be promising solar absorbers. In addition, we further calculate the open-circuit voltages (Voc), electron affinities (EAs) and ionization potentials (IPs) for the screened semiconductors, and the work functions (WFs) for those with no bandgaps.
3. Results and discussion
3.1. Structure and electronic properties
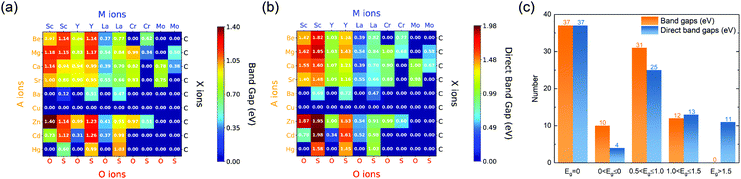
The formation energies and the corresponding competing phases of the oxycarbide and sulcarbide MAOX, with all possible combinations of atoms, are listed in Table S1 (ESI†). The negative formation energies indicate that they are thermally stable. The band structure based on HSE06 is used to determine whether a MAOX phase is a semiconductor or not. The distributions of fundamental bandgaps (the smaller of direct and indirect bandgaps) and indirect bandgaps are shown in Fig. 2a and b. The histogram of bandgaps Eg is exhibited in Fig. 2c, where fundamental bandgaps are grouped into five ranges. The detailed lattice parameters and fundamental bandgaps are summarized in Table S2 (ESI†). One-third of the 2121 MAOX phases are metallic with no bandgaps. The remaining two-thirds of MAOX are semiconductors with the fundamental bandgaps ranging from 0.12 to 1.40 eV and the direct bandgaps from 0.34 to 1.98 eV. About a quarter of the MAOX semiconductors have bandgaps in the range of 1.0 to 1.5 eV, which is desirable for potential solar absorbers since the maximum PV conversion efficiency is around the direct-allowed bandgap of 1.3 eV according to the Shockley–Queisser (SQ) limit.27,28Fig. 2 shows that MAOX semiconductors are generally indirect, while the direct ones are all La-based. In addition, that an oxycarbide MAOX phase is semiconducting does not imply the corresponding sulcarbide MAOX phase is semiconducting and vice versa. It is found that the Sc-, Y-, and La-based series of MAOX mostly are semiconductors in Fig. S2 (ESI†). In particular, the Sc- and Y-based series of MAOX have more flexibility in bandgap selections to tune and optimize the efficiency of the MAOX semiconductor. According to the bandgaps in Fig. S2 (ESI†), the La-, Cr- and Mo-based series have narrow distributions from 0.34 to 1.03. Therefore they are not suitable to tune bandgaps. Also when A = Cu, MAOX are metallic.
The band structures and densities of states (DOS) of the ninety MAOX compositions are plotted in Fig. S3–S12 (ESI†) to help us in understanding the electronic properties of the Sc-, Y-, La-, Cr- and Mo-based series of MAOX. MAOX semiconductors generally have curvy conduction bands and flat valence bands, indicating high electron mobility and poor hole mobility. According to our previous study,15 both the valence band maximum (VBM) and the conduction band minimum (CBM) mainly consist of M-derived and O-derived states, followed by X-derived ones, while the contribution from A's orbitals can be ignored, suggesting that the M component determines the bandgap. On the other hand, it is observed that the fundamental bandgaps of MAOX consisting of the same compositions of M, O, and X vary with A compounds. This observation makes us assume that the tailoring of bandgaps could be achieved by changing the A species for geometrical reasons. However, there are no obvious distinguishing features in the valence and conduction bands between semiconducting and metallic MAOX. The origin of the metallic MAOX remains unclear and requires further investigation in the future.
3.2. Absorbers for solar cells
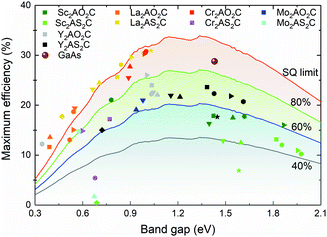
The SQ limit efficiencies are associated with the efficiencies of solar absorbers with fundamental bandgaps.27 However, the bandgap is not the sole factor associated with the PV performance, the absorption coefficient of the material, the thickness of the sample, and electron and hole recombination also play important roles. Beyond the SQ limit, SLME will be a suitable descriptor correlating with PV performance that considers all the factors mentioned above.29 The SLME with a film thickness of 0.2 μm is plotted versus the direct-allowed gap for fifty-three MAOX semiconductors in Fig. 3. It is found that there are a series of compounds having SLME values above the SQ limit. To understand this issue, a previously published paper37 could help. Accordingly, using SLME calculations, ten MAOX semiconductors with maximum efficiencies larger than 25.0% are selected. Importantly, their efficiencies are all above 80% of the SQ limit, indicating that they can utilize solar energy very efficiently. These ten MAOX semiconductors have sharp absorption onsets and large absorption coefficients (α) greater than 104 cm−1 near the band edge (Fig. S13, ESI†), and thus they exhibit high conversion efficiencies.Surprisingly, La2CdS2C, La2HgS2C, Cr2MgO2C and Cr2ZnO2C with direct bandgaps of 0.98, 1.03, 1.00 and 0.99 eV exhibit the best PV performance among all MAOX semiconductors, whose SLMEs are 30.41%, 30.88%, 30.77% and 30.28%, respectively, higher than the power conversion efficiency (PCE) of GaAs.30 La2CdS2C and La2HgS2C are direct bandgap semiconductors and show strong absorption across the visible light region and as such exhibit high efficiencies. Likewise, although both Cr2MgO2C and Cr2ZnO2C have indirect bandgaps, the corresponding direct bandgaps are 0.02 eV larger than the fundamental ones, and their bandgaps can be considered as “near-direct”. For this reason, the conversion loss due to non-radiation recombination can be neglected. In addition to their strong absorption, the efficiencies of Cr2MgO2C and Cr2ZnO2C are also high.
3.3. Descriptors for inverse design
In double-layer or multilayer systems, such as BN, MoS2, WSe2, and phosphorene, interlayer distance enlargement will widen the fundamental bandgaps.31–34 Hypothetically, a similar effect can be found in MAOX semiconductors given that MAOX geometrically are combinations of alternatively arranged oxycarbide/sulcarbide layers (AO2) and MXene layers (MX2).15 If there is evidence to support this hypothesis, we can tune the bandgaps by adjusting the distances between the adjacent MX2 through the alternations of AO2 layers.
In order to confirm this hypothesis, the relationships between the fundamental bandgaps and possible geometrical descriptors, such as a, c, a/c ratios, cell volumes, and interlayer distances, are explored. It is found that the a/c ratio is the suitable descriptor to correlate with the bandgap. The ninety 2121 MAOX phases are categorized according to M, A, O, AO and MO. The relationships between the bandgaps and a/c ratios are visualized for each category in Fig. S14 (ESI†). In most cases, Fig. S14 (ESI†) shows that the oxycarbide and sulcarbide MAOX demonstrate very different patterns since the sulcarbide MAOX generally possess smaller a/c ratios and have wider bandgaps.
In the Sc, Y, La, Cr, and Mo-based (five) series of oxycarbide MAOX or sulcarbide MAOX, the bandgap demonstrates a near-linear relationship with the a/c ratio as shown in Fig. 5a and b. To further examine the relationship, Fig. 5b is further separated into Fig. 5c for the oxycarbide MAOX and Fig. 5d for the sulcarbide MAOX. Interestingly, the majority of data generally follow a near-linear relationship in the MAOX phases with the ScO, ScS, YS, LaS, MoO, and MoS compounds as shown by the colored solid lines in Fig. 5c and d. However, the LaO-based series shows a negative near-linear relationship as shown by the green dashed line in Fig. 5c. We thought that the positive relationship might be available over a specific range of a/c ratios.
To further confirm the positive linear relationship for the ScO-, ScS-, YS-, LaS-, MoO- and MoS-based series, a computational test is carried out by manually adjusting the a/c ratio of the lattice. With the same cell volumes and fractional coordinates of each atom, different a/c ratios were produced for the ten representative oxycarbide and sulcarbide MAOX semiconductors. The target a/c ratios are maximum 10% larger or smaller than the equilibrium ones. In Fig. S15 (ESI†), for both oxycarbide and sulcarbide MAOX, the representative Sc- and Y-based MAOX demonstrate a broad positive near-linear region during the stretch and compression. This strongly confirms the positive near-linear relationship between the fundamental bandgaps and a/c ratios in the ScO-, ScS- and YS-based series of MAOX. On the other hand, the bandgaps of Cr2CaO2C and Cr2CaS2C have a negative near-linear relationship with the a/c ratios, while Mo2CaO2C and Mo2CaS2C show no obvious relationship. Based on the computational test, within a narrow range around the small a/c ratio (0.40–0.42 and 0.35–0.37 for oxycarbide and sulcarbide MAOX, respectively), the fundamental bandgaps are linearly correlated with the a/c ratios. However, it is noted that La2CaO2C and Cr2CaS2C can become metallic at a very small (95% of equilibrium) or a large a/c ratio (105% of equilibrium), and therefore the a/c ratio descriptor has limitations. The a/c ratio is demonstrated to be a descriptor for the bandgaps of MAOX. But it is still unclear why the MAOX phases with moderate ratios are metallic. Nevertheless, the a/c ratio can act as a descriptor for qualitatively predicting the fundamental bandgaps, and thereby aid in designing a semiconductor with an optimal bandgap for high conversion efficiency.
The valence and conduction band alignment with respect to the vacuum level was calculated using the core-level alignment approach and is shown in Fig. S16a–e (ESI†). The MAOX phases with the five M components (Sc, Y, La, Cr or Mo) are grouped together. The oxycarbide and sulcarbide pairs are marked with the two adjacent bars with different colors and no spacing in the histograms in Fig. S16a–e (ESI†). In general, the sulcarbide MAOX show higher EAs and IPs (or WFs) than the oxycarbide ones.
For A in Group IIA, the EA and IP (or WF) increases monotonically from Be to Ba. However, for A in Group IIB, the trend goes up-and-down. Histograms of EAs and WFs for all the A's in Group IIA and Group IIB including Cu from Group IB are plotted in Fig. S17 (ESI†). The trends in Fig. S17 (ESI†) indicated with arrows are in excellent agreement with the trends of EAs and IPs (or WFs) of MAOX. The agreement indicates that the AO layers are not only the bridges between the carbide layers of MAOX but also strongly influence the valence and conduction band alignment. The AO should be considered in the design of MAOX semiconductors for solar absorbers. The open-circuit voltage Voc values of thin films versus EAs and IPs are shown in Fig. S18 (ESI†). It is observed that MAOX semiconductors with low EAs of 2.4–3.0 eV and moderate IPs of 3.7–4.3 eV tend to produce high Voc values above 0.9 eV. EA and IP can be considered as descriptors to engineer Voc.
4. Conclusions
SLMEs, a/c ratios, EAs, and IPs are identified as good descriptors. All the 2121 MAOX phases are thermally stable and fifty-three of them are semiconductors with bandgaps from 0.12 to 1.40 eV. Most of the MAOX semiconductors exhibit desirable strong absorption in the visible light region, and the SLMEs of twenty-three MAOX phases exceed 80% of the SQ limit. Some of them have maximum efficiencies larger than 25.0%. In particular, La2CdS2C, La2HgS2C, Cr2MgO2C, and Cr2ZnO2C have SLMEs of 30.41%, 30.88%, 30.77%, and 30.28%, and are highly promising candidates for solar absorbers. Ceramic MAOX semiconductors exhibit excellent PV properties and can be optimized and tuned via design of absorber layers of solar cells based on the descriptors. In the future, MAOX phase materials can find more applications beyond photovoltaics. Furthermore, what we learn about MAOX, such as descriptors, and structural information, can be transferred to help screen and discover more novel ceramic energy materials computationally. The systematic screening scheme incorporating more Artificial Intelligence (AI) techniques and more computational and experimental data in the future will enhance the efficiency of classification and the accuracy of prediction.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Xin Chen and Chunming Niu acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 21773182 (B030103) and No. 51201175).Notes and references
- A. Jain, G. Hautier, C. J. Moore, S. P. Ong, C. C. Fischer, T. Mueller, K. A. Persson and G. Ceder, Comput. Mater. Sci., 2011, 50, 2295–2310 CrossRef CAS.
- D. Morgan, G. Ceder and S. Curtarolo, Meas. Sci. Technol., 2005, 16, 296–301 CrossRef CAS.
- S. Curtarolo, G. L. W. Hart, M. B. Nardelli, N. Mingo, S. Sanvito and O. Levy, Nat. Mater., 2013, 12, 191–201 CrossRef CAS PubMed.
- E. O. Pyzer-Knapp, C. Suh, R. Gomez-Bombarelli, J. Aguilera-Iparraguirre and A. Aspuru-Guzik, Annu. Rev. Mater. Res., 2015, 45, 195–216 CrossRef CAS.
- S. M. Hasnain, Energy Convers. Manage., 1998, 39, 1127–1138 CrossRef CAS.
- M. C. Orilall and U. Wiesner, Chem. Soc. Rev., 2011, 40, 520–535 RSC.
- T. Pfeifer, J. Matyáš, P. Balaya, D. Singh, J. Wei, T. Ohji, M. Singh and A. Michaelis, Ceramics for Energy Conversion, Storage, and Distribution Systems, Wiley, 2016 Search PubMed.
- A. D. B. L. Ferreira, P. R. O. Novoa and A. T. Marques, Compos. Struct., 2016, 151, 3–35 CrossRef.
- A. Brotchie, Nat. Rev. Mater., 2017, 2, 17044 CrossRef.
- P. Greil, Adv. Eng. Mater., 2000, 2, 339–348 CrossRef CAS.
- V. M. Fedirko, I. M. Pohrelyuk and O. I. Yas’kiv, Mater. Sci., 2006, 42, 299–308 CrossRef CAS.
- J. Homeny, G. G. Nelson, S. W. Paulik and S. H. Risbud, J. Am. Ceram. Soc., 1987, 70, C114–C116 CrossRef.
- A. Vladescu, M. A. Surmeneva, C. M. Cotrut, R. A. Surmenev and I. V. Antoniac, in Handbook of Bioceramics and Biocomposites, ed. I. V. Antoniac, Springer International Publishing, Cham, 2014, pp. 1–31 Search PubMed.
- F. A. Memon, F. Morichetti and A. Melloni, ACS Photonics, 2018, 5, 2755–2759 CrossRef CAS.
- Z. Wang, X. Chen and C. Niu, J. Phys. Chem. C, 2018, 122, 14240–14247 CrossRef CAS.
- R. Hoogenboom, M. A. R. Meier and U. S. Schubert, Macromol. Rapid Commun., 2003, 24, 16–32 Search PubMed.
- E. M. Chan, Chem. Soc. Rev., 2015, 44, 1653–1679 RSC.
- S. M. Senkan, Nature, 1998, 394, 350–353 CrossRef CAS.
- N. Malo, J. A. Hanley, S. Cerquozzi, J. Pelletier and R. Nadon, Nat. Biotechnol., 2006, 24, 167–175 CrossRef CAS PubMed.
- J. R. Hattrick-Simpers, J. M. Gregoire and A. G. Kusne, APL Mater., 2016, 4, 053211 CrossRef.
- N. Mounet, M. Gibertini, P. Schwaller, D. Campi, A. Merkys, A. Marrazzo, T. Sohier, I. E. Castelli, A. Cepellotti, G. Pizzi and N. Marzari, Nat. Nanotechnol., 2018, 13, 246 CrossRef CAS PubMed.
- B. Meredig and C. Wolverton, Nat. Mater., 2013, 12, 123–127 CrossRef CAS PubMed.
- J. Carrasquilla and R. G. Melko, Nat. Phys., 2017, 13, 431 Search PubMed.
- R. Ramprasad, R. Batra, G. Pilania, A. Mannodi-Kanakkithodi and C. Kim, npj Comput. Mater., 2017, 3, 54 Search PubMed.
- K. T. Butler, D. W. Davies, H. Cartwright, O. Isayev and A. Walsh, Nature, 2018, 559, 547–555 CrossRef CAS PubMed.
- B. Sanchez-Lengeling and A. Aspuru-Guzik, Science, 2018, 361, 360–365 CrossRef CAS PubMed.
- W. Shockley and H. J. Queisser, J. Appl. Phys., 1961, 32, 510–519 CrossRef CAS.
- L. P. Yu, R. S. Kokenyesi, D. A. Keszler and A. Zunger, Adv. Energy Mater., 2013, 3, 43–48 CrossRef CAS.
- L. P. Yu and A. Zunger, Phys. Rev. Lett., 2012, 108, 068701 CrossRef PubMed.
- M. A. Green, K. Emery, Y. Hishikawa, W. Warta and E. D. Dunlop, Prog. Photovoltaics, 2015, 23, 1–9 Search PubMed.
- G. Giovannetti, P. A. Khomyakov, G. Brocks, P. J. Kelly and J. van den Brink, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 073103 CrossRef.
- H. Peelaers and C. G. Van de Walle, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 241401 CrossRef.
- A. Ramasubramaniam, D. Naveh and E. Towe, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 205325 CrossRef.
- V. Tran, R. Soklaski, Y. F. Liang and L. Yang, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 235319 CrossRef.
- A. M. Ganose, C. N. Savory and D. O. Scanlon, Chem. Commun., 2017, 53, 20–44 RSC.
- F. Hong, W. J. Lin, W. W. Meng and Y. F. Yan, Phys. Chem. Chem. Phys., 2016, 18, 4828–4834 RSC.
- M. Bercx, N. Sarmadian, R. Saniz, B. Partoensa and D. Lamoena, Phys. Chem. Chem. Phys., 2016, 18, 20542–20549 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Sections S1 and S2, Tables S1 and S2, and Fig. S1–S18. See DOI: 10.1039/c9tc01078e |
| ‡ Zhenyu Wang and Xin Chen contributed equally to this article. |
| This journal is © The Royal Society of Chemistry 2019 |




![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or S and (b) the categories according to the ScO(S), YO(S), LaO(S), CrO(S), and MoO(S) compounds corresponding to the oxycarbide and sulcarbide MAOX. The compounds in the same group are in the same color. The corresponding two subgroups are shown in (c and d).
O or S and (b) the categories according to the ScO(S), YO(S), LaO(S), CrO(S), and MoO(S) compounds corresponding to the oxycarbide and sulcarbide MAOX. The compounds in the same group are in the same color. The corresponding two subgroups are shown in (c and d).