Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Interplay between H2O and CO2 coadsorption and space-charge on Y-doped BaZrO3 surfaces
Jonathan M.
Polfus
 *ab,
Jing
Yang
*ab,
Jing
Yang
 b and
Bilge
Yildiz
*bc
b and
Bilge
Yildiz
*bc
aSINTEF Industry, Sustainable Energy Technology, PO Box 124 Blindern, NO-0314 Oslo, Norway. E-mail: jonathan.polfus@sintef.no
bDepartment of Materials Science and Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139, USA
cDepartment of Nuclear Science and Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139, USA. E-mail: byildiz@mit.edu
First published on 30th August 2019
Abstract
Correction for ‘Interplay between H2O and CO2 coadsorption and space-charge on Y-doped BaZrO3 surfaces’ by Jonathan M. Polfus et al., J. Mater. Chem. A, 2018, 6, 24823–24830.
The calculated entropies of H2O and CO2 chemisorption presented in Table 2 in the published article, −6.37 × 10−4 eV K−1 and −8.78 × 10−4 eV K−1, respectively, were not correct due to an error in the reference value used for gaseous H2O and CO2. The correct adsorption entropies are −1.39 × 10−3 eV K−1 for H2O and −1.68 × 10−3 eV K−1 for CO2, respectively.
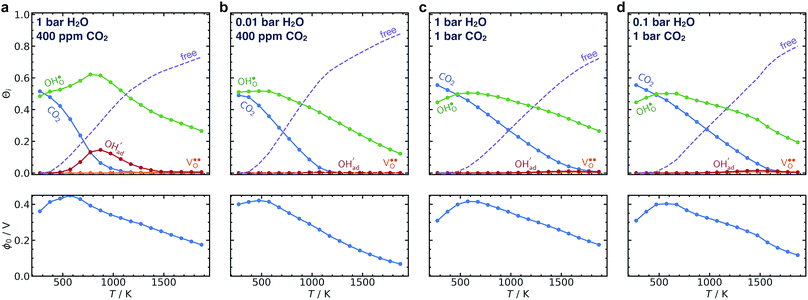
The less favorable adsorption entropies lead to somewhat lower surface coverages, especially as the temperature increases (Fig. 4). Overall, surface protons become relatively more favored and predominate over carbonate adsorbates across a wider range of temperature and pressure. Hydroxide species become less relevant, and consequently, the maximum space-charge potential increases from about 0.3 V at 500–700 K to about 0.4 V at 500–600 K.
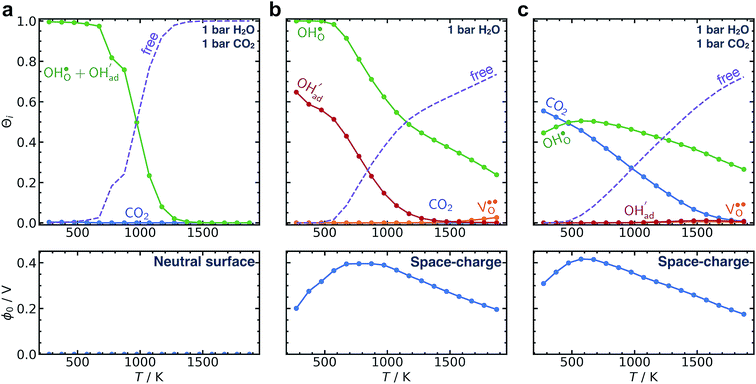
Fig. 7 illustrates that taking into account coadsorption and space-charge formation remains crucial for the calculated surface coverages. Despite the current numerical corrections, the overall findings and conclusions in the original manuscript remain the same.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2019 |