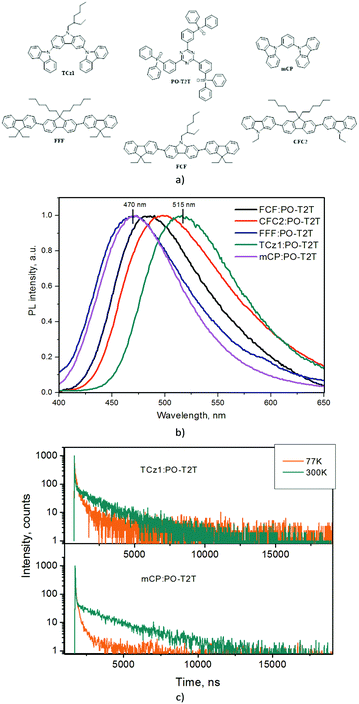
Dual nature of exciplexes: exciplex-forming properties of carbazole and fluorene hybrid trimers†
Matas
Guzauskas
a,
Dmytro
Volyniuk
 a,
Ausra
Tomkeviciene
a,
Anna
Pidluzhna
a,
Ausra
Tomkeviciene
a,
Anna
Pidluzhna
 b,
Algirdas
Lazauskas
c and
Juozas Vidas
Grazulevicius
b,
Algirdas
Lazauskas
c and
Juozas Vidas
Grazulevicius
 *a
*a
aKaunas University of Technology, Department of Polymer Chemistry and Technology, Radvilenu pl. 19, LT-50254, Kaunas, Lithuania. E-mail: juozas.grazulevicius@ktu.lt
bLviv Polytechnic National University, S. Bandera 12, 79013 Lviv, Ukraine
cInstitute of Materials Science, Kaunas University of Technology, K. Baršausko St. 59, LT51423 Kaunas, Lithuania
First published on 6th November 2018
Abstract
Two exciplexes were detected for the first time for the known exciplex-forming system consisting of electron donating 1,3-bis(N-carbazolyl)benzene and electron accepting 2,4,6-tris[3-(diphenylphosphinyl)phenyl]-1,3,5-triazine. Exploiting thermal treatment, sky-blue (high-energy) and orange (low-energy) exciplexes were observed for a solid-state mixture of the compounds under electrical excitation. Similarly, stable high-energy and low-energy exciplexes were observed for new exciplex-forming systems consisting of 2,4,6-tris[3-(diphenylphosphinyl)phenyl]-1,3,5-triazine and one of four selected carbazole and fluorene hybrid trimers as donors. The high-energy exciplexes were observed when there was a small energy barrier between the locally excited state and the high-energy exciplex state. A large energy barrier between the locally excited state and the low-energy exciplex state was the reason that the dual nature of exciplexes was not discovered yet. Emission of both exciplexes was observed in electroluminescence spectra of exciplex-interface based devices using developed exiplex-forming systems as emitters. Observed under optical and electrical excitations, the low-energy exciplexes were separated using thermal treatment of the studied exciplex-forming systems. The exciplex-forming system consisting of 2,4,6-tris[3-(diphenylphosphinyl)phenyl]-1,3,5-triazine and 3,6-di(9-carbazolyl)-9-(2-ethylhexyl)carbazole, which exhibits thermally activated delayed fluorescence, showed the best performance in organic light-emitting diodes (OLEDs) based on interface and volume exciplex emitters. The best device showed maximum external quantum and maximum current efficiencies of 18% and 54 cd A−1 respectively. Additionally, white OLEDs were fabricated exploiting sky-blue and orange emissions from a single exciplex-forming system. Our findings provide evidence of the dual nature of exciplexes and pave the way towards design of new exciplex-forming systems with high photoluminescence quantum yields and efficient exciplex-based devices.
Introduction
Even through spacers,1,2 intermolecular interactions between donor and acceptor molecules can cause formation of bound excited complexes known as exciplexes.3 Radiative decay of the exciplexes results in emission with red-shifted and broader spectra in comparison to photoluminescence spectra of a separate donor and acceptor.4,5 The energy of the emission maximum of an exciplex can be described as:6| hνmaxex ≃ IDP − EAA − EC | (1) |
Very recently, broad exciplex spectra were explained by formation of different dimers in an exciplex layer between tris(4-carbazoyl-9-ylphenyl)amine (TCTA) and 4,6-bis(3,5-di(pyridin-4-yl)phenyl)-2-methylpyrimidine (B4PyMPM).22 Thus, exciplex emission can consist of multicomponent emission with different energies falling in the range from high energy to low energy due to the different distances (r) between donor and acceptor molecules. Because of the Coulomb interaction proportional to r−1, exciplexes with longer donor–acceptor distances have higher emission energy, while those with lower donor–acceptor distances have lower emission energy.22 Since exciplexes can be formed between appropriate donor and acceptor molecules even when they are separated by spacers,1 it is not clear how formation of different dimers affects the emission of such exciplexes. It is also not understood yet why energy from the high-energy exciplex is not transferred to the low-energy exciplex. Therefore, deeper fundamental knowledge on exciplex emission has to be gained.
In this manuscript, we report on an unusual phenomenon i.e. high-energy and low-energy exciplex emission for the same donor–acceptor exciplex-forming system (dual nature of exciplexes) which was experimentally discovered. Two exciplex emission bands, i.e. sky-blue (high-energy) and orange (low-energy), were observed for the exciplex-forming solid mixture mCP and PO-T2T. Unusual orange emission for the mCP:PO-T2T system was clearly observed in electroluminescence spectra of exciplex-interface based devices. Moreover, one colour sky-blue or orange electroluminescence was observed for the same devices based on thermally non-annealed or thermally annealed light-emitting layers respectively with volume exciplex-forming molecular mixture mCP:PO-T2T. Thus, we separated the low-energy exciplex by thermal annealing due to the more efficient intermolecular interactions in donor–acceptor mixtures restricting formation of high-energy exciplexes. The contributions of both high-energy and low-energy exciplexes in the emission of the exciplex-forming system strongly depend on energy barriers between the locally excited state and corresponding exciplex states. Similar exciplex emission was observed for four new exciplex-forming systems developed in this work. We show a strong influence of the contribution of low-energy exciplex emission on the efficiency of emission of the high-energy exciplex (usual exciplex). Because of leakage of energy through low-energy exciplex states, exciplex-forming systems with a high contribution of low-energy exciplex emission showed low efficiency, i.e. TADF properties and lower external quantum efficiencies (EQEs) of OLEDs in contrast to exciplex-forming systems with a low contribution of low-energy exciplex emission. We proposed an approach for developing highly-efficient exciplex-forming systems which is based on the test of the contribution of low-energy exciplex emission by thermal annealing. Using this approach, we have developed exciplex-forming systems which were used as interface and volume emitters in OLEDs, the best of which showed a maximum EQE of 18%. We suppose that the discovered low-energy excited complex plays an important role in highly efficient exciplex-forming systems.
Results and discussion
mCP is widely used as a donor in exciplex-forming systems such as mCP:PO-T2T.14,20 However it suffers from a low glass transition temperature (Tg = 55 °C) and relatively low hole mobility (5 × 10−4 cm2 V−1 s−1 at an electric field of ca. 4.2 × 105 V cm−1).23 Taking this information into account we selected promising carbazole and fluorene hybrid trimers, i.e. 3,6-di(9-carbazolyl)-9-(2-ethylhexyl)carbazole (TCz1), 2,7-bis(9,9-diethylfluoren-2-yl)-9-(2-ethylhexyl)carbazole (FCF), 2,7-bis(9-ethylcarbazol-2-yl)-9,9-dihexylfluorene (CFC2), and 2,7-bis(9,9-diethylfluoren-2-yl)-9,9-di(2-ethylhexyl)fluorene (FFF) as the donors for replacing mCP in exciplex-forming systems (Fig. 1a).24,25 They were characterized by appropriate ionization potential values for sky-blue exciplex formation with the acceptor PO-T2T, by high hole mobilities (>1 × 10−3 cm2 V−1 s−1 at high electric fields), and by higher Tg values than mCP.24,25All the selected donors TCz1, FCF, CFC2, and FFF formed exciplexes with acceptor PO-T2T. In comparison to PL spectra of the pure donors and acceptor, the spectra of solid molecular mixtures of the studied donors with PO-T2T showed red shifts similar to that of the mixture of mCP with PO-T2T (Fig. 1b and Fig. S1, ESI†).20 Exciplex emissions of the studied molecular mixtures were characterized by longer PL decays (up to the μs region) and broader full widths at half maxima (FWHM) as compared to those pure compounds studied. Such observations are usually attributed to the exciplexes (Fig. S1, S2 (ESI†) and Table 1).16 The short and long-lived components of PL decays of the studied exciplex-forming molecular mixtures are respectively attributed to prompt and thermally activated delayed fluorescence similarly to those of previously studied mixture mCP:PO-T2T (Fig. 1c and Fig. S3, ESI†).20 The PL maxima of exciplexes were observed in the range from 470 to 515 nm due to the different ionization potentials for the selected donors. The energies of PL maxima were in good agreement with those calculated by formula 1.
| Exciplex-forming systems | PL λmax (FWHM), nm | PL λmax (FWHM), nm | area2/area1a | EQEmax, % | EL λmax (FWHM), nm | EL λmax (FWHM), nm |
|---|---|---|---|---|---|---|
| Non-annealed | Annealed | Fitting | Non-annealed | Annealed | ||
| a Area2/area1 was obtained using the Guassian fitting of PL spectra with two peaks for the annealed exciplex-forming systems. b – shoulder. | ||||||
| FCF:PO-T2T | 475 (96) | 482, 534b (267) | 1.03 | 6.2 | 492 (90) | 575 (112) |
| CFC2:PO-T2T | 483 (108) | 524 (245) | 2.76 | 2.6 | 522 (113) | 576 (120) |
| FFF:PO-T2T | 470 (127) | 537 (128) | 3.1 | 0.8 | 477 (102) | 570 (115) |
| TCz1:PO-T2T | 501 (88) | 517 (98) | 1.13 | 18.0 | 524 (92) | 524 (92) |
| mCP:PO-T2T | 472 (88) | 472 (106) | 1.07 | 8.2 | 497 (94) | 570 (109) |
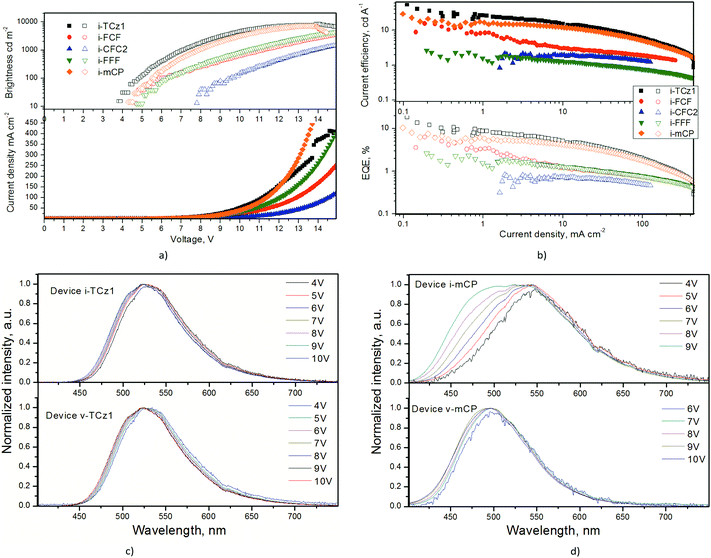
To develop non-doped OLEDs, exciplexes of TCz1:PO-T2T, FCF:PO-T2T, CFC2:PO-T2T, FFF:PO-T2T, and mCP:PO-T2T were used as interface TADF emitters in devices with structures ITO/MoO3 (0.4 nm)/NPB (30 nm)/TCTA (15 nm)/TCz1, FCF, CFC2, FFF or mCP (10 nm)/PO-T2T (10 nm)/TPBi (60 nm)/LiF (0.4 nm)/Al (60 nm). These devices were named as devices i-TCz1, i-FCF, i-CFC2, i-FFF and i-mCP, respectively. Equilibrium energy diagrams of the fabricated interface exciplex-based devices are shown in Fig. S4 (ESI†). Due to the energy barriers formed between selected functional materials of the devices, the exciplex-forming zone (recombination zone of hole–electron pairs) is formed between PO-T2T and one of the donors. Charges and excitons are blocked in this zone because of the appropriate HOMO/LUMO and triplet energy levels. In the devices, the layers of molybdenum oxide (MoO3) and lithium fluoride (LiF) were used as charge injecting layers, while, the layers of N,N′-bis(naphthalen-1-yl)-N,N′-bis(phenyl)benzidine (NPB), tris(4-carbazoyl-9-ylphenyl)amine (TCTA), and 2,2′,2′′-(1,3,5-benzinetriyl)-tris(1-phenyl-1-H-benzimidazole) (TPBi) were used as charge transporting layers.
Turn-on voltages for the devices were observed in the range from 3.8 V (for device i-TCz1) to 7.8 V (for device i-CFC2). To form blue exciplexes, we selected donors with HOMO values ranging from 5.65 to 6.1 eV forming relatively large energy barriers between ITO and the donors. We used additional functional layers in the device structure for lowering these energy barriers. Apparently, the low HOMO values of the selected donors and the usage of additional layers had an effect on turn-on voltages of our exciplex-based devices. In addition, different hole-drift mobilities of the selected donors had an effect on turn-on voltages. Summarizing, we can state that the device thickness, low HOMO values of the selected donors, the used additional layers in the devices and different hole-drift mobilities of the selected donors resulted in turn-on voltages exceeding 3.8 V. Since charge-injecting and charge-transporting properties of the selected donors are similar, such big differences in turn-on voltages of the devices are hardly understandable. Apparently, exciplex-forming properties of the donors played additional roles in working mechanisms of the series of devices having only one different layer, i.e. that of TCz1, FCF, CFC2, FFF or mCP. The highest luminance was observed for device i-TCz1. Current and external quantum efficiencies of device i-TCz1 exceeded 30 cd A−1 and 10%, while its maximum current and external quantum efficiencies were 53 cd A−1 and 18%, respectively (Fig. 2b and Table 1). Such an EQE value is among the best for devices in which exciplex emission is exploited.14 This result can be partly explained by bipolar charge-transporting properties of TCz1 owing to which TCz1 was utilized as a host or non-doped light-emitting layer in efficient OLEDs.26,27 The maximum EQE of 8.2% for device i-mCP based on the interface mCP:PO-T2T emitter is in good agreement with previously described devices based on the volume emitter mCP:PO-T2T.20 Relatively lower maximum EQEs of 6.2, 2.6, and 0.8% were observed for devices i-FCF, i-CFC2 and i-FFF (Fig. 2b). This observation can be partly explained by relatively low triplet energy levels of fluorene-based donors FCF, CFC2 and FFF in comparison to carbazole-based donors TCz1 and mCP (Fig. S5, ESI†).24 Jablonski diagrams of the tested exciplex-forming systems clearly show the energy leakage pathways for the systems FCF:PO-T2T, CFC2:PO-T2T, and FFF:PO-T2T resulting in low device efficiencies of the corresponding devices in comparison with those of devices based on the systems mCP:PO-T2T and TCz1:PO-T2T (Fig. S6, ESI†). In addition, emission of FCF, CFC2 and FFF in devices i-FCF, i-CFC2 and i-FFF is apparently of lower efficiency (Fig. S7, ESI†).
Slight blue-shifts of EL spectra were obtained with increasing applied voltages. Similar blue-shifts for interface exciplex-based devices were previously investigated and the blue-shifts were attributed to electron–hole separation at an interface exciplex-forming site.28 The blue-shifts were found to be dependent on the electrical structure of the interface, the relative geometrical structure of the donor and acceptor molecules at the interface and the characteristics of the applied field.28 Different EL spectra of device i-mCP were recorded at different voltages (Fig. 2d). They were not simultaneously shifted with increasing voltage. Therefore, it seems that the differences of EL spectra of device i-mCP can not be only explained by the different extent of electron–hole separation. Apparently, they resulted from two different emitting species. Similar behaviour of EL spectra was observed for devices i-FCF, i-CFC2 and i-FFF (Fig. S7, ESI†).
Electroluminescence (EL) spectra (with a maximum of 530 nm at 4 V) of device i-TCz1 were slightly red-shifted in comparison to the PL spectrum of the TCz1:PO-T2T exciplex (Fig. 1b and 2c). Analogous red shifts were previously observed for exciplex-forming systems and were explained by the enhancement of the delayed fluorescence of exciplex emission under electrical excitation.15
In contrast to EL spectra of interface exciplex-based devices, EL spectra of volume exciplex-based devices were very similar to PL spectra of the corresponding exciplex-forming molecular mixtures (Fig. 2c, d and Fig. S7, ESI†). Their differences at different voltages were observed due to the different extent of electron–hole separation and due to the enhancement of the delayed fluorescence of the exciplex emission under increased voltages. The exciplex-forming layers of TCz1:PO-T2T, FCF:PO-T2T, CFC2:PO-T2T, FFF:PO-T2T, and mCP:PO-T2T were deposited as doped light-emitting layers with concentrations of 50% of donor and 50% of acceptor (PO-T2T) in the volume exciplex-based devices with the structures ITO/MoO3 (0.4 nm)/NPB (30 nm)/TCTA (15 nm)/donor (10 nm)/donor:PO-T2T (10 nm)/PO-T2T (10 nm)/TPBi (60 nm)/LiF (0.4 nm)/Al (60 nm) where donor is TCz1, FCF, CFC2, FFF or mCP. These devices were named as devices v-TCz1, v-FCF, v-CFC2, v-FFF and v-mCP, respectively. The blocking layers /donor/ and /PO-T2T/ were deposited to avoid formation of interface exciplexes.
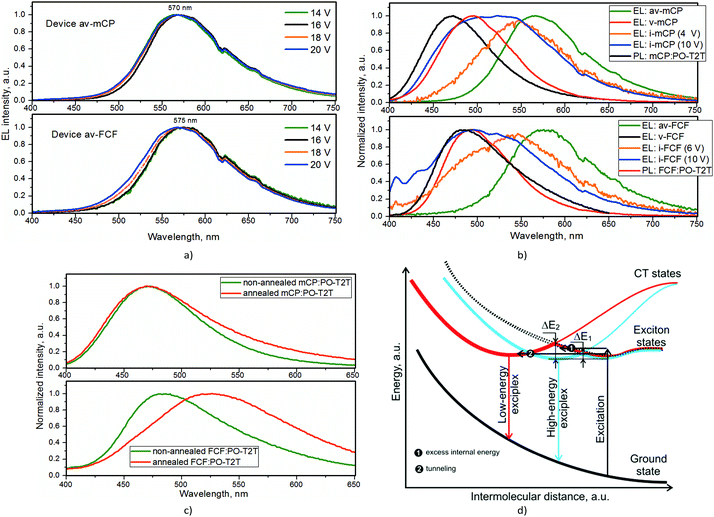
Additionally, the devices v-TCz1, v-FCF, v-CFC2, v-FFF and v-mCP were annealed at a temperature of ca. 100 °C which is higher than Tg of used donors and PO-T2T but much lower than their melting temperatures. The annealed devices were marked as av-TCz1, av-FCF, av-CFC2, av-FFF and av-mCP. EL spectra of these devices were red-shifted with respect to EL spectra of non-annealed devices v-TCz1, v-FCF, v-CFC2, v-FFF and v-mCP as well as with respect to PL spectra of exciplex-forming molecular mixtures TCz1:PO-T2T, FCF:PO-T2T, CFC2:PO-T2T, FFF:PO-T2T, and mCP:PO-T2T (Fig. 3a, b and Fig. S8, ESI†). The wavelengths of EL maxima of these devices av-TCz1, av-FCF, av-CFC2, av-FFF and av-mCP ranged from 548 to 580 nm depending on the wavelengths of low-energy exciplex emission. With the increase of applied voltages, slight blue-shifts of EL spectra of annealed volume exciplex-based devices were observed. The blue-shifts can be attributed to the different extent of electron–hole separation at interface exciplex-forming sites.28 Very similar low-energy emission bands to those of annealed volume interface-based devices av-TCz1, av-FCF, av-CFC2, av-FFF and av-mCP were also observed in EL spectra of the non-annealed interface exciplex-based devices i-TCz1, i-FCF, i-CFC2, i-FFF and i-mCP at wavelengths ranging from 546 to 552 nm (Fig. 2d, 3b and Fig. S7, ESI†). Thus, either high-energy and low-energy exciplex emission bands or their overlapping were observed for one exciplex-forming systems depending on the device configurations (Fig. 3b). The low-energy emission of the studied devices is also assigned to the exciplex but not to other emission species taking into account the following considerations. The low-energy emission of the devices av-TCz1, av-FCF, av-CFC2, av-FFF and av-mCP is not related to electroplex, electromer or exciplex formation between functional layers since the red-shifts of emission of annealed exciplex-forming systems were also observed under optical excitation (Fig. 3c). We investigated the exciplex-forming systems using a wide range of annealing temperatures (Fig. S9, ESI†). Clearly seen red-shifts of PL spectra were observed for the studied samples at annealing temperatures higher than 100 °C (Fig. S9, ESI†). The emission intensity of the low-energy exciplex increased and the emission intensity of the high-energy exciplex decreased with increasing annealing temperature. Excimer formation can also be excluded. This assumption is supported by the absence of emission in the yellow-red region in PL spectra of non-doped layers of donors or acceptors before and after thermal annealing (Fig. S10, ESI†). In addition, excimer formation is not common for solid molecular mixtures of two different compounds. No additional bands were observed in UV spectra of the molecular mixtures after annealing (Fig. S11, ESI†). Thus, degradation can be excluded. In addition, both high-energy and low-energy exciplex emission bands were found to be relatively stable. They did not change during three weeks (Fig. S12, ESI†).
Atomic force microscopy (AFM) measurements were additionally performed for the molecular mixtures on glass substrates before and after annealing. Characteristic AFM topographical images are shown in Fig. S13 (ESI†). Almost in all cases, the root mean square roughness (Rq) slightly increased after annealing. However, Rq values for the annealed and non-annealed solid mixtures were found to be low and acceptable for the fabrication of efficient exciplex-based devices. In addition, X-ray diffraction patterns at grazing incidence of the layers of the molecular mixtures on glass substrates before and after annealing are shown in Fig. S14 (ESI†) with crystallinity values indicated. As deposited films of FFF:PO-T2T, mCP:PO-T2T, and TCz1:PO-T2T exhibited predominantly amorphous structure with very small crystallinity, while FCF:PO-T2T, CFC:PO-T2T were found to be fully amorphous. After annealing crystallinity slightly increased for the films of FCF:PO-T2T, FFF:PO-T2T and CFC:PO-T2T. The observed crystallinity is surprising since mixtures with molar mass concentrations of 50% of donor and 50% of acceptor were studied. However, this result is in good agreement with the recently reported observation that mixtures of clusters of donors and acceptors were observed in exciplex-forming donor:acceptor films.8 Apparently, some clusters exhibit a certain degree of crystallinity.
Exciplex formation is attributed to strong mixing of charge transfer (CT) states with the exciton states (the locally excited states).29 There is a barrier in the adiabatic potential surface between the minima of the locally excited state and the exciplex state.29 Similar geometries between the locally excited states and the exciplex states result in a small barrier; while very different geometries result in a large barrier.29 The authors of ref. 29 schematically showed that exciplex formation can occur due to the overcoming of small or large barriers depending on the studied donor–acceptor mixtures. As it was shown above, high-energy and low-energy exciplexes were observed for single exciplex-forming system TCz1:PO-T2T, FCF:PO-T2T, CFC2:PO-T2T, FFF:PO-T2T, or mCP:PO-T2T (Fig. 3d). Formation of two kinds of exciplexes (high-energy and low-energy exciplexes) for a single exciplex-forming system can apparently be explained by two different mixtures of CT states and exciton states with small (ΔE1) and large (ΔE2) barriers at the same time. Formation of two kinds of exciplexes is schematically presented in Fig. 3d. Commonly, one exciplex formation is observed due to internal energy in excess of the small barrier ΔE1 which can be caused by similar geometries of the locally excited states and the exciplex states (Fig. 3d). Formation of the second exciplex can be observed due to the tunnelling of the large barrier or when there is enough excitation energy for overcoming of a large barrier.
The intermolecular distance between donor and acceptor components plays an important role in the exciplex energy. This is schematically demonstrated in Fig. 3d. Therefore, TCz1, FCF, CFC2 and FFF with steric bulky alkyl chains formed exciplex emission having a clear red-shift, while the red-shift of the mCP based exciplex is little. To further demonstrate that the intermolecular distance between donor and acceptor components plays an important role in the exciplex energy, we selected two donors without alkyl chains, i.e., N,N′-di(1-naphthyl)-N,N′-diphenyl-(1,1′-biphenyl)-4,4′-diamine (NPB) and tris(4-carbazoyl-9-ylphenyl)amine (TCTA) which formed exciplexes with PO-T2T. As seen from Fig. S12, ESI†, the red-shift of NPB and TCTA based exciplexes is also little as it was observed for mCP based exciplex emission. Therefore, we assume that thermal annealing leads to more efficient intermolecular interactions in donor–acceptor mixtures restricting formation of high-energy exciplexes (Fig. 3d). This assumption is in good agreement with the results of recently published theoretical predictions.22 Formation of the low-energy exciplexes affects the efficiency of exciplex-forming systems through a tunnelling process (Fig. 3b). Large red-shift of the emission of low-energy exciplexes can lead to additional energy losses. The results of characterization of OLEDs support this presumption. Worse parameters (turn-on voltages exceding 10 V, EQE of less than 1%) were obtained for annealed interface exciplex-based devices ai-FCF, ai-CFC2, and ai-mCP (Fig. S15, ESI†). Consequently, the most effective exciplex-forming systems should be characterized by high barriers ΔE2. Indeed, after thermal annealing, low-energy exciplexes were practically not observed for the systems TCz1:PO-T2T and mCP:PO-T2T under optical excitation, which are, apparently, characterized by high barriers ΔE2 (Fig. 3c and Fig. S7, ESI†). To obtain the ratio of the low-energy state which contributed to device efficiencies, we fitted the PL spectra of annealed exciplex-forming systems by Guassian fitting with two peaks (Fig. S16, ESI†). It is seen that high EQE values are related to low area2/area1 values, thus a low ratio of the low-energy state (Table 1). Low EQE values are related to high area2/area1 values, thus a high ratio of the low-energy state.
In our opinion, the possibility of formation of low-energy exciplexes has to be taken into account in the design of highly efficient exciplex-forming systems. It also has to be taken into account in interpretation of the electroluminescent behaviour of exciplex-based devices. The proposed schematic diagram which illustrates the transformation to low-energy and high-energy exciplexes when there are small (ΔE1) and large (ΔE2) barriers (ΔE2 > ΔE1) between the locally excited states and the exciplex states can be used for the development of further theoretical models of exciplex emission. The efficient exciplex TCz1:PO-T2T can be utilized in different devices. Finally, white OLEDs can be fabricated mixing sky-blue and orange exciplex emissions generated by one exciplex-forming system (Fig. S17, ESI†). To obtain OLEDs with stable white at different voltages, new approaches have to be developed. We can predict that such white OLEDs will include annealed and non-annealed light-emitting layers based on the same exciplex-forming system emitting in different spectral regions.
Conclusions
Two high-energy and low-energy exciplex species were observed for five different exciplex-forming solid-state mixtures. Emission bands of both exciplexes were well observed in electroluminescence spectra of exciplex-interface based devices using developed exiplex-forming systems as emitters. The low-energy exciplexes were separated using thermal treatment of the studied exciplex-forming systems. Thus, only (mainly) the low-energy exciplex emission bands were observed under optical and electrical excitations for solid films and exciplex-based devices. The main contribution of high-energy exciplexes was observed since there was a small energy barrier between the locally excited state and the high-energy exciplex state. The small contribution of the low-energy exciplexes was detected since there was a large energy barrier between the locally excited state and the low-energy exciplex state. Analysis of exciplex-forming properties of five donor–acceptor systems revealed that the most efficient are those in which the contribution of low-energy exciplexes is the least. Among the studied exciplex-forming systems characterized by thermally activated delayed fluorescence, the most efficient one showed good performance in organic light-emitting devices with the maximum external quantum efficiency reaching 18%.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the European Social Fund according to the activity ‘Improvement of researchers’ qualification by implementing world-class R&D projects’ with Measure No. 09.3.3-LMT-K-712.y acknowledged.Notes and references
- H. Nakanotani, T. Furukawa, K. Morimoto and C. Adachi, Sci. Adv., 2016, 2, e1501470 CrossRef.
- V. Cherpak, P. Stakhira, B. Minaev, G. Baryshnikov, E. Stromylo, I. Helzhynskyy, M. Chapran, D. Volyniuk, Z. Hotra, A. Dabuliene, A. Tomkeviciene, L. Voznyak and J. V. Grazulevicius, ACS Appl. Mater. Interfaces, 2015, 7, 1219–1225 CrossRef CAS.
- J. Kalinowski, Excimers and exciplexes in organic electroluminescence, Mater. Sci.-Pol., 2009, 27, 735–756 CAS.
- V. Jankus, P. Data, D. Graves, C. McGuinness, J. Santos, M. R. Bryce, F. B. Dias and A. P. Monkman, Adv. Funct. Mater., 2014, 24, 6178–6186 CrossRef CAS.
- D. Volyniuk, J. Sutaite, A. Tomkeviciene, N. Kostiv, G. Buika and J. V. Grazulevicius, J. Lumin., 2017, 192, 534–540 CrossRef CAS.
- J. Kalinowski, Organic Light-Emitting Diodes, CRC Press, 2004, vol. 92 Search PubMed.
- K. Goushi, K. Yoshida, K. Sato and C. Adachi, Nat. Photonics, 2012, 6, 253–258 CrossRef CAS.
- T.-C. Lin, M. Sarma, Y.-T. Chen, S.-H. Liu, K.-T. Lin, P.-Y. Chiang, W.-T. Chuang, Y.-C. Liu, H.-F. Hsu, W.-Y. Hung, W.-C. Tang, K.-T. Wong and P.-T. Chou, Nat. Commun., 2018, 9(3111), 1–8 CAS.
- B.-Y. Lin, C. J. Easley, C.-H. Chen, P.-C. Tseng, M.-Z. Lee, P.-H. Sher, J.-K. Wang, T.-L. Chiu, C.-F. Lin, C. J. Bardeen and J.-H. Lee, ACS Appl. Mater. Interfaces, 2017, 9, 10963–10970 CrossRef CAS.
- G. Grybauskaite-Kaminskiene, K. Ivaniuk, G. Bagdziunas, P. Turyk, P. Stakhira, G. Baryshnikov, D. Volyniuk, V. Cherpak, B. Minaev, Z. Hotra, H. Ågren and J. V. Grazulevicius, J. Mater. Chem. C, 2018, 6, 1543–1550 RSC.
- X.-K. Liu, Z. Chen, J. Qing, W.-J. Zhang, B. Wu, H. L. Tam, F. Zhu, X.-H. Zhang and C.-S. Lee, Adv. Mater., 2015, 27, 7079–7085 CrossRef CAS.
- Y. Liu, X. Wei, Z. Li, J. Liu, R. Wang, X. Hu, P. Wang, T. Qi and Y. Wang, Adv. Opt. Mater., 2018, 1800978 CrossRef.
- P. Pander, S. Gogoc, M. Colella, P. Data and F. B. Dias., ACS Appl. Mater. Interfaces, 2018, 10(34), 28796–28802 CrossRef CAS.
- M. Sarma and K.-T. Wong, ACS Appl. Mater. Interfaces, 2018, 10, 19279–19304 CrossRef CAS PubMed.
- G. Sych, J. Simokaitiene, O. Bezvikonnyi, U. Tsiko, D. Volyniuk, D. Gudeika and J. V. Grazulevicius, J. Phys. Chem. C, 2018, 122, 14827–14837 CrossRef CAS.
- E. Skuodis, A. Tomkeviciene, R. Reghu, L. Peciulyte, K. Ivaniuk, D. Volyniuk, O. Bezvikonnyi, G. Bagdziunas, D. Gudeika and J. V. Grazulevicius, Dyes Pigm., 2017, 139, 795–807 CrossRef CAS.
- T. Deksnys, J. Simokaitiene, J. Keruckas, D. Volyniuk, O. Bezvikonnyi, V. Cherpak, P. Stakhira, K. Ivaniuk, I. Helzhynskyy, G. Baryshnikov, B. Minaev and J. V. Grazulevicius, New J. Chem., 2017, 41, 559–568 RSC.
- V. Jankus, C.-J. Chiang, F. Dias and A. P. Monkman, Adv. Mater., 2013, 25, 1455–1459 CrossRef CAS.
- M. Cekaviciute, J. Simokaitiene, D. Volyniuk, G. Sini and J. V. Grazulevicius, Dyes Pigm., 2017, 140, 187–202 CrossRef CAS.
- W.-Y. Hung, G.-C. Fang, S.-W. Lin, S.-H. Cheng, K.-T. Wong, T.-Y. Kuo and P.-T. Chou, Sci. Rep., 2015, 4, 5161 CrossRef.
- C. Duan, C. Han, R. Du, Y. Wei and H. Xu, Adv. Opt. Mater., 2018, 1800437 CrossRef.
- C.-K. Moon, J.-S. Huh, J.-M. Kim and J.-J. Kim, Chem. Mater., 2018, 30, 5648–5654 CrossRef CAS.
- M.-F. Wu, S.-J. Yeh, C.-T. Chen, H. Murayama, T. Tsuboi, W.-S. Li, I. Chao, S.-W. Liu and J.-K. Wang, Adv. Funct. Mater., 2007, 17, 1887–1895 CrossRef CAS.
- A. Tomkeviciene, J. V. Grazulevicius, D. Volyniuk, V. Jankauskas and G. Sini, Phys. Chem. Chem. Phys., 2014, 16, 13932 RSC.
- M.-H. Tsai, Y.-H. Hong, C.-H. Chang, H.-C. Su, C.-C. Wu, A. Matoliukstyte, J. Simokaitiene, S. Grigalevicius, J. V. Grazulevicius and C.-P. Hsu, Adv. Mater., 2007, 19, 862–866 CrossRef CAS.
- V. V. Cherpak, P. Y. Stakhira, D. Y. Volynyuk, J. Simokaitiene, A. Tomkeviciene, J. V. Grazulevicius, A. Bucinskas, V. M. Yashchuk, A. V. Kukhta, I. N. Kukhta, V. V. Kosach and Z. Y. Hotra., Synth. Met., 2011, 161, 1343–1346 CrossRef CAS.
- E. Skuodis, O. Bezvikonnyi, A. Tomkeviciene, D. Volyniuk, V. Mimaite, A. Lazauskas, A. Bucinskas, R. Keruckiene, G. Sini and J. V. Grazulevicius, Org. Electron., 2018, 63, 29–40 CrossRef CAS.
- H. A. Al Attar and A. P. Monkman, Adv. Mater., 2016, 28, 8014–8020 CrossRef CAS PubMed.
- W. T. Yip and D. H. Levy, Phys. Chem. Chem. Phys., 1996, 28, 11539–11545 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8tc04708a |
| This journal is © The Royal Society of Chemistry 2019 |