Zinc–nickel–cobalt ternary hydroxide nanoarrays for high-performance supercapacitors†
Zi-Hang
Huang
a,
Fang-Fang
Sun
a,
Munkhbayar
Batmunkh
 b,
Wen-Han
Li
a,
Hui
Li
a,
Ying
Sun
a,
Qin
Zhao
a,
Xue
Liu
b,
Wen-Han
Li
a,
Hui
Li
a,
Ying
Sun
a,
Qin
Zhao
a,
Xue
Liu
 a and
Tian-Yi
Ma
a and
Tian-Yi
Ma
 *c
*c
aInstitute of Clean Energy Chemistry, Key Laboratory for Green Synthesis and Preparative Chemistry of Advanced Materials, College of Chemistry, Liaoning University, Shenyang 110036, China
bAustralian Institute for Bioengineering and Nanotechnology, University of Queensland, St. Lucia, QLD 4072, Australia
cDiscipline of Chemistry, University of Newcastle, Callaghan, NSW 2308, Australia. E-mail: Tianyi.Ma@newcastle.edu.au
First published on 10th April 2019
Abstract
The development of high-capacity, stable cycling, and high mass loading cathode materials for asymmetric supercapacitors has been the subject of intense exploration. In this work, a well-aligned zinc–nickel–cobalt ternary (oxy)hydroxide (Zn–Ni–Co TOH) nanostructure with a controlled morphology is used, for the first time, as a high-performance cathode material for supercapacitors. Our findings demonstrate that precursor Zn–Ni–Co TOH materials can deliver superior capacity and rate capability to the Zn–Ni–Co oxide. A high mass loading of 7 mg cm−2 on a carbon cloth substrate is achieved, accompanied by substantially improved facile ionic and electronic transport due to the highly open well-defined nanoarray architecture. The growth mechanism of Zn–Ni–Co TOH was studied in depth by scanning electron microscopy analysis. The optimized Zn–Ni–Co TOH-130 nanowire array electrode delivered an outstanding areal capacitance of 2.14 F cm−2 (or a specific capacitance of 305 F g−1) at 3 mA cm−2 and an excellent rate capability. Moreover, the asymmetric supercapacitor assembled with our Zn–Ni–Co TOH-130 cathode exhibited a maximum volumetric energy density of 2.43 mW h cm−3 at a volumetric power density of 6 mW cm−3 and a long-term cycling stability (153% retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles), which is superior to the majority of the state-of-the-art supercapacitors. This work paves the way for the construction of high-capacity cathode materials for widespread applications including next-generation wearable energy-storage devices.
000 cycles), which is superior to the majority of the state-of-the-art supercapacitors. This work paves the way for the construction of high-capacity cathode materials for widespread applications including next-generation wearable energy-storage devices.
Introduction
Electrochemical energy storage technologies have shown great promise in addressing energy and environmental issues.1–4 Among various types of electrochemical energy storage devices, supercapacitors have attracted much interest owing to their high power density, stable cycling and fast charge–discharge rate.5–7 However, the rather moderate energy density is the major limitation of this cutting-edge technology. Over the past several years, considerable attention has been focused on pseudocapacitive type electrode materials, with the charge stored through not only ion adsorption but also near surface redox reactions.8,9 Among various pseudocapacitive type electrode materials, transition metal oxides/hydroxides have been considered the most promising candidates for high-performance electrode materials because of their high theoretical capacitance, low fabrication cost, and environmental benignity.10–12 Nevertheless, satisfactory electrochemical performance has not been achieved with the increase of mass loading due to their relatively poor electrical conductivity and sluggish ionic transportation. Further improving the specific capacitance of single-component oxides/hydroxides remains a significant challenge which limits the practical application of supercapacitors in high energy devices.A feasible strategy to enhance the electrochemical performance of high mass loading materials is to construct multi-component transition metal oxides/hydroxides with 3D hierarchical structures and establish conductive linkages.13 The direct attachment of each component of transition metal oxides/hydroxides to the current collector not only improves the electrode charge transfer but also provides abundant electroactive sites to the liquid electrolyte.14–16 Recently, various self-supported hierarchical nanostructured electrodes have been reported to exhibit enhanced electrochemical performances.17–20 In spite of these advances, rational design and synthesis of multi-component (≥3 metal components) transition metal oxides/hydroxides with a hierarchical nanostructure directly grown on a current collector using a simple process is extremely challenging.
It has been demonstrated that the incorporation of zinc elements in nickel and cobalt hydroxides or cobalt-doped nickel hydroxides is able to significantly enhance their electrochemical performance.17,21 The enhancement originates from their complex chemical compositions involving different mixed valence states at the multi-metal centers and synergetic effects on the redox reactions.17,22 In such Zn–Ni–Co ternary electrode materials, the Co element provides increased electronic conductivity and the Ni element offers a high capacity and can improve the active site density. Meanwhile, the Zn element possesses good electrical conductivity that can result in the improvement of capacitive performance.23–25 Moreover, the incorporation of several metal ions may yield multi-phase metal materials and introduce abundant structural defects, which can be beneficial to electrochemical energy storage. Until recently, there have been a limited number of studies utilizing ternary metal oxides of Zn–Ni–Co combination as cathode materials for supercapacitors.16,19,23 As compared to the Zn–Ni–Co ternary metal oxide electrode, the precursor ternary (oxy)hydroxides can deliver better capacity due to the high theoretical specific capacitance of each component in hydroxides.26,27 Meanwhile, layered hydroxides have a large surface area for redox reactions and allow facile ion migration.27,28 However, Zn–Ni–Co ternary (oxy)hydroxides are hardly used as cathode materials for supercapacitors.
Herein, we perform an in-depth investigation on the designing of hierarchical nanostructured zinc–nickel–cobalt ternary (oxy)hydroxides with various morphologies. Using a facile hydrothermal method, a hierarchical architecture of well-aligned zinc–nickel–cobalt ternary (oxy)hydroxide nanowire arrays was grown on the surface of carbon fibers in carbon cloth, with a large mass loading of 7 mg cm−2. The electrode with optimized hierarchical nanostructures through careful control of temperature and reaction time exhibited a high areal capacitance of 2.14 F cm−2 at 3 mA cm−2 and an excellent rate capability in an alkaline electrolyte. The assembled asymmetric supercapacitor (ASC) can deliver an excellent volumetric capacitance of 5.4 F cm−3 as well as a high volumetric energy density of 2.43 mW h cm−3. More importantly, our ASC devices are able to reversibly cycle with a high operating voltage of 1.8 V with no capacitive decay after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.
000 cycles.
Results and discussion
Materials characterization and formation mechanism
We used a facile hydrothermal method for the synthesis of nanostructured Zn–Ni–Co TOH on carbon fibers of carbon cloth at different temperatures. The morphologies of the as-prepared samples depicted in Fig. 1 reveal a strong trend with the reaction temperature. A uniform Zn–Ni–Co TOH coating was obtained on each carbon fiber. At 110 °C, inter-connected Zn–Ni–Co TOH nanosheets (Zn–Ni–Co TOH-110) were grown on the surface of the carbon fiber substrate (Fig. 1b), with a diameter of ∼28 nm (Fig. 1c). At more elevated temperatures, the nanosheets disappeared and well-aligned nanowire arrays were grown (Zn–Ni–Co TOH-130, Fig. 1e). The carbon fiber was wrapped with radial nanowires with a diameter of ∼20 nm (Fig. 1f). Similar nanowires were also observed in the Zn–Ni–Co TOH reacted at 150 °C (Zn–Ni–Co TOH-150), with some micron-sized sheets shown on top (Fig. 1h and i).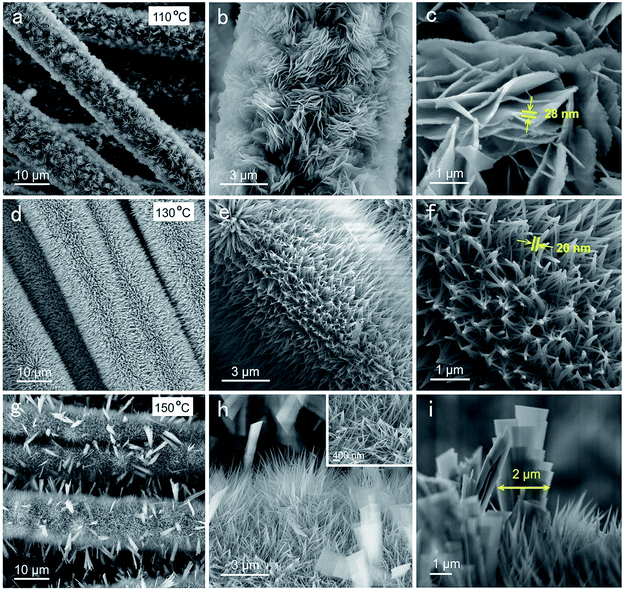 | ||
| Fig. 1 SEM images of Zn–Ni–Co TOH electrodes obtained using different hydrothermal reaction temperatures: (a–c) 110 °C, (d–f) 130 °C and (g–i) 150 °C. | ||
The detailed growth process of Zn–Ni–Co TOH was further characterized by a series of time-dependent experiments at temperatures of 110 °C, 130 °C and 150 °C, respectively. Fig. 2a–o show the SEM images of Zn–Ni–Co TOH obtained using various hydrothermal reaction times (1, 3, 5, 8, and 12 h) at three different temperatures, which discloses the morphological evolution of Zn–Ni–Co TOH. At 110 °C, thin and small Zn–Ni–Co TOH nanosheets were grown on the surface of the carbon fiber substrate and started to grow larger continuously up to 8 h (Fig. 2a–d). However, during 8 h to 12 h, nanosheets were transformed into aligned nanowires and resembled the morphology of the Zn–Ni–Co TOH-130 sample (Fig. 2e). At 130 °C, in contrast, the nanosheets started to grow at an earlier stage. The morphology of the sample at 130 °C for 1 h already resembled that of the one at 110 °C for 8 h. During 1 h to 3 h, we can see that nanowires appear looming in the nanosheet (Fig. 2g). Upon further increasing the reaction time (from 5 to 12 h), the nanowires continued to grow and resulted in the final radial nanowire structure. At 150 °C, nanosheets are similarly grown on the substrate at the beginning stage of hydrothermal reaction (Fig. 2k). The process of morphological evolution accelerates with increasing reaction temperature. The morphology of the sample reacted at 150 °C for 3 h already resembled that of the one at 130 °C for 5 h. When the reaction time increased from 5 to 12 h, nanowires aggregated to form micron-sized sheets (Fig. 2m–o). The energy dispersive X-ray spectroscopy (EDS) elemental mapping reveals the uniform distribution of Zn, Ni and Co elements in the Zn–Ni–Co TOH (Fig. 3a). It can be seen from the high-resolution transmission electron microscopy (HRTEM) image shown in Fig. 3b and c that Zn–Ni–Co TOH nanowires have an ∼4 nm thick disordered surface overlayer, suggesting plenty of defects on the surface of Zn–Ni–Co TOH nanowires, which are highly desirable for electrochemical applications.29Fig. 3d shows the HRTEM images of the Zn–Ni–Co TOH-130 sample. The selected areas in the HRTEM image are presented in Fig. 3e–i, in which both the mix-phases are observed. The lattice fringe spacings of about 0.21 and 0.23 nm could be indexed to the (101) and (121) planes of Co(OH)2 and CoOOH phases. Besides, the lattice fringe spacings of about 0.32, 0.14 and 0.22 nm could be indexed to the (110), (006) and (215) planes of Ni(OH)2, NiOOH and Zn(OH)2 phases, respectively. The selected area electron diffraction (SAED) pattern revealed that the nanowire has a poly-crystalline nature (Fig. 3j).
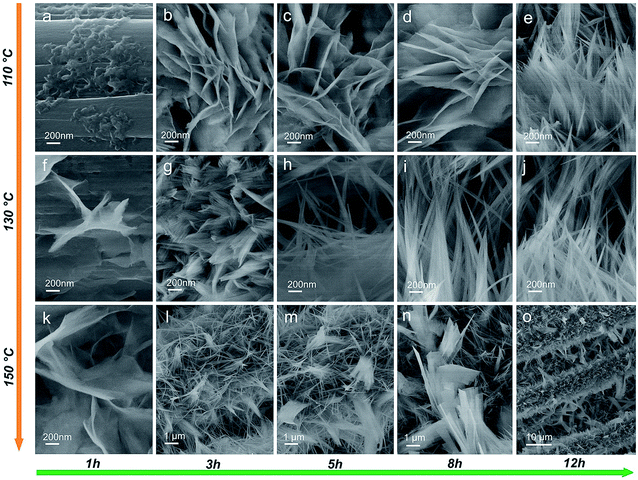 | ||
| Fig. 2 SEM images of (a–e) Zn–Ni–Co TOH-110, (f–j) Zn–Ni–Co TOH-130 and (k–o) Zn–Ni–Co TOH-150 afforded from different hydrothermal periods: 1 h, 3 h, 5 h, 8 h, and 12 h. | ||
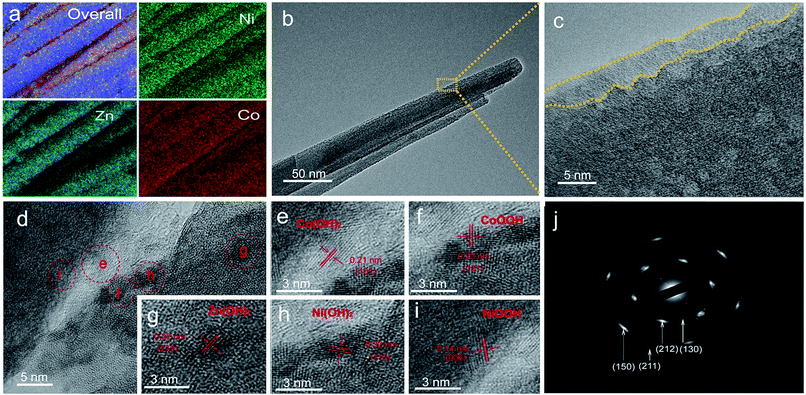 | ||
| Fig. 3 (a) Elemental mapping (Zn Kα1, Ni Kα1 and Co Kα1) images of Zn–Ni–Co TOH-130. (b–i) TEM images of the Zn–Ni–Co TOH-130 nanowire and (j) the corresponding SAED pattern. | ||
Our results demonstrate a temperature dependent stepwise growth mechanism of the Zn–Ni–Co TOH. Despite the final morphology varieties, the nanosheets are preferentially grown on the carbon fiber substrate at the beginning stage of hydrothermal reaction; however, the specific growth process depends on the hydrothermal temperature. The above results indicate that the morphology evolution of Zn–Ni–Co TOH is followed by “nanosheet growth—a larger nanosheet separated into nanowires—nanowires aggregated into microscale sheets”. The well-aligned nanowire array is the intermediate state of the entire evolution process of Zn–Ni–Co TOH and has ordered hierarchical nanostructures. The hierarchical structure of the three-dimensional distributed carbon fibers in a carbon cloth and the upwardly grown Zn–Ni–Co TOH nanowires provides a facile ion diffusion as well as a fast electron transport path, which are critical for electrochemical performance. It was also found that the hydrothermal temperature affects the morphology evolution rate of the Zn–Ni–Co TOH. Higher temperature accelerates new nucleation and growth of the material, accelerating the entire morphology evolution process of the Zn–Ni–Co TOH materials.
The crystal structures of the Zn–Ni–Co TOH materials were examined by X-ray diffraction (XRD, Fig. 4a). The XRD peaks of three Zn–Ni–Co TOH materials correspond to Co(OH)2 (JCPDS card 30-0443), CoOOH (JCPDS 26-0480), Ni(OH)2 (JCPDS 38-0715), NiOOH (JCPDS 06-0075) and Zn(OH)2 (JCPDS 38-0356). This suggests that the Zn–Ni–Co TOH nanostructures belong to the multi-phase nature, which is consistent with the SAED result. The broad XRD peaks were the result of the nano-sized material and/or the lattice defects created during the reaction in which multi-phases may diffuse into each other at the interphases.30–32 This can be extremely beneficial to electrochemical energy storage. Furthermore, X-ray photoelectron spectroscopy (XPS) was used to characterize the Zn–Ni–Co TOH materials. The XPS survey spectrum of Zn–Ni–Co TOH materials confirms the presence of Zn, Ni, and Co elements (Fig. S1†) in the sample. The atomic percentages of the Zn, Ni and Co for the Zn–Ni–Co TOHs are shown in Table S1.† The atomic percentage of Ni in Zn–Ni–Co TOH-130 was higher than that in Zn–Ni–Co TOH-110 and Zn–Ni–Co TOH-150, and the same trend is observed for Zn and Co. As the amount of Ni is closely related to pseudo-capacitance contribution and the active site density and Co/Zn related to the electrical conductivity, the chemical nature of each sample is expected to play an important role in determining the electrochemical performance.23Fig. 4b and c show the Co 2p, Ni 2p, Zn 2p and O 1s XPS spectra of the representative Zn–Ni–Co TOH-130 sample, while the others are depicted in Fig. S2 and S3.† In the Co 2p spectrum, two fitted peaks at the binding energies of 780.3 and 795.9 eV were assigned to the Co(III) oxidation state while two fitted peaks at 782.1 and 797.7 eV can be assigned to the Co(II) oxidation state.3,20,33 In the Ni 2p spectrum, two peaks at the binding energies of 855.5 eV (Ni 2p3/2) and 873.0 eV (Ni 2p1/2) were assigned to the Ni(II) oxidation state.33 In the Zn 2p spectrum, two well-defined peaks at binding energies of 1021.2 eV (Zn 2p3/2) and 1044.5 eV (Zn 2p1/2) were detected, which can be assigned to the Zn(II) oxidation state in the hydroxide.34 Finally, the O 1s peak is fitted with three components. The O1 peak at 530.3 eV is ascribed to the O2−. The peak at 531.1 eV (O2) can be assigned to the oxygen from defect sites with a low oxygen coordination in the material,35 which will be beneficial for charge storage capacity. The peak at 532.1 eV (O3) belongs to the O–H from the structural or physisorbed water.35,36 These results confirm the formation of Zn–Ni–Co ternary (oxy)hydroxides on the carbon fiber substrate.
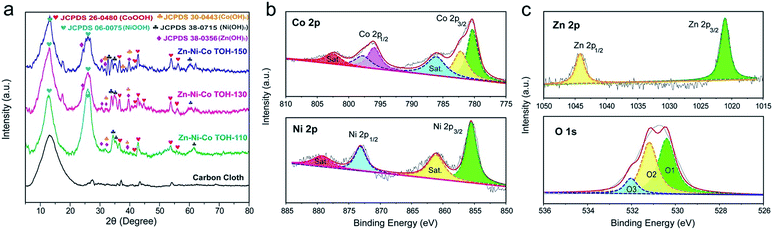 | ||
| Fig. 4 (a) XRD patterns of the as-prepared Zn–Ni–Co TOH-based materials. XPS spectra of (b) Co 2p and Ni 2p and (c) Zn 2p and O 1s of the Zn–Ni–Co TOH-130. | ||
Electrochemical performance
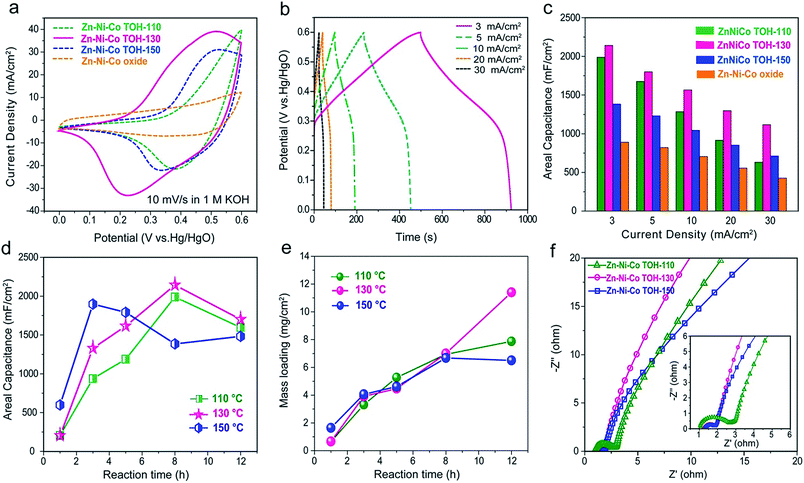
The electrochemical performances of the Zn–Ni–Co TOH and Zn–Ni–Co oxide electrodes were studied using a three electrode cell containing 1 M KOH electrolyte with a Hg/HgO electrode as the reference electrode and graphite foil as the counter electrode (Fig. S4†). Fig. 5a compares the cyclic voltammetry (CV) profiles of the Zn–Ni–Co TOH electrodes reacted at different hydrothermal temperatures as well as the Zn–Ni–Co oxide electrode. The CV profile of Zn–Ni–Co TOH-130 showed increased current as compared to the Zn–Ni–Co TOH-110 and Zn–Ni–Co TOH-150 electrodes in the whole potential range, demonstrating its high capacitive nature of charge storage. This increased current for the Zn–Ni–Co TOH-130 can be attributed to the fact that the well-aligned nanowire array nanostructures directly attached to the current collector, which enables the full exposure and good accessibility of electroactive sites to the liquid electrolyte. The Zn–Ni–Co oxide was achieved by annealing the precursor Zn–Ni–Co TOH-130 electrode. The annealing treatment of the electrode, however, leads to a capacitance decrease. The same trend is observed when the electrodes were tested by galvanostatic charge–discharge (GCD) analysis (Fig. S5†). A dramatic reduction in the capacitance of the Zn–Ni–Co oxide sample is probably due to the conversion to the corresponding oxide. In addition, to study the influence of multi-element transition metals on the electrochemical characteristics, CV curves of the Zn–Ni–Co TOH-130 and cobalt (oxy)hydroxide electrodes are compared in Fig. S6.† Evidently, the area surrounded by the CV curve of Zn–Ni–Co TOH-130 electrodes is considerably larger than that of the cobalt (oxy)hydroxide electrode, indicating the superior capacitive performance of the multi-element transition metal (oxy)hydroxide electrode. This could be due to the fact that the addition of nickel and zinc elements provides high capacity and good electrical conductivity, thus resulting in the improvement of capacitive performance.23–25Fig. 5b, S7 and S8† show the GCD profiles of the Zn–Ni–Co TOH electrodes collected at current densities between 3 and 30 mA cm−2. The calculated capacitances are illustrated in Fig. 5c and S9.† Among all the electrodes, the Zn–Ni–Co TOH-130 electrode displayed the largest capacity under all tested conditions. For example, at 3 mA cm−2, the Zn–Ni–Co TOH-130 yielded a high capacitance of 2.14 F cm−2 (equivalent to a gravimetric capacitance of 305 F g−1 normalized to a mass loading of 7 mg cm−2), which is larger than that of the other electrodes (Zn–Ni–Co TOH-110: 1.98 F cm−2, Zn–Ni–Co TOH-150: 1.38 F cm−2 and Zn–Ni–Co oxide: 0.89 F cm−2). The Zn–Ni–Co TOH-130 electrode also exhibited excellent rate capability performance. When the current density increases from 3 to 30 mA cm−2, the Zn–Ni–Co TOH-130 retained 52.3% of its initial capacitance, demonstrating a considerably high areal capacitance of 1.12 F cm−2 at high current density. In contrast, the capacitances of other samples decreased faster with the increase of the current density, with only 31.8% (0.63 F cm−2 for Zn–Ni–Co TOH-110), 51.4% (0.71 F cm−2 for Zn–Ni–Co TOH-150) and 47.9% (0.42 F cm−2 for Zn–Ni–Co oxide) areal capacitance remaining. Similar results were also obtained by CV measurements with scan rates normalized with the total charge–discharge time of the GCD processes (Fig. S10 and S11†). For example, the capacitance is calculated to be 2.30 F cm−2 for the Zn–Ni–Co TOH-130 electrode based on the data measured at 1.3 mV s−1 scan rate, close to the 2.14 F cm−2 capacitance measured by GCD at a current density of 3 mA cm−2, both with a similar discharging time. Close values were obtained for other conditions and other electrodes as well, verifying the results by the GCD measurements.
Discussion
The results reveal that the chemical nature of each transition metal and morphology play an important role in the charge storage capability of the Zn–Ni–Co TOH materials. The optimized material (Zn–Ni–Co TOH-130) with a hierarchical nanostructure is consisting of the 3D conductive carbon fiber in a carbon cloth skeleton interconnected with aligned electroactive nanowire arrays atop.On the one hand, the novel chemical combination of Zn, Ni and Co in the electrodes plays a critical role in the charge storage capability of the Zn–Ni–Co TOH. We then took a closer look at the material itself and found that in such Zn–Ni–Co ternary electrodes, Ni offers a high pseudo-capacitance contribution while Co and Zn elements possess good electrical conductivities. The mixed valence states at these multi-metal centers and synergetic effects in the complex chemical compositions endow the electrode with excellent electrochemical performance.22–25 XPS analysis suggests a higher atomic percentage of Zn, Ni and Co in the Zn–Ni–Co TOH-130 electrode, essentially establishing its excellent electrochemical performance.
On the other hand, to gain insight into the relationship between the morphology, mass loading and enhanced electrochemical performance, electrochemical tests of Zn–Ni–Co TOH electrodes obtained from different hydrothermal times (1, 3, 5, 8, and 12 h) were conducted. Fig. 5d and e show the areal capacitance and corresponding mass loading of the Zn–Ni–Co TOH collected at different hydrothermal temperatures as a function of reaction times. At 110 and 130 °C, the areal capacitance and mass loading increases drastically with the increase of reaction time up to 8 h, reaching the maximum value (1.98 F cm−2 for Zn–Ni–Co TOH-110 and 2.14 F cm−2 for Zn–Ni–Co TOH-130), followed by a decrease when the reaction time reached 12 h. We collected the SEM images of the electrode reacted for 12 h (11.4 mg cm−2, Fig. S12†). The sample shows an apparent stack of the deposited layers in carbon fiber substrate. This is not conducive to electrolyte diffusion into the entire electrode, which impedes thorough interaction, leading to its poor capacitance performance. At 150 °C, higher temperature accelerates nucleation and growth of the material, the aligned nanowire structure is formed in 3 h (Fig. 2l) and the capacitance reaches the maximum value in 3 h (Fig. 5d). Upon further reaction from 5 to 12 h, the nanowires started to agglomerate and resulted in a decrease of areal capacitance. These results reveal that the morphology plays one of the key roles in determining the final electrochemical performance. The Zn–Ni–Co TOH-130 material contains a nanoarray architecture with 3D conductive carbon fibers in a carbon cloth skeleton interconnected with aligned electro-active nanowires atop. The multiple connection points create a “super highway” for a fast electron transportation pathway. At the same time, the highly open well-defined nanoarray structure allows sufficient immersion of the electrolyte into the electrode, providing a rapid ionic transportation. The charge-transfer process in the Zn–Ni–Co TOH-130 electrode is thus fast enough to ensure large capacity and good rate capability with a high mass loading. In addition, the Zn–Ni–Co TOH-130 material has a poly-crystalline nature, as demonstrated earlier, exhibiting structural defects at the intersection of the phases and thus providing increased electrochemically active sites. More importantly, the Zn–Ni–Co hydroxides contain layered 2D structures for facile ion migration and high theoretical specific capacitance, which can be a significant contribution to the capacitive charge storage and rate capability as compared with the Zn–Ni–Co oxide.
Electrochemical impedance spectroscopy (EIS) was further utilized to explore the electrochemical performance of the Zn–Ni–Co TOH-based electrodes. As shown in the low-frequency domain (Fig. 5f), the ionic transport is more efficient in the Zn–Ni–Co TOH-130 as compared to the other electrodes. In addition, Zn–Ni–Co TOH-130 exhibited a considerably smaller semi-circle (correlated with the charge transfer resistance, Rct) compared to those of Zn–Ni–Co TOH-110 and Zn–Ni–Co TOH-150 owing to its large capacitance and good rate capability. We have also performed long-term cycling of Zn–Ni–Co TOH-based electrodes in a three-electrode system at a scan rate of 100 mV s−1. Fig. S13† compares the capacitance retention of the Zn–Ni–Co TOH-based electrodes. After 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, the Zn–Ni–Co TOH-110, 130, and 150 electrodes maintain 100.3%, 108.6% and 110.6% of the initial capacitance, respectively, demonstrating their high stability.
000 cycles, the Zn–Ni–Co TOH-110, 130, and 150 electrodes maintain 100.3%, 108.6% and 110.6% of the initial capacitance, respectively, demonstrating their high stability.
Asymmetric supercapacitor
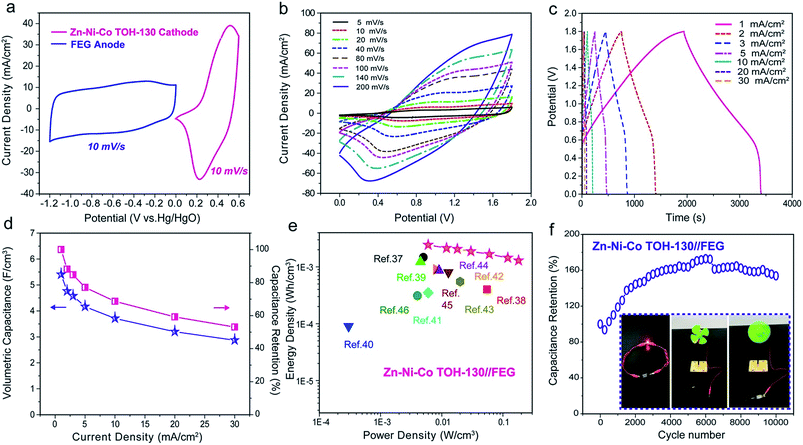
To evaluate the performance of the Zn–Ni–Co TOH electrode in the supercapacitor, an asymmetric supercapacitor (ASC) was fabricated by assembling a Zn–Ni–Co TOH-130 cathode, a functionalized partial-exfoliated graphite (FEG) anode and a piece of separator (see the Experimental section for details). The ASC is denoted as Zn–Ni–Co TOH-130//FEG. The FEG shows superior electrochemical performance within a potential window of 1.2 V (Fig. S14 and S15†). Fig. 6a shows the CV curves of the Zn–Ni–Co TOH-130 cathode (0 to 0.6 V) and FEG anode (−1.2 to 0 V) at 10 mV s−1, which in combination give rise to a large stable voltage window up to 1.8 V for the ASC. The capacitive performances of the ASC devices were investigated using the CV curves at different scan rates and GCD curves at various current densities. As shown in Fig. 6b, no significant distortion with the increase of scan rate was observed from the CV curves, suggesting the good rate capability of the ASC device. Fig. 6c depicts the GCD curves of the ASC devices collected at different current densities (1–30 mA cm−2). The symmetric and linear profiles demonstrate an ideal capacitive behavior. It can be seen from the GCD profile that the ASC displays a high volumetric capacitance of 5.4 F cm−3 at 1.0 mA cm−2. Interestingly, the ASC device still displayed a considerably high volumetric capacitance of 2.9 F cm−3 when the current density increased to 30 mA cm−2, demonstrating a good rate capability (Fig. 6d).Energy and power densities are two important factors to evaluate the energy storage performance of supercapacitors. Fig. 6e shows the Ragone plot of our Zn–Ni–Co TOH-130//FEG ASC device. The device displayed a high volumetric energy density of 2.43 mW h cm−3 at a power density of 6 mW cm−3. At a high power density of 180 mW cm−3, the ASC also displayed a significantly high energy density of 1.29 mW h cm−3. These values are essentially greater than the values obtained from other capacitor devices reported to date (Table S2†).37–46 We have calculated the gravimetric energy density and power density normalized to the total mass loadings of active materials on two electrodes (Fig. S16†). Our device achieved a gravimetric energy density of 33.6 W h kg−1 at a power density of 83 W kg−1 and an energy density of 17.9 W h kg−1 at a power density of 2490 W kg−1, which are higher than or comparable to those of other ASCs/SSCs assembled with nanostructured electrodes, such as Ni–Co oxide//AC (12.5 W h kg−1 at 100 W kg−1 and 7 W h kg−1 at 2000 W kg−1),47 Co3O4//Co3O4 (8 W h kg−1 at 700 W kg−1 and 4 W h kg−1 at 1600 W kg−1),48 rGO (reduced graphene oxide)/MoO3//rGO/MoO3 (2.2 W h kg−1 at 480 W kg−1 and 0.2 W h kg−1 at 580 W kg−1),49 rGO/CMOF-5//rGO/CMOF-5 (17.2 W h kg−1 at 250 W kg−1 and 7 W h kg−1 at 2200 W kg−1),50 CNT/PPy//CNT/MnO2 (22.8 W h kg−1 at 0.86 kW kg−1 and 6.2 W h kg−1 at 2.7 kW kg−1),51 vanadium nitride (VN) nanowire//VOx nanowire (2.1 W h kg−1 at 0.003 kW kg−1),52 and rGO/carbon cloth//SnO2/carbon cloth (22.8 W h kg−1 at 0.85 kW kg−1 and 4.84 W h kg−1 at 5.6 kW kg−1).53 Our ASC was also highly stable with a capacitance retention rate of 153% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at a scan rate of 50 mV s−1 (Fig. 6f), which is substantially better than previously reported capacitor devices (Table S2†). It can be observed that the specific capacity of the ASC device increases gradually, which may be due to the gradual activation of the electrode14,16 and fades very slowly after that, illustrating the excellent long-term electrochemical stability of the ASC (Fig. S17†). In addition, our ASC can effectively operate a red light-emitting diode (LED) or an electrical fan at the fully charged voltage, demonstrating its potential use for portable energy storage systems (see the insets in Fig. 6f).
000 cycles at a scan rate of 50 mV s−1 (Fig. 6f), which is substantially better than previously reported capacitor devices (Table S2†). It can be observed that the specific capacity of the ASC device increases gradually, which may be due to the gradual activation of the electrode14,16 and fades very slowly after that, illustrating the excellent long-term electrochemical stability of the ASC (Fig. S17†). In addition, our ASC can effectively operate a red light-emitting diode (LED) or an electrical fan at the fully charged voltage, demonstrating its potential use for portable energy storage systems (see the insets in Fig. 6f).
We attribute the outstanding electrochemical performances of the ASC device to the following key aspects: first, excellent capacitive performance is achieved for the ASC by assembling a high-performance pathway Zn–Ni–Co TOH-130 cathode and the FEG anode. The wide voltage window of 1.8 V dramatically increases the energy density of the device; second, the large loading of ordered nanoarrays allows efficient ion diffusion with a reduced diffusion length, thus resulting in a large pseudocapacitance. “Super highways” for fast electron transportation pathways are achieved by the highly open well-defined nanoarray architecture together with the ideal 3D conductive scaffold provided by the carbon fiber in the carbon cloth substrate; third, the direct growth of the nanoarrays on carbon fibers without the need for any binder provides small internal resistance of the ASC (this was supported by a negligible IR drop in the GCD profiles at all current densities in Fig. 5b). Such a small internal resistance can greatly reduce the energy dissipation and enhance the energy density.
Conclusions
In summary, a well-aligned zinc–nickel–cobalt ternary hydroxide nanoarray was grown on conductive carbon cloth as a promising cathode material for supercapacitors for the first time. Owing to the synergistic effects of novel chemical combination and morphological structures, the optimized Zn–Ni–Co hydroxide electrode displayed extraordinary rate performance and impressive capacity as compared to the Zn–Ni–Co oxide. The morphologies of the nanoarrays were carefully controlled by the hydrothermal temperature and reaction time to maximize the electrochemical performances. The optimized material (Zn–Ni–Co TOH-130), which is composed of the 3D conductive carbon fiber skeleton with the highly open nanoarray architecture atop, exhibited excellent ionic and electronic conductivities with a high mass loading of 7 mg cm−2. The Zn–Ni–Co TOH-130 electrode provides a high areal capacitance of 2.14 F cm−2 at 3 mA cm−2 and an excellent rate capability in an alkaline electrolyte. The ASC assembled with the Zn–Ni–Co TOH-130 cathode and FEG anode can deliver an excellent volumetric capacitance of 5.4 F cm−3 as well as a high volumetric energy density of 2.43 mW h cm−3. More importantly, our ASC devices are able to reversibly cycle with a high operating voltage of 1.8 V with no capacitive decay after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. This work provides key findings for the growth mechanism and corresponding electrochemical energy storage performances of ternary (oxy)hydroxides.
000 cycles. This work provides key findings for the growth mechanism and corresponding electrochemical energy storage performances of ternary (oxy)hydroxides.
Experimental section
Materials
All chemicals were of analytical grade and used as received. Carbon cloth and graphite foil were purchased from Fuel Cell Earth (United States) and SGL group (Germany), respectively.Synthesis of zinc–nickel–cobalt ternary (oxy)hydroxides on carbon cloth
The Zn–Ni–Co ternary (oxy)hydroxide was directly prepared on carbon cloth using a simple hydrothermal method. An aqueous solution (35 mL) containing 0.87 g Co(NO3)2·6H2O, 0.43 g Ni(NO3)2·6H2O, 0.44 g Zn(NO3)2·6H2O, 0.36 g CO(NH2)2 and 0.38 g CH3COONH4 was added into a 40 mL volume Teflon-lined autoclave. A piece of carbon cloth (3 cm × 4 cm) cleaned with ethanol and deionized water was immersed in the above solution. The sealed autoclave was heated in an electric oven at various temperatures such as 110 °C, 130 °C and 150 °C for 8 h to investigate the influence of temperature on the Zn–Ni–Co ternary (oxy)hydroxide growth. After the hydrothermal reaction, the autoclave was cooled down slowly at room temperature. The product was washed thoroughly with distilled water and dried in a vacuum at 60 °C for 24 h. Then the product was cycled between 0 and 0.8 V at 50 mV s−1 in 1 M KOH aqueous solution for 1200 cycles for electrochemical activation of the electrode, using graphite foil and Hg/HgO as the counter and reference electrodes, respectively. The products were denoted as Zn–Ni–Co TOH-110, Zn–Ni–Co TOH-130 and Zn–Ni–Co TOH-150. The active material mass loadings of Zn–Ni–Co TOH-110, Zn–Ni–Co TOH-130 and Zn–Ni–Co TOH-150 were ≈6.9, ≈7.0 and ≈6.7 mg cm−2, respectively. The Zn–Ni–Co oxide was obtained by annealing the Zn–Ni–Co TOH-130 at a temperature of 350 °C for 2 h in air.To investigate the detailed growth mechanism and morphological evolution of Zn–Ni–Co TOH-based samples, hydrothermal reaction was also conducted at 110 °C, 130 °C and 150 °C for different periods (1, 3, 5, 8, and 12 h).
Assembly of the Zn–Ni–Co TOH-130//FEG asymmetric electrochemical capacitor
The functionalized partial-exfoliated graphite (FEG) anode was fabricated as follows:54 first, graphite foil (1 × 1 cm2) was treated using cyclic voltammetry between 0.6 and 1.8 V at 20 mV s−1 in 0.5 M K2CO3 electrolyte for 7 cycles, using a Pt plate and a SCE as the counter and reference electrodes, respectively. Subsequently, the working electrode was further treated in 0.5 M KNO3 at 1.8 V vs. SCE for 3 h. To recover the electrical conductivity of the electrode, the working electrode was then reduced using cyclic voltammetry from −1.0 to 0.9 V vs. SCE for 60 cycles at a scan rate of 50 mV s−1 in 3 M KCl electrolyte. Subsequently, the Zn–Ni–Co TOH-130 (cathode), FEG (anode), and a piece of cellulose paper (NKK separator, Japan) as a separator were soaked in 1 M KOH aqueous electrolyte for 5 min. The entire device was wrapped and sealed with parafilm to prevent the electrolyte from drying. The working area of the device was 1.0 cm2. The volume of the device (including the volume of the two electrodes and separator) was 150 mm3 [10 mm (L) × 10 mm (W) × 1.5 mm (H)].Characterization
A scanning electron microscope (HITACHI, SU8010, Japan) and a TEM (JEM-2010) were used to probe the morphologies of the as-prepared samples. XPS was performed on an XPS spectrometer (ESCALAB 250Xi, Thermo Scientific Escalab, USA) with Al-Kα radiation (8.34 Å) as the excitation source. The data were calibrated by referencing the C 1s peak to 284.6 eV. The mass loading of the active materials was measured using the weight difference of the electrode before and after hydrothermal reaction, using a microbalance with a sensitivity of 0.01 mg (BT 25 S, Sartorius, Germany). The crystal structures of the samples were characterized by X-ray diffraction (X'Pert Pro, PANalytical B.V.).Electrochemical tests
Electrochemical measurements were conducted using a CHI 760E electrochemical work station. The electrochemical performance of Zn–Ni–Co TOH samples was tested in a three-electrode cell with a Hg/HgO electrode and graphite foil as the reference and counter electrodes, respectively, in 1 M KOH electrolyte. EIS was carried out at open-circuit potentials with a perturbation of 10 mV and in a frequency range from 0.01 Hz to 40 kHz. Electrochemical performance of the Zn–Ni–Co TOH-130//FEG was evaluated using a two-electrode testing system.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Thousand Talents Program of China, the Pan Deng Scholars Program of Liaoning Province, the Science and Technology Innovative Talents Support Program of Shenyang (RC180166), the Australian Research Council (ARC) through Discovery Early Career Researcher Award (DE150101306) and the Linkage Project (LP160100927).References
- P. Zhang, B. Y. Guan, L. Yu and X. W. Lou, Angew. Chem., Int. Ed., 2017, 56, 7141–7145 CrossRef CAS PubMed.
- P. Simon, Y. Gogotsi and B. Dunn, Science, 2014, 343, 1210–1211 CrossRef CAS PubMed.
- Y. Zeng, Z. Lai, Y. Han, H. Zhang, S. Xie and X. Lu, Adv. Mater., 2018, 1802396 CrossRef PubMed.
- Z. H. Huang, T. Y. Liu, Y. Song, Y. Li and X. X. Liu, Nanoscale, 2017, 9, 13119–13127 RSC.
- M. Yu, D. Lin, H. Feng, Y. Zeng, Y. Tong and X. Lu, Angew. Chem., Int. Ed., 2017, 56, 1–6 CrossRef.
- Z. H. Huang, Y. Song, D. Y. Feng, Z. Sun, X. Sun and X. X. Liu, ACS Nano, 2018, 12, 3557–3567 CrossRef CAS PubMed.
- L. Hu, C. Shi, K. Guo, T. Zhai, H. Li and Y. Wang, Angew. Chem., Int. Ed., 2018, 130, 8346–8350 CrossRef.
- Q. Yun, Q. Lu, X. Zhang, C. Tan and H. Zhang, Angew. Chem., Int. Ed., 2018, 57, 626–646 CrossRef CAS PubMed.
- Z. H. Huang, Y. Song, X. X. Xu and X. X. Liu, ACS Appl. Mater. Interfaces, 2015, 7, 25506–25513 CrossRef CAS PubMed.
- F. Ma, L. Yu, C. Xu and X. Lou, Energy Environ. Sci., 2016, 9, 862–866 RSC.
- S. Peng, L. Li, Y. Hu, M. Srinivasan, F. Cheng, J. Chen and S. Ramakrishna, ACS Nano, 2015, 9, 1945–1954 CrossRef CAS PubMed.
- Z. H. Huang, Y. Song and X. X. Liu, Chem. Eng. J., 2019, 358, 1529–1538 CrossRef CAS.
- S. Peng, L. Li, H. Wu, S. Madhavi and X. W. Lou, Adv. Energy Mater., 2015, 5, 1401172 CrossRef.
- L. Shen, Q. Che, H. Li and X. Zhang, Adv. Funct. Mater., 2014, 24, 2630–2637 CrossRef CAS.
- F. Zhang, C. Yuan, X. Lu, L. Zhang, Q. Che and X. Zhang, J. Power Sources, 2012, 203, 250–256 CrossRef CAS.
- X. Xia, J. Tu, Y. Zhang, X. Wang, C. Gu, X. Zhao and H. J. Fan, ACS Nano, 2012, 6, 5531–5538 CrossRef CAS PubMed.
- Q. Zhang, B. Zhao, J. Wang, C. Qu, H. Sun and K. Zhang, Nano Energy, 2016, 28, 475–485 CrossRef.
- K. Xiao, L. Xia, G. Liu, S. Wang, L. Ding and H. Wang, J. Mater. Chem. A, 2015, 3, 6128–6135 RSC.
- J. Wang, X. Zhang, Q. Wei, H. Lv, Y. Tian, Z. Tong, X. Liu, J. Hao, H. Qu, J. Zhao, Y. Li and L. Mai, Nano Energy, 2016, 19, 222–233 CrossRef CAS.
- W. Hu, H. Wei, Y. She, X. Tang, M. Zhou, Z. Zang, J. Du, C. Gao and D. Bao, J. Alloys Compd., 2017, 708, 146–153 CrossRef CAS.
- J. Tang, D. Liu, Y. Zheng, X. Li, X. Wang and D. He, J. Mater. Chem. A, 2014, 2, 2585–2591 RSC.
- C. Yuan, H. Wu, Y. Xie and X. W. Lou, Angew. Chem., Int. Ed., 2014, 53, 1488–1504 CrossRef CAS PubMed.
- C. Wu, J. Cai, Q. Zhang, X. Zhou, Y. Zhu, P. Shen and K. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 26512–26521 CrossRef CAS PubMed.
- B. Liu, B. Liu, Q. Wang, X. Wang, Q. Wang, D. Chen and G. Shen, ACS Appl. Mater. Interfaces, 2013, 5, 10011–10017 CrossRef CAS PubMed.
- J. H. Zhong, A. L. Wang, G. R. Li, J. W. Wang, Y. N. Ou and Y. X. Tong, J. Mater. Chem., 2012, 22, 5656–5665 RSC.
- J. Yang, C. Yu, X. Fan and J. Qiu, Adv. Energy Mater., 2014, 1400761 CrossRef.
- C. Shang, S. Dong, S. Wang, D. Xiao, P. Han, X. Wang, L. Gu and G. Cui, ACS Nano, 2013, 7, 5430–5436 CrossRef CAS PubMed.
- J. Yang, C. Yu, C. Hu, M. Wang, S. Li, H. Huang, K. Bustullo, X. Han, C. Zhao, W. Guo, Z. Zeng, H. Zheng and J. Qiu, Adv. Funct. Mater., 2018, 1803272 CrossRef.
- Z. Lei, J. Zhang and X. S. Zhao, J. Mater. Chem., 2012, 22, 153–160 RSC.
- Q. Wu, J. Xu, X. Yang, F. Lu, S. He, J. Yang, H. J. Fan and M. Wu, Adv. Energy Mater., 2015, 5, 1401756 CrossRef.
- Y. Zheng, Y. Jiao, M. Jaroniec and S. Z. Qiao, Angew. Chem., Int. Ed., 2015, 54, 52–65 CrossRef CAS PubMed.
- D. Yang, H. Liu, Z. Zheng, Y. Yuan, J. C. Zhao, E. R. Waclawik, X. Ke and H. Zhu, J. Am. Chem. Soc., 2009, 131, 17885–17893 CrossRef CAS PubMed.
- J. X. Feng, S. H. Ye, A. L. Wang, X. F. Lu and Y. X. Tong, Adv. Funct. Mater., 2014, 24, 7039–7101 CrossRef.
- L. Qian, L. Gu, L. Yang, H. Yuan and D. Xiao, Nanoscale, 2013, 5, 7388–7396 RSC.
- C. Guan, X. Liu, W. Ren, X. Li, C. Cheng and J. Wang, Adv. Energy Mater., 2017, 7, 1602391 CrossRef.
- C. Zhu, S. Fu, D. Du and Y. Lin, Chem.–Eur. J., 2016, 22, 4000–4007 CrossRef CAS PubMed.
- G. Yilmaz, C. X. Guo and X. M. Lu, ChemElectroChem, 2016, 3, 158–164 Search PubMed.
- X. H. Lu, Y. X. Zeng, M. H. Yu, T. Zhai, C. L. Liang, S. L. Xie, M. Balogun and Y. X. Tong, Adv. Mater., 2014, 26, 3148–3155 CrossRef CAS PubMed.
- Z. Zhang, K. Chi, F. Xiao and S. Wang, J. Mater. Chem. A, 2015, 3, 12828–12835 Search PubMed.
- F. M. Wang, Y. C. Li, Z. Z. Cheng, K. Xu, X. Y. Zhan, Z. X. Wang and J. He, Phys. Chem. Chem. Phys., 2014, 16, 12214–12220 RSC.
- J. X. Feng, S. H. Ye, X. F. Lu, Y. X. Tong and G. R. Li, ACS Appl. Mater. Interfaces, 2015, 7, 11444–11451 CrossRef CAS PubMed.
- Z. Zhang, F. Xiao and S. Wang, J. Mater. Chem. A, 2015, 3, 11215–11223 RSC.
- P. H. Yang, Y. Ding, Z. Y. Lin, Z. W. Chen, Y. Z. Li, P. F. Qiang, M. Ebrahimi, W. J. Mai, C. P. Wong and Z. L. Wang, Nano Lett., 2014, 14, 731–736 CrossRef CAS PubMed.
- T. Zhai, X. H. Lu, Y. C. Ling, M. H. Yu, G. M. Wang, T. Y. Liu, C. L. Liang, Y. X. Tong and Y. Li, Adv. Mater., 2014, 26, 5869–5875 CrossRef CAS PubMed.
- D. Y. Feng, Y. Song, Z. H. Huang, X. X. Xu and X. X. Liu, J. Power Sources, 2016, 324, 788–797 CrossRef CAS.
- Q. Q. Tang, M. M. Chen, C. Y. Yang, W. Q. Wang, H. Bao and G. C. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 15303–15313 Search PubMed.
- C. H. Tang, Z. Tang and H. Gong, J. Electrochem. Soc., 2012, 159, A651–A656 CrossRef CAS.
- R. R. Salunkhe, J. Tang, Y. Kamachi, T. Nakato, J. H. Kim and Y. Yamauchi, ACS Nano, 2015, 9, 6288–6296 CrossRef CAS PubMed.
- X. Cao, B. Zheng, W. Shi, J. Yang, Z. Fan, Z. Luo, X. Rui, B. Chen, Q. Yan and H. Zhang, Adv. Mater., 2015, 27, 4695–4701 CrossRef CAS PubMed.
- P. Wen, Z. Li, P. Gong, J. Sun, J. Wang and S. Yang, RSC Adv., 2016, 6, 13264–13271 RSC.
- J. Liu, L. Zhang, H. Wu, J. Lin, Z. Shen and X. W. Lou, Energy Environ. Sci., 2014, 7, 3709–3719 RSC.
- X. Lu, M. Yu, T. Zhai, C. Wang, S. Xie, T. Liu, C. Liang, Y. Tong and Y. Li, Nano Lett., 2013, 13, 2628–2633 Search PubMed.
- Y. Zhang, Z. Hu, Y. Liang, Y. Yang, N. An, Z. Li and H. Wu, J. Mater. Chem. A, 2015, 3, 15057–15067 RSC.
- Y. Song, T. Y. Liu, G. L. Xu, D. Y. Feng, B. Yao, T. Y. Kou, X. X. Liu and Y. Li, J. Mater. Chem. A, 2016, 4, 7683–7688 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ta01995b |
| This journal is © The Royal Society of Chemistry 2019 |