A 3D ordered hierarchically porous non-carbon electrode for highly effective and efficient capacitive deionization†
Yuchen
Wu‡
ab,
Gaopeng
Jiang‡
 a,
Guihua
Liu
a,
Gregory
Lui
a,
Zachary Paul
Cano
a,
Qian
Li
a,
Zhen
Zhang
a,
Guihua
Liu
a,
Gregory
Lui
a,
Zachary Paul
Cano
a,
Qian
Li
a,
Zhen
Zhang
 a,
Aiping
Yu
a,
Aiping
Yu
 *a,
Zisheng
Zhang
*b and
Zhongwei
Chen
*a,
Zisheng
Zhang
*b and
Zhongwei
Chen
 *a
*a
aDepartment of Chemical Engineering, Waterloo Institute for Nanotechnology, University of Waterloo, Waterloo, Ontario N2L3G1, Canada. E-mail: zhwchen@uwaterloo.ca; aipingyu@uwaterloo.ca
bDepartment of Chemical and Biological Engineering, University of Ottawa, Ottawa, Ontario K1N 6N5, Canada. E-mail: Jason.zhang@uottawa.ca
First published on 7th June 2019
Abstract
In this work, three-dimensional ordered mesoporous titanium nitride (3DOM-TiN) has been synthesized via a templating method as a novel high-performance non-carbon capacitive deionization (CDI) electrode material. The dual ion electrosorption mechanisms, fast ion diffusion and rapid charge transfer enabled by the multiple advantageous features of the 3DOM-TiN electrode facilitate an outstanding CDI salt adsorption capacity (SAC) as high as 23.6 mg g−1 and a record-breaking maximum salt adsorption rate (SAR) of 3.2 mg g−1 min−1 in 500 mg L−1 NaCl solution at an applied voltage of 1.2 V. Such excellent CDI performance in addition to excellent cycling stability not only ranks the 3DOM-TiN electrode as one of the most promising electrodes for future CDI applications but also confirms the great potential of nanoengineered non-carbon electrodes for next-generation CDI cells.
The freshwater crisis has become one of the most challenging and urgent global issues due to the growing human population and pollution levels.1 The ideal solution to this issue is the development of sustainable desalination technology, which can treat abundant saline and brackish water.2–4 Current technologies include distillation, reverse osmosis, and electrodialysis. However, these technologies suffer from major drawbacks such as high capital cost and secondary pollution.4 Alternatively, a highly efficient, low-cost, scalable and environmentally benign desalination technology known as capacitive deionization (CDI) has emerged as a viable and promising solution over the last two decades.5–8 CDI applies the principle of ion electrosorption to remove charged ions in low salinity solutions such as brackish water.9 Due to their similarity to a supercapacitor,10,11 CDI electrode materials play a pivotal role in the CDI performance. The majority of reported CDI electrodes are carbonaceous materials, from the earliest activated carbon (AC) electrode to the most recent carbon nanomaterial electrodes.12–18 However, these carbonaceous CDI electrodes still suffer from low CDI performance, especially low salt adsorption capacity (SAC) and low salt adsorption rate (SAR).9 Due to the strong dependence on electrostatic double-layer capacitance (EDLC) to remove ions from the solution, the improvement of SAC for carbonaceous CDI electrodes will be limited. The lack of tuning of the pore structure for these hydrophobic carbonaceous electrodes causes inaccessible surfaces and unfavorable tortuous microporous channels for ions to diffuse, leading to the decrease of both SAC and SAR. Therefore, to further enhance the CDI performance for practical applications, new ion adsorption mechanisms and fine tuning of the pore structure must be considered in a new strategy for the fabrication of CDI electrodes.
In this work, a strategy of using a nanoengineered porous non-carbon electrode for a CDI cell is proposed for the first time. As shown in Fig. 1, the as-prepared 3D ordered mesoporous titanium nitride (3DOM-TiN) functions as the nanoengineered non-carbon electrode for high-performance CDI. Such a novel and rational design adapts the following advantages to maximize CDI performance (Fig. 1): (i) the application of dual ion electrosorption mechanisms, namely EDLC and pseudocapacitance (PC), which significantly enhances the SAC and SAR; (ii) a well-designed ordered hierarchically porous structure together with high surface area and high pore volume, which effectively boosts ion adsorption and decreases ion diffusion resistance, hence maximizing both SAC and SAR; and, (iii) an interconnected nanoframework and nitrogen-doped carbon residual coating, which ensure high electrical conductivity and fast charge transfer, hence further improving CDI performance. As a result, in a batch mode flow-by CDI cell, the 3DOM-TiN electrode delivers a high SAC of 23.6 mg g−1 and maximum SAR of 3.2 mg g−1 min−1 in 500 mg L−1 NaCl solution at an applied voltage of 1.2 V. Furthermore, it demonstrates a superior regeneration and cycling stability to that of the AC electrode. The superior CDI performance of the 3DOM-TiN electrode not only provides promising CDI applications but also highlights the effective strategy of employing nanoengineered non-carbon electrode designs for next-generation CDI devices.
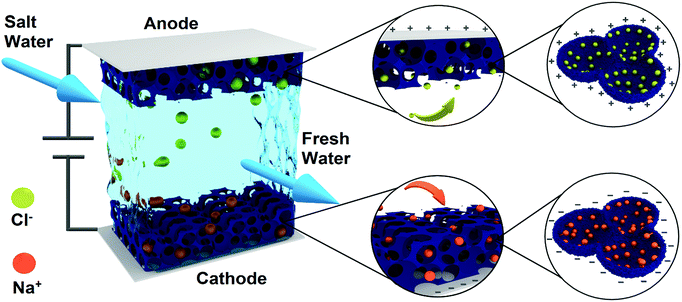 | ||
| Fig. 1 Schematic of 3D hierarchically ordered mesoporous titanium nitride as the electrode for capacitive deionization. | ||
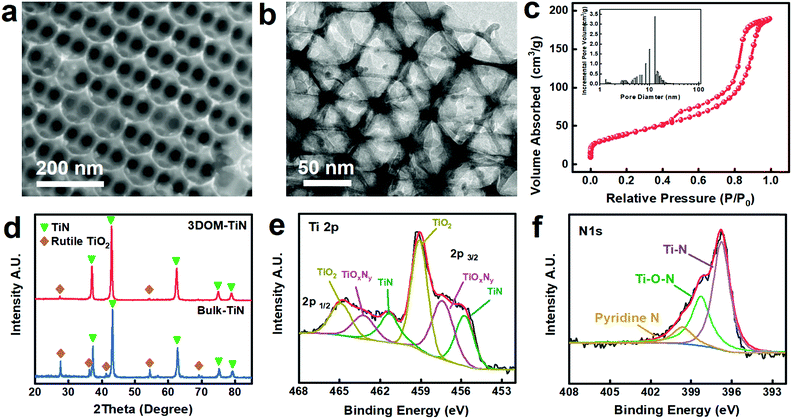
The synthesis of 3DOM-TiN follows the typical templating method depicted in Fig. S1.†19,20 The Experimental details can be seen in the ESI.† A 3D ordered polystyrene (PS) template was infiltrated with titanium butoxide to form the PS/sol–gel composite. Then, the composite was annealed under an argon atmosphere to remove the PS template followed by a nitridation process with NH3 at 800 °C to obtain 3DOM-TiN. For comparison, a bulk-TiN sample was synthesized through a similar sol–gel-annealing-nitridation process without the introduction of the PS template. As demonstrated by scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images in Fig. 2a, b & S2,† the interconnected ordered hierarchically porous 3D nanostructure is obtained through such a synthesis procedure. These images match with the BET analysis results in Fig. 2c, showing the ordered hollow macropores with a size of approximately 100 nm and the connecting mesopores with a size of 12.7 nm. The scattered nanoparticles and the rough pore walls in the magnified TEM images are related to the sol–gel and nitridation process. This process creates small mesopores of less than 10 nm for both 3DOM-TiN and bulk-TiN (Fig. 2c & S3†). The hierarchical macro-/mesoporous structure provides 3DOM-TiN with a high surface area of 141.6 m2 g−1, over four times larger than that of bulk-TiN (31.65 m2 g−1, Table S1†) and significantly larger than the surface areas of previously reported TiN materials.21,22 This maximizes the electrolyte–electrode interfaces and improves the electrosorption capacity of Na+ and Cl− onto the electrode during the CDI process. Moreover, according to Table S1,† the interconnected macropores also provide 3DOM-TiN with nearly ten times higher pore volume in comparison with that of bulk-TiN (0.291 cm3 g−1vs. 0.030 cm3 g−1). This allows the absorption of bulk saline electrolyte into its structure, which will extend the electrolyte–electrode interface into 3DOM-TiN and minimize the ion diffusion distance from bulk saline water to the electrode surface during the CDI process.
The successful nitridation of both 3DOM-TiN and bulk-TiN is confirmed using the X-ray diffraction (XRD) patterns (Fig. 2d). The major peaks at 36°, 42°, 62°, 75°, and 80° are assigned to TiN crystal planes (111), (200), (220), (311), and (420), respectively. Despite the existence of the two minor rutile TiO2 peaks, 3DOM-TiN shows much higher purity in comparison with bulk-TiN. The slight downshift of XRD peaks in 3DOM-TiN as opposed to bulk-TiN further proves the higher degree of nitridation in 3DOM-TiN according to the calculation in Table S2.†23,24 Such a high purity and degree of nitridation are attributed to the 3D interconnected porous structure which allows high diffusion and thorough reaction with NH3 during the nitridation process.
Both the energy-dispersive X-ray spectroscopy (EDX) results in Fig. S4† and the X-ray photoelectron spectroscopy (XPS) survey in Fig. S5a† reveal the existence of O and C on the surface of 3DOM-TiN (Table S3†). In addition, according to the EDX map in Fig. S4c–e,† they are well distributed together with Ti and N. This indicates that surface oxidation and residual carbon coating from the removal of PS templates occur at the surface of 3DOM-TiN.20 The surface oxidation of 3DOM-TiN occurs when it is exposed to air,25 generating high oxidation state Ti species such as TiOxNy and TiO2 on its surfaces according to the high-resolution Ti 2p, N 1s, and O 1s XPS spectra in Fig. 2e, f & S5b.† These Ti species with various oxidation states on the high surface area 3DOM-TiN potentially allow fast surface faradaic reactions through the oxidation/reduction of these Ti species during the electrochemical charge and discharge processes.26,27 Also, these species can facilitate fast surface intercalation/deintercalation of sodium ions to further enhance ion electrosorption capacity.28–30 The residual carbon coating from the PS template is found to be doped with nitrogen, mainly forming pyridinic nitrogen in the carbon structure (Fig. 2f & S5c†). Such a nitrogen-doped carbon (NCR) coating can not only enhance the electronic conductivity of 3DOM-TiN to indirectly improve CDI performance, but also directly contribute potential extra SAC for 3DOM-TiN during the CDI process.31,32 Additionally, these polar oxygenated species on the surface of 3DOM-TiN shown in Fig. S5b† can form hydrogen bonds with water and hence enhance the wettability of the electrode and benefit the ion storage process in 3DOM-TiN.33,34
The electrosorption performance of the electrodes was first investigated via cyclic voltammetry (CV) in a typical three-electrode system in 1 mol L−1 NaCl aqueous solution, employing a saturated calomel electrode (SCE) and a graphite rod as the reference electrode and the counter electrode, respectively. The CV curves of 3DOM-TiN, bulk-TiN, and AC electrodes in Fig. 3a are all quasi-rectangular in shape, showing the considerable contribution of EDLC in all three electrodes. The additional distinct redox peaks located at −0.23 V vs. SCE in the CV curves of both 3DOM-TiN and bulk-TiN show the existence of pseudocapacitance. Overall, the specific capacitance of the 3DOM-TiN electrode (142.9 F g−1) is larger than that of the AC electrode (88.9 F g−1) and the bulk-TiN electrode (45.4 F g−1) at a scan rate of 50 mV s−1 (Fig. 3a). The dramatic enhancement of the specific capacity of the 3DOM-TiN electrode is strongly related to the increase of surface area brought by the 3D hierarchically porous interconnected nanostructure, which increased the efficiency of utilizing the active sites and enhanced the ion diffusion from the electrolyte to the internal porous structure of 3DOM-TiN.35,36 When varying scan rates are applied to the 3DOM-TiN electrode, the CV curves maintain the quasi-rectangular shape (Fig. 3b), and the obtained specific capacitance can reach as high as 171.1 F g−1 at 5 mV s−1 (Fig. 3c). At every scan rate, the 3DOM-TiN electrode outperforms the AC and bulk-TiN electrodes with approximately 50% and 200% larger specific capacitance, respectively (Fig. 3c & S6†), thus indicating its superior electrosorption performance during the CDI process. The specific capacitance of 3DOM-TiN also increases with the concentration of NaCl (Fig. S7†) in the low electrolyte concentration range (0.1–1.0 mol L−1). Furthermore, the 3DOM-TiN electrode demonstrates excellent stability, showing over 90% retention of its initial capacitance after 5000 cycles at a scan rate of 50 mV s−1 in 1.0 mol L−1 NaCl solution (Fig. 3d), indicating its excellent stability in the CDI cell. Additionally, the high-resolution C 1s XPS of 3DOM-TiN after cycling shows an insignificant decrease of sp2 hybridized carbon (∼4%) and slight increase of oxygenated carbon species and sp3 hybridized carbon (Fig. S8†), indicating sufficient stability and resistivity to oxidation of the NCR-coating for CDI applications.
To further understand the electrochemical properties and electrosorption behavior of the electrodes, electrochemical impedance spectroscopy (EIS) was conducted for both 3DOM-TiN and bulk-TiN electrodes. The Nyquist plots of both electrodes in Fig. 4a show an incomplete semicircle in the high-frequency region and a steep straight line in the low-frequency region. The associated equivalent circuit model, shown as an inset, in Fig. 4a successfully accounted for the resistive and capacitive features of the electrodes.37,38 Both the smaller equivalent series resistance (Rs) as shown in Fig. S9a† (4.07 Ω) and the larger electronic conductivity (562.7 S m−1) of 3DOM-TiN as shown in Fig. 4b relative to bulk-TiN (8.69 Ω, 317.5 S m−1) suggest an enhanced electronic transfer due to the interconnected 3D nanostructure and NCR-coating within the 3DOM-TiN electrode. This inevitable stable NCR-coating will contribute to the CDI performance indirectly via the enhancement of electrical conductivity. According to the literature,34,37,38 the two constant phase elements (CPEs), namely CPE1 and CPE2, are assigned to EDLC and PC, respectively. The charge transfer resistance (Rct) is connected in series with CPE2 and mainly indicates the resistive behavior at the electrode–electrolyte interface occurring during the pseudocapacitive charge storage process. The observed lower Rct of the 3DOM-TiN electrode as opposed to that of the bulk-TiN electrode (9.62 Ω vs. 17.61 Ω) (Fig. S9a†) reveals the fast kinetics of the faradaic reactions occurring at the highly exposed accessible active sites on the surface of the interconnected hierarchically porous 3DOM-TiN electrode. It is also observed in Fig. 4a that the linear straight line exhibited by 3DOM-TiN in the low-frequency region is steeper than that of the bulk-TiN electrode, implying faster ion diffusion in the 3DOM-TiN electrode. The calculated Warburg coefficient σ (Ω s−0.5) in Fig. S9b† demonstrates that the ion diffusion resistance of 3DOM-TiN is only 12% of the value of that in the bulk-TiN electrode. These results prove that the 3D interconnected hierarchically porous nanostructure enables fast ion diffusion from the electrolyte to the electrode, fast pseudocapacitive charge storage and high electronic conductivity, thus endowing the 3DOM-TiN electrode with a high specific capacitance and great potential as a CDI electrode.
Investigations of the electrochemical behavior of 3DOM-TiN as both the anode and cathode were also conducted via CV measurement. As illustrated in Fig. 4c, the 3DOM-TiN cathode delivers a slightly higher specific capacity than the 3DOM-TiN anode at a scan rate of 10 mV s−1 (90.2 F g−1vs. 66.5 F g−1). Such a difference between electrosorption towards the cation (Na+) and anion (Cl−) lies in the difference in sorption mechanisms of each specific ion.39,40 Nevertheless, Fig. 4c indicates that the 3DOM-TiN electrode has high electrosorption capacity towards both cations and anions, which is a desirable property for electrodes used in the symmetric design of CDI. To quantify the contribution of capacitive ion sorption mechanisms, Dunn's method is employed for the 3DOM-TiN electrode in the anodic and cathodic ranges as well as the full potential range.9,37,38 The calculation in Fig. S10† and the shaded areas in Fig. 4d reveal that EDLC and PC contribute equally in the 3DOM-TiN cathode while EDLC dominates the electrosorption in the 3DOM-TiN anode (81.8%). Therefore, Cl− is mainly adsorbed onto the hierarchically porous 3DOM-TiN electrode via the formation of EDL, which is in line with the literature.26 Given the highly oxygen-deficient and reductive atmosphere during the nitridation process, a large amount of oxygen vacancies (OVs) must be generated on the surface of 3DOM-TiN during ammonia heat treatment.19 These OVs on TiN or hydrogenated TiO2 have been reported to be responsible for their excellent pseudocapacitance via the oxidation/reduction of surface hydroxyl groups and OH− ion adsorption in the chloride-free aqueous electrolyte.28,41–43 Therefore, it is assumed that the OVs on the surface of 3DOM-TiN can act as non-selective adsorbing sites towards anions in NaCl solution (Cl−, OH−, etc.) to contribute to the pseudocapacitance at the anode. On the other hand, Na+ tends to be electro-adsorbed onto the 3DOM-TiN electrode via the fast surface faradaic reaction in addition to the EDL formation. This faradaic Na+ storage mechanism takes place at the interface between partially oxidized TiOxNy moieties and the electrolyte via the oxidation/reduction of Ti during chemisorption or intercalation/deintercalation which is represented by the following reaction.26–28
| (TiOxNy)surface + Na+ + e− ⇌ (TiOxNy−Na+)surface |
Therefore, the pseudocapacitive electrosorption of Na+ accounts for the majority of PC in the 3DOM-TiN electrode in the full range (Fig. 4d). As a result, the 3DOM-TiN electrode is proved to apply the dual ion electrosorption mechanism to store NaCl simultaneously. Such a mechanism resembles the working principle of a hybrid system of batteries and supercapacitors, such as lithium or sodium ion supercapacitors.44,45 In this case, Na+ ions are stored at the electrode–electrolyte interface at the cathode via EDLC and PC, while the counter ions, Cl− ions, are mainly stored in the EDL to balance the charges and transfer extra electrons at the anode. The novel mechanism will enable efficient desalination in the CDI process.
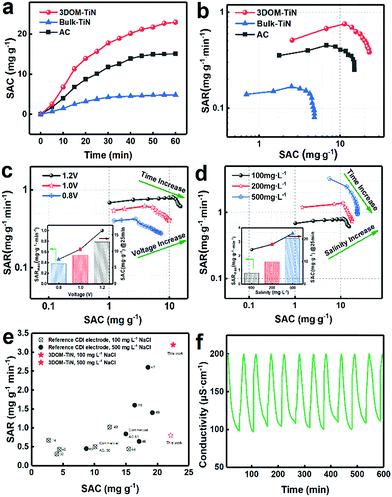
The evaluation of CDI performance was conducted in a flow-by mode symmetrical CDI cell equipped with the 3DOM-TiN, bulk-TiN or AC electrode. Fig. S11† shows the comparison of the conductivity profiles of desalinated water against the operation time for the three electrodes in a full batch-mode CDI process at an applied voltage of 1.2 V with 100 mg g−1 NaCl solution pumped through the cell at a flow rate of 5 mL min−1. This shows that all three electrodes took roughly 25 minutes to reduce the solution conductivity to its minimum and then gradually reached a saturated adsorption state around 60 minutes. Correspondingly, the SACs of each electrode at different desalination times were calculated according to Fig. S12† and were plotted as a function of time in Fig. 5a. 3DOM-TiN achieves the highest SAC among these three electrodes, specifically 22.1 mg g−1 at 60 min, which is roughly 4 times and 40% higher than the SAC of bulk-TiN and AC electrodes, respectively. Most importantly, such a high SAC at low salinity ranks the 3DOM-TiN electrode at the top of the best previously reported titanium-based CDI electrodes (Table S4†). To compare the desalination efficiency of the CDI electrodes, the SARs of three electrodes were calculated and plotted against SAC in the CDI Ragone plot in Fig. 5b. The SAR increases quickly within the initial 10 min due to the rapid infusion of salt water and then decreases due to the gradual saturation of ion adsorption. Clearly, the 3DOM-TiN electrode shows the highest SAR among these three samples, reaching a maximum SAR of 0.81 mg g−1 min−1 which is 2 times and 4 times higher than that of AC and bulk-TiN electrodes, respectively. Such effective and efficient CDI performance of the 3DOM-TiN electrode, showing both high capacity and high rate, is ideal for the CDI process.46 The dramatic increase of CDI performance with respect to bulk-TiN is strongly related to the nanoengineered features of 3DOM-TiN. First, the high surface area of 3DOM-TiN exposes a large amount of accessible active sites at the electrode–electrolyte interface for dual capacitive deionization mechanisms,18,39 which greatly increases the SAC. Second, the 3D hierarchically porous structure and high pore volume reduce the ion diffusion resistance, which enhances the SAC and SAR simultaneously. Third, the interconnected nanoframework and nitrogen-doped carbon residual coating ensure high electronic conductivity and efficient charge transfer, which further improves the total CDI performance. All these features together make 3DOM-TiN a superior electrode material for CDI.
To further assess the potential of the 3DOM-TiN electrode for practical CDI applications, different operational parameters, namely cell voltage, salinity, and flow rate, were evaluated in the continuous CDI cell, with all desalination processes lasting until the lowest conductivity was reached. The obtained Ragone plots are displayed in Fig. 5c, d & S13.† Both SAC and SAR increase together with the applied voltage (Fig. 5c), resulting from the thicker electrical double layer and the stronger coulombic force under the more intense electric field.47,48 Similarly, the curve in Fig. 5d shifts to the top-right of the CDI Ragone plot as the salinity of the solution increases, indicating the increase of both SAC and SAR at a high salt concentration. When the continuous CDI cell is fed with a solution of 500 mg L−1 NaCl, the SAC further reached 23.6 mg g−1 at the saturated adsorption moment, with a maximum SAR of 3.2 mg g−1 min−1. Such a maximum SAR is the highest-ever reported value among all those obtained from carbon and non-carbon-based CDI electrodes (Tables S5 & S6†). The 3DOM-TiN electrode outperforms all recently reported titanium-containing CDI electrodes in terms of the combined performance of SAC and SAR in Fig. 5e.16,34,49–58 This excellent performance is attributed to two factors; the first being that a higher concentration of ions can enable the formation of a more coherent and denser EDL, and thus more pseudocapacitive ion adsorption and storage at the electrode–electrolyte interfaces.18 Second, the interconnected hierarchically porous structure and high electronic conductivity of the 3DOM-TiN electrode lower the ion diffusion and charge transfer resistances even at high salinity, hence maximizing the SAR. When the flow rate is adjusted up to 15 mL min−1, both the SAC and SAR of the 3DOM-TiN electrode are inferior to those obtained at lower flow rates as shown in Fig. S13.† This decrease could be attributed to the insufficient residence time of the ions in the CDI cell stemming from the high flow rate.18
Finally, salt adsorption–desorption cycling tests of the 3DOM-TiN electrode in the CDI cell were conducted to evaluate its stability during the CDI process. The regeneration curve in Fig. 5f demonstrates the continuous and stable performance of over 600 minutes (10 cycles) at an applied potential of 1.2 V and feeding saline water of 100 mg L−1 NaCl at a flow rate of 5 mL min−1. The SAC of the 3DOM-TiN electrode after cycling remains at 15.3 mg g−1, representing over 91% retention. It is significantly higher than the retention rate of the AC electrode (73.4%) after 600 minutes cycling (Fig. S14†). Therefore, the 3DOM-TiN electrode exhibited exellcent and stable cycling and regeneration abilities. Such high CDI performance of the 3DOM-TiN electrode, especially its high SAC and SAR, demonstrates its promising application as a novel non-carbon CDI electrode for future large-scale desalination treatment of brackish water and seawater.
Conclusions
In this work, we successfully synthesized 3DOM-TiN using a facile template method and fabricated it into electrodes for batch-mode symmetric CDI cells. The following distinct advantageous features were observed: (i) the high specific capacitance as both the anode and cathode, owing to both the cohesive electrical double layer formed at large accessible electrolyte–electrode interfaces and the fast surface pseudocapacitive reactions involving the bonding of Na+; (ii) the well-designed hierarchical 3DOM structure together with high pore volume dramatically decreased the ion diffusion resistances from saline solution to the electrode; and, (iii) the interconnected nano framework and nitrogen-doped carbon residue coating ensured the high electronic conductivity and the low charge transfer resistance. All these features made 3DOM-TiN a promising electrode material in the CDI cell. The 3DOM-TiN electrode delivered an outstanding SAC as high as 23.6 mg g−1 and a record high maximum SAR of 3.2 mg min−1 g−1 in a symmetric CDI cell fed with 500 mg L−1 NaCl solution. The superior CDI performance not only demonstrates the great potential of the 3DOM-TiN electrode for future CDI applications, but also proposes a feasible and effective strategy of using nanoengineered porous non-carbon electrodes for next-generation CDI cells.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the Natural Sciences and Engineering Research Council of Canada, the Nanomaterials and Clean Energy Laboratory at the University of Waterloo, the Department of Chemical and Biological Engineering at the University of Ottawa, and the China Scholarship Council.Notes and references
- M. Catley-Carlson, Nature, 2011, 473, 27 CrossRef CAS.
- A. Subramani and J. G. Jacangelo, Water Res., 2015, 75, 164–187 CrossRef CAS PubMed.
- J. Choi, P. Dorji, H. K. Shon and S. Hong, Desalination, 2019, 449, 118–130 CrossRef CAS.
- Y. H. Teow and A. W. Mohammad, Desalination, 2019, 451, 2–17 CrossRef CAS.
- M. Andelman, Sep. Purif. Technol., 2011, 80, 262–269 CrossRef CAS.
- K. Singh, S. Porada, H. de Gier, P. Biesheuvel and L. de Smet, Desalination, 2019, 455, 115–134 CrossRef CAS.
- Y. Zhao, Y. Wang, R. Wang, Y. Wu, S. Xu and J. Wang, Desalination, 2013, 324, 127–133 CrossRef CAS.
- M. Qin, A. Deshmukh, R. Epsztein, S. K. Patel, O. M. Owoseni, W. S. Walker and M. Elimelech, Desalination, 2019, 455, 100–114 CrossRef CAS.
- W. Bao, X. Tang, X. Guo, S. Choi, C. Wang, Y. Gogotsi and G. Wang, Joule, 2018, 2, 778–787 CrossRef CAS.
- V. Chabot, D. Higgins, A. Yu, X. Xiao, Z. Chen and J. Zhang, Energy Environ. Sci., 2014, 7, 1564–1596 RSC.
- L. Lim, Y. Liu, W. Liu, R. Tjandra, L. Rasenthiram, Z. Chen and A. Yu, ACS Appl. Mater. Interfaces, 2017, 9, 39576–39583 CrossRef CAS PubMed.
- G. Luo, Y. Wang, L. Gao, D. Zhang and T. Lin, Electrochim. Acta, 2018, 260, 656–663 CrossRef CAS.
- Y. Li, Y. Liu, M. Wang, X. Xu, T. Lu, C. Q. Sun and L. Pan, Carbon, 2018, 130, 377–383 CrossRef CAS.
- Y. Wimalasiri and L. Zou, Carbon, 2013, 59, 464–471 CrossRef CAS.
- T. Gao, F. Zhou, W. Ma and H. Li, Electrochim. Acta, 2018, 263, 85–93 CrossRef CAS.
- H. Yin, S. Zhao, J. Wan, H. Tang, L. Chang, L. He, H. Zhao, Y. Gao and Z. Tang, Adv. Mater., 2013, 25, 6270–6276 CrossRef CAS PubMed.
- H. Wang, L. Shi, T. Yan, J. Zhang, Q. Zhong and D. Zhang, J. Mater. Chem. A, 2014, 2, 4739–4750 RSC.
- J. Zhang, J. Fang, J. Han, T. Yan, L. Shi and D. Zhang, J. Mater. Chem. A, 2018, 6, 15245–15252 RSC.
- G. Liu, J. Li, J. Fu, G. Jiang, G. Lui, D. Luo, Y. P. Deng, J. Zhang, Z. P. Cano and A. Yu, Adv. Mater., 2018, 1806761 CrossRef PubMed.
- G. Lui, G. Li, X. Wang, G. Jiang, E. Lin, M. Fowler, A. Yu and Z. Chen, Nano Energy, 2016, 24, 72–77 CrossRef CAS.
- D. C. Higgins, J.-Y. Choi, J. Wu, A. Lopez and Z. Chen, J. Mater. Chem., 2012, 22, 3727–3732 RSC.
- Z. Xing, G. Li, S. Sy and Z. Chen, Nano Energy, 2018, 54, 1–9 CrossRef CAS.
- I. G. Morozov, O. Belousova, O. Belyakov, I. Parkin, S. Sathasivam and M. Kuznetcov, J. Alloys Compd., 2016, 675, 266–276 CrossRef CAS.
- R. A. Sait and R. B. M. Cross, Appl. Surf. Sci., 2017, 424, 290–298 CrossRef CAS.
- B. M. Gray, A. L. Hector, M. Jura, J. R. Owen and J. Whittam, J. Mater. Chem. A, 2017, 5, 4550–4559 RSC.
- C. Zhu, P. Yang, D. Chao, X. Wang, X. Zhang, S. Chen, B. K. Tay, H. Huang, H. Zhang and W. Mai, Adv. Mater., 2015, 27, 4566–4571 CrossRef CAS PubMed.
- G. Hasegawa, A. Kitada, S. Kawasaki, K. Kanamori, K. Nakanishi, Y. Kobayashi, H. Kageyama and T. Abe, J. Electrochem. Soc., 2015, 162, A77–A85 CrossRef CAS.
- X. Lu, G. Wang, T. Zhai, M. Yu, S. Xie, Y. Ling, C. Liang, Y. Tong and Y. Li, Nano Lett., 2012, 12, 5376–5381 CrossRef CAS PubMed.
- J. Dong, Y. Jiang, Q. Li, Q. Wei, W. Yang, S. Tan, X. Xu, Q. An and L. Mai, J. Mater. Chem. A, 2017, 5, 10827–10835 RSC.
- Z. Le, F. Liu, P. Nie, X. Li, X. Liu, Z. Bian, G. Chen, H. B. Wu and Y. Lu, ACS Nano, 2017, 11, 2952–2960 CrossRef CAS PubMed.
- R. Tjandra, W. Liu, L. Lim and A. Yu, Carbon, 2018, 129, 152–158 CrossRef CAS.
- W. Lei, W. Xiao, J. Li, G. Li, Z. Wu, C. Xuan, D. Luo, Y.-P. Deng, D. Wang and Z. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 28604–28611 CrossRef CAS PubMed.
- M. Hashemi, M. S. Rahmanifar, M. F. El-Kady, A. Noori, M. F. Mousavi and R. B. Kaner, Nano Energy, 2018, 44, 489–498 CrossRef CAS.
- A. S. Yasin, H. O. Mohamed, I. M. Mohamed, H. M. Mousa and N. A. Barakat, Sep. Purif. Technol., 2016, 171, 34–43 CrossRef CAS.
- A. Achour, J. B. Ducros, R. L. Porto, M. Boujtita, E. Gautron, L. Le Brizoual, M. A. Djouadi and T. Brousse, Nano Energy, 2014, 7, 104–113 CrossRef CAS.
- X. Zhou, C. Shang, L. Gu, S. Dong, X. Chen, P. Han, L. Li, J. Yao, Z. Liu, H. Xu, Y. Zhu and G. Cui, ACS Appl. Mater. Interfaces, 2011, 3, 3058–3063 CrossRef CAS PubMed.
- Z. Zhou, T. Liu, A. U. Khan and G. Liu, Sci. Adv., 2019, 5, eaau6852 CrossRef PubMed.
- F. Zhang, T. Liu, M. Li, M. Yu, Y. Luo, Y. Tong and Y. Li, Nano Lett., 2017, 17, 3097–3104 CrossRef CAS PubMed.
- C. Costentin, T. R. Porter and J.-M. Savéant, ACS Appl. Mater. Interfaces, 2017, 9, 8649–8658 CrossRef CAS PubMed.
- T. Brousse, D. Bélanger and J. W. Long, J. Electrochem. Soc., 2015, 162, A5185–A5189 CrossRef CAS.
- A. Achour, R. L. Porto, M.-A. Soussou, M. Islam, M. Boujtita, K. A. Aissa, L. Le Brizoual, A. Djouadi and T. Brousse, J. Power Sources, 2015, 300, 525–532 CrossRef CAS.
- A. Achour, J. Ducros, R. L. Porto, M. Boujtita, E. Gautron, L. Le Brizoual, M. Djouadi and T. Brousse, Nano Energy, 2014, 7, 104–113 CrossRef CAS.
- X. Lu, G. Wang, T. Zhai, M. Yu, J. Gan, Y. Tong and Y. Li, Nano Lett., 2012, 12, 1690–1696 CrossRef CAS PubMed.
- J. Ding, Z. Li, K. Cui, S. Boyer, D. Karpuzov and D. Mitlin, Nano Energy, 2016, 23, 129–137 CrossRef CAS.
- Q. Xia, H. Yang, M. Wang, M. Yang, Q. Guo, L. Wan, H. Xia and Y. Yu, Adv. Energy Mater., 2017, 7, 1701336 CrossRef.
- T. Kim and J. Yoon, RSC Adv., 2015, 5, 1456–1461 RSC.
- G. Trefalt, S. H. Behrens and M. Borkovec, Langmuir, 2015, 32, 380–400 CrossRef PubMed.
- M. M. Hatlo, R. Van Roij and L. Lue, EPL, 2012, 97, 28010 CrossRef.
- A. S. Yasin, I. M. Mohamed, H. M. Mousa, C. H. Park and C. S. Kim, Sci. Rep., 2018, 8, 541 CrossRef PubMed.
- A. S. Yasin, M. Obaid, I. Mohamed, A. Yousef and N. Barakat, ZrO2 Nanofibers/Activated Carbon Composite as a Novel and Effective Electrode Material for the Enhancement of Capacitive Deionization Performance, 2017 Search PubMed.
- A. G. El-Deen, J.-H. Choi, C. S. Kim, K. A. Khalil, A. A. Almajid and N. A. Barakat, Desalination, 2015, 361, 53–64 CrossRef CAS.
- P. Srimuk, M. Zeiger, N. Jäckel, A. Tolosa, B. Krüner, S. Fleischmann, I. Grobelsek, M. Aslan, B. Shvartsev and M. E. Suss, Electrochim. Acta, 2017, 224, 314–328 CrossRef CAS.
- P.-I. Liu, L.-C. Chung, H. Shao, T.-M. Liang, R.-Y. Horng, C.-C. M. Ma and M.-C. Chang, Electrochim. Acta, 2013, 96, 173–179 CrossRef CAS.
- P. Srimuk, F. Kaasik, B. Krüner, A. Tolosa, S. Fleischmann, N. Jäckel, M. C. Tekeli, M. Aslan, M. E. Suss and V. Presser, J. Mater. Chem. A, 2016, 4, 18265–18271 RSC.
- S. Kim, J. Lee, C. Kim and J. Yoon, Electrochim. Acta, 2016, 203, 265–271 CrossRef CAS.
- B. H. Min, J.-H. Choi and K. Y. Jung, Electrochim. Acta, 2018, 270, 543–551 CrossRef CAS.
- R. Zhao, O. Satpradit, H. Rijnaarts, P. Biesheuvel and A. Van der Wal, Water Res., 2013, 47, 1941–1952 CrossRef CAS PubMed.
- K. Dermentzis, J. Eng. Sci. Technol. Rev., 2016, 9, 130–133 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ta04025k |
| ‡ These authors have contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |