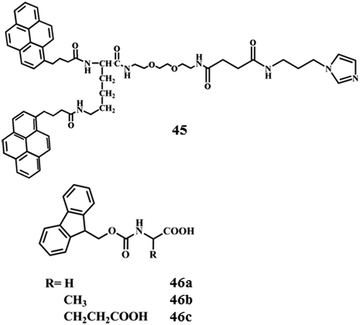
Fabrication of soft-nanocomposites from functional molecules with diversified applications
Pritam
Choudhury
,
Soumik
Dinda
and
Prasanta
Kumar Das
 *
*
School of Biological Sciences, Indian Association for the Cultivation of Science Jadavpur, Kolkata-700 032, India. E-mail: bcpkd@iacs.res.in
First published on 2nd October 2019
Abstract
With the increasing demand for new soft materials having excellent physical and biological characteristics and functionality, the design of hybrid materials offers a simple, yet versatile platform for the development of materials with specific and tunable properties. By definition a “soft-nanocomposite” is the combination of supramolecular self-assemblies with nanomaterials of different origins (inorganic/metallic nanoparticles and carbonaceous allotropes like carbon nanotubes and graphene) through covalent/non-covalent interactions. Dynamic supramolecular self-assemblies can serve as excellent hosts for the incorporation of these dimensionally different nanomaterials. Nanomaterials within the matrix of supramolecular self-assemblies can give rise to new characteristics due to the synergistic contribution of both materials. Although the very initial work intended to use molecular gels as media for the preparation and stabilization of nanoparticles, recent reports have suggested that amalgamation of different supramolecular self-assemblies with nanoparticles is advantageous for both constituents. These newly developed soft-nanocomposites have interesting properties including electrical conductivity, viscoelasticity, thermal robustness, magnetic, phase-selective, redox and near-infrared radiation sensitive properties and so on. This review will focus on some of the most recent advancements in the development of novel soft-nanocomposites. In particular, we intend to correlate various design strategies for synthesis as well as composite preparation from functional molecules with interesting applications in the area of supercapacitors, nanoelectronics, photovoltaic devices, chemical and biosensors, biomedicine and so on. We expect that this article will be a general and conceptual demonstration of various approaches to develop different soft-nanocomposites and will highlight their applications across disciplines.
1 Introduction
After the first advent of functional hybrid materials combining the best specific properties of organic and inorganic materials, it is time to focus on the advancement of soft-nanocomposites which have widespread applications across the disciplines from materials sciences to biological sciences.1–7 With the continuous surge of increasing demand for new soft materials, the design of hybrid materials offers a simple, yet versatile, platform for the development of materials with specific and tunable properties. The formation of “soft-nanocomposites” is governed by different covalent/non-covalent interactions through the amalgamation of supramolecular soft-materials with multidimensional nanomaterials (inorganic/metallic nanoparticles and carbonaceous allotropes like carbon nanotubes and graphene). Suitably tailored nanoscopic entities have the tendency to interact at the molecular level with supramolecular soft-materials derived from either small molecules or polymers. Therefore, these self-assembled entities can act as a “host” to integrate multidimensional nanomaterials as interactive “guest” systems to form soft-nanocomposites. Several of these aspects are described in the following sections.1.1 Supramolecular self-assemblies as hosts
Supramolecular aggregation of small amphiphilic molecules often leads to different kinds of soft-materials like micelles, reverse micelles, vesicles, fibres, supramolecular gels and so on.1,2 Dynamic supramolecular self-assemblies can serve as excellent hosts for the incorporation of dimensionally different nanomaterials.8–18 Nanomaterials within the matrix of supramolecular self-assemblies can give rise to altogether new characteristics due to the synergistic contribution of both materials.17,18 Although the very initial work intended to use molecular gels as the host/template for the preparation and stabilization of nanoparticles, recent reports have suggested that amalgamation of different supramolecular self-assemblies with nanoparticles is advantageous for both constituents.11,13,17 Each of the manifestations of the self-assembled structures of amphiphilic molecules in the chosen solvent has its individual prominences and finds noteworthy applications in the area of chemistry, physics, biology, engineering and others.1,5,19 To this end, multiple cargos and biologically relevant molecules have been successfully loaded within the matrix of the different self-assembled soft-materials.20,21 All these incorporations of foreign substances within the nanoscopic space of the three-dimensional (3D) network of soft-materials mainly focused on the targeted task specific applications.20,21 With the advent of nanoscience and nanotechnology, advancement in the development of hybrid nanocomposites is considered as an attractive, versatile, technological platform for future electronic, optical, magnetic, and biomedical applications. The common thread between the two constituents in soft nanocomposites is that both are governed by colloidal stability where weak forces provide stability from the nanoscopic to microscopic domains.19 The progress observed in this area is primarily due to the availability of diverse ways of preparing soft-nanocomposites of different nature.18,19 It is indeed a challenge to make a homogeneous dispersion of inorganic nano-objects into a macroscopic supramolecular matrix formed by either polymers or amphiphilic molecules. For instance, gold, silver, and other metal nanoparticles when capped with suitable organic amphiphiles produce stable dispersions in common lipophilic solvents. Such surface capping agents enable them to exert van der Waals and other non-covalent interactions with the host.18,19 Thus the precursors with multimodal functionality plays a crucial role as superior hosts.1,181.2 Nano-objects as guests
Various nanomaterials including metal and non-metal nanoparticles as well as carbon nanomaterials (CNMs) have been extensively exploited toward the formation of soft-nanohybrids.17–19 Incorporation of these nanomaterials within the organic matrices of supramolecular self-assemblies may lead to interesting properties including viscoelasticity, thermal robustness, magnetic, phase-selective, redox, near-infrared radiation sensitive properties and so on.1,18 More specifically, metal nanoparticle based soft-nanocomposites have gained enormous importance due to their interesting optical, electronic, magnetic, and catalytic properties. Similarly, silica nanoparticles (SiNP) can be utilised for hydrogel-storing which is a critical feature for nano-medical applications.19 Soft-nanocomposites based on other nanomaterials like TiO2, ZrO2, ZnO, and WO3 are also well-known for their unique properties.19 To this end, another fascinating class of soft-nanocomposites that has received enormous attention in the recent past is carbon nanomaterial-based soft-composites. According to the shape, nanoscopic carbon allotropes vary from 3-dimensional (3D), fullerenes, to zero-dimensional, carbon dots (CDs). Hence, the incorporation of multiple dimensional carbon-based nanomaterials as well as different types of metal-based nanoparticles within the matrices of soft-materials has led to the formation of integrated systems with unprecedented properties having potential applications in the area of materials science, biomedicine, nano-biotechnology and others. For example, inclusion of fullerenes is extensively applied for the development of organic solar cells where the fullerene acts as an electron acceptor.18 CNTs are next generation 1D nanocarbons which consist of graphene sheets rolled up into a tubular shape and this property makes them very useful for delivery purposes.18,191.3 Host–guest interactions: formation of soft-nanocomposites
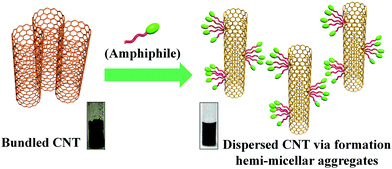
The strategy to design and develop soft-nanocomposites with task specific properties is one of the exciting ongoing research domains. To prepare soft-nanocomposites, both constituents get amalgamated through either covalent or non-covalent interactions that include π–π stacking, van der Waals forces, hydrogen bonding, dipolar interactions, electrostatic interactions and others. One of the prime reasons for the preparation of these hybrid nanocomposites is that they often lead to novel materials with superior properties compared to the individual constituents. For example, inclusion of nanomaterials in the gel matrix improves the physical properties of the gel like an increase in its viscoelasticity, gel-melting temperature, variation in thermal mesophases behaviour, etc.18 On the other hand, gold, silver, and other metal nanoparticles when capped with suitable organic ligands produce a stable dispersion in otherwise immiscible lipophilic solvents.17 Decoration of the highly hydrophobic carbon nanotube (CNT) surface by water soluble cytocompatible vesicular aggregates can significantly reduce the toxic effect of the CNT and make it more suitable as a cellular transporter.22 Hence, the synergistic contribution of each component of hybrid materials plays a pivotal role in the notable improvement of their individual properties.2 Metal nanoparticle based soft-nanocomposites
Metal nanoparticles (MNPs) like Au, Ag, Pt and Pd and other metal based nanoparticles such as CdS, ZnO, TiO2, Fe2O3, etc. have gained attention for their catalytic properties and opto-electronic and magnetic applications.23,24 Various supramolecular self-aggregates are utilized as templates for the synthesis of inorganic nanoparticles to prepare soft-nanocomposites. Such MNPs were synthesized either externally and added to a different self-assembled system or were synthesized in situ with or without the assistance of soft-materials. According to the mode of development of different MNP-based soft-nanocomposites and their applications, we discuss these areas in the following sub-sections.2.1 Gold nanoparticle-based soft-nanocomposites
Reports on gold nanoparticle (GNP)-based soft-nanocomposites that have been developed using diverse soft-material templates like micelles, reverse micelles, Langmuir–Blodgett (LB) films, vesicles, gel matrix and so forth are the most abundant. For example, Mandal et al. synthesized GNPs via UV irradiation of HAuCl4 in a poly(oxyethylene)isooctyl phenyl ether (TX-100) based micellar system.11 The photoactivation technique offers a very simple way by which almost monodispersed (tight size distribution) metal nanoparticles can be generated. Here TX-100 serves as a reducing agent (triggered by UV light) and simultaneously acts as a stabilizer and provides the required template for linear aggregation of gold nanoparticles. The size and shape of the GNPs were regulated by varying the surfactant (TX-100) concentration (Fig. 1a). Using higher concentrations of TX-100 (8.1 × 10−2 mol dm−3) and keeping the concentration of HAuCl4 constant, different shapes (rod, cubic, and tetragonal) of GNPs were obtained along with spherical particles (Fig. 1a). The formation of various shaped GNPs were formed due to successive growth of GNPs and this phenomenon made them haphazard. This haphazardly formed NPs often forms different shaped (spherical, triangular, rod, cubic) GNPs. Instead of micelles, Herrera et al. developed a soft-nanocomposite of GNPs in a sodium bis-(2-ethylhexyl) sulfoccinate (AOT) based reverse micelle in isooctane.12 A core–shell Mie model depicted that GNPs are either retained within the water pool of the reverse micelles or layered by AOT.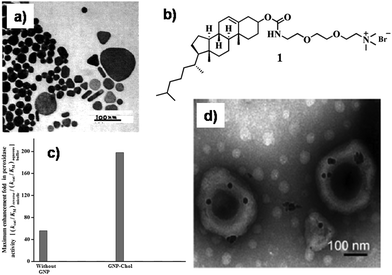 | ||
| Fig. 1 (a) TEM image of different shaped GNPs. Conditions: [TX-100] = 8.1 × 10−2 mol dm−3; [Au] = 9.5 × 10−4 mol dm−3. Irradiation time = 25 min. Reproduced with permission from ref. 11, Langmuir, 2002, 18, 7792–7797, Copyright 2000 American Chemical Society. (b) Chemical structure of compounds 1. (c) Maximum peroxidase activity enhancement for cyt c in CTAB (50 mm)/water/isooctane/n-hexanol (z = 16) reverse micelles with respect to aqueous buffer in the presence of GNP525 capped with amphiphiles with varying hydrophobicity at 25 °C and pH = 7.0 (25 mm phosphate buffer). [Cyt c] = 0.8 mm; [GNP525] = 10 nm; diameter of GNPs = 8–10 nm; [pyrogallol] = 10 mm; [H2O2] = 0.5–30 mm. (d) TEM image of GNPs synthesized in vesicles. Reproduced with permission from ref. 13 and 14, Wiley VCH. | ||
Surface functionalized GNPs often regulate the activity of biologically important molecules such as enzymes, proteins, etc. in compartmentalized systems. In this context, Maiti et al. monitored the peroxidase activity of cytochrome c (cyt c) within cetyltrimethylammonium bromide (CTAB) reverse micelles.13 In native CTAB reverse micelles, cyt c showed 56-fold and 1.3-fold higher activity than in pure water and AOT based reverse micelles, respectively. However, the peroxidase activity of cyt c got remarkably enhanced within CTAB reverse micelles by altering their structure due to different surface-functionalized GNPs. The hydrophobicity of the surface functionalized GNPs was judiciously modulated from a long alkyl chain to rigid cholesterol residue 1 (Fig. 1b). The hydrophobic moiety on the surface of the GNPs was headed towards the interface inducing huge steric strain. The hydrophobic cholesterol moiety of the surface-functionalized GNP (GNP-Chol) remarkably enhanced the cyt c activity up to 200-fold compared to that found in aqueous buffer (Fig. 1c). The combined crowding effect due to the surfactant/co-surfactant shell and the GNPs and their surface-functionalized hydrophobicity around cyt c was sufficient to disrupt the tertiary structure of the protein. The unfolding in the tertiary structure of cyt c due to the favorable hydrophobic interactions provided easier substrate accessibility to the exposed heme part of the protein and resulted in a notable improvement in the peroxidase activity of cyt c within the compartmentalized system in the presence of surface-functionalized GNPs. Thus, novel soft-nanocomposites formed using surface functionalized GNP doped reverse micelles can act as a peroxidase activity regulator for mitochondrial membrane proteins.
Apart from micelles/reverse micelles, vesicles constitute another template that has been used for the development of GNP-based soft-nanocomposites. For example, Shome et al. synthesized GNPs in vesicles devoid of any exterior reducing agent (Fig. 1d).14 The vesicles were formed by mixing amino acid-based cationic surfactants with two anionic surfactants [sodium dodecylbenzene sulfonate (SDBS) and sodium dodecyl sulfate (SDS)]. L-Tryptophan or L-tyrosinate residue containing surfactants have the ability to synthesize in situ GNPs in the vesicular system. The presence of an electron rich indole group (L-tryptophan unit) or phenoxide group (L-tyrosinate unit) facilitates in situ reduction of HAuCl4 to form colloidal Au(0) particles. Simultaneously, these GNPs also get stabilized in the presence of these amino acid tagged cationic surfactants. These GNP entrapped vesicles (soft-nanocomposite) will have significant applications in determining the internalization of drug/protein-loaded vesicles into cells. Song et al. developed plasmonic vesicles assembled from surface-enhanced Raman scattering (SERS)-encoded amphiphilic GNPs for targeted drug delivery.15 In general, Raman active molecules are non-specifically adsorbed on the GNP surface. In this study, the small molecular ligand 2,2′-dithiobis[1-(2-bromo-2-methylpropionyloxy)]ethane (DTBE) can easily substitute the weakly bound Raman molecules due to the formation of the more favorable Au–S bond. In contrast, the Raman dye 2-(4-(bis(4-(diethylamino)phenyl) (hydroxy)methyl)phenoxy)ethyl 5-(1,2-dithiolan-3-yl)pentanoate (BGLA) with a multivalent disulfide anchoring group can survive over the ligand exchange reaction with polyethylene glycol (PEG) and DTBE. These Raman active GNPs were amalgamated with polymeric vesicles to develop a pH-sensitive plasmonic soft-nanocomposite which can be utilized as a multifunctional drug carrier by stimulated intracellular drug release in the acidic micro-environment of specific organelles. This soft-nanocomposite can be used to give unique optical and spectroscopic feedback on the cargo release by plasmonic imaging and SERS spectroscopy.
Supramolecular gels are comprised of nano-fibrillar networks, which are held together by non-covalent interactions among the gelators. Such soft materials have been used significantly as matrices for the development of GNP based soft-nanocomposites. At the early stage, GNPs were synthesized externally with different capping ligands and then mixed within supramolecular self-assemblies. For instance, Bhattacharya and co-workers incorporated functionalized GNPs with different thiol-based capping agents in the gel matrix of amino acid based small amphiphilic molecule 2 (Fig. 2a).25 Incorporation of GNPs altered the microstructure of the gel aggregate without compromising the gelation ability. Among these capping agents, long chain (AuC12) and cholesterol containing (AuChol) GNPs were prone to stabilize the supramolecular aggregates through van der Waals interaction. However, the rigid, small capping agent tailored GNPs (AuPhMe) showed poor stabilization of the gel matrix due to the lack of hydrophobic interaction. The microscopy images clearly indicated that the interaction of GNPs with the gelator molecules (2) was intimately related to the molecular structure of the capping agent tethered on the GNP surface (Fig. 2b and c). These findings were also reflected in the viscoelastic properties of the soft-nanocomposites. With the increment of chain length and the hydrophobicity of the capping agent, the mechanical stiffness of the organogel got increased (∼450 Pa for AuChol) (Fig. 2d). Other amino acid appended gelators have been utilized subsequently for the integration of GNPs. To this end, Coates et al. synthesized bola-amphiphilic type gelator molecules 3a and 3b (Fig. 2a) comprised of Boc-protected L-cysteine and L-tryptophan at both the terminals, respectively.26 The presence of a cysteinyl moiety in the molecular backbone (3a) facilitates the S–Au interaction to develop a GNP-based soft-nanocomposite. The GNPs may actually be inside the larger hierarchically assembled fibers. The GNPs also seem to be spread out along the fibers rather than clustered. It is possible that the smaller, more highly curved gold nanoparticles are better able to interact with the thioethers decorating the surface of the gel nanofibrils via the formation of S–Au interactions. Certainly it appears in this case that gelator:GNP interactions are sufficiently strong to dominate the GNP:GNP interactions which would be expected to lead to nanoparticle clustering. The nanoparticles appear to be actually on, or embedded in, the hierarchically assembled nanofibers, rather than simply adjacent to them. The resulting nanohybrids were highly dynamic in nature and could also respond to an external stimulus (e.g., heating) as a consequence of the non-covalent interactions. When the xerogel embedded with GNPs was heated, Ostwald ripening of the nanoparticles was observed within the xerogel, with an increase in the diameter of the fiber. A degree of nanoparticle alignment into ‘wire-like’ aggregates can be observed, but in general it appears that the nanoparticles become detached from the fibres and that a ‘negative staining’ effect is being observed. It seems likely that under heating conditions, the thioether–gold (S–Au) interactions become weak. Simultaneously the embedded GNPs get detached from the fibrillar network and arrange in an ordered array. These hybrid materials have potential applications in nanoscale electronics and as flow through reusable catalysts for chemical transformations.
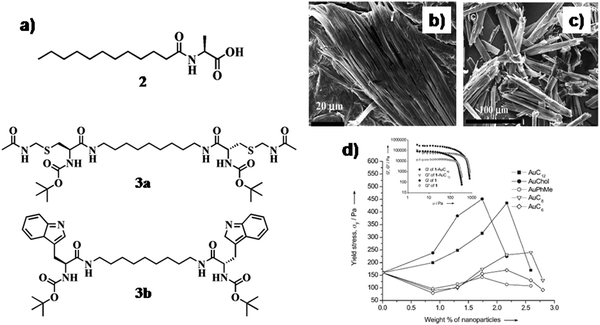 | ||
| Fig. 2 (a) Chemical structure of compounds 2 and 3; SEM images of xerogels of the (b) 2-AuC12 composite and (c) 2-AuChol composite. (d) Plots of yield stress vs. nanoparticle concentration. Inset: Plots of storage modulus G′ and loss modulus G′′ vs. stress for a gel of 2 in toluene and a 2-AuC12 composite containing 2.17 wt% of nanoparticles. Reproduced with permission from ref. 25, Wiley VCH. | ||
In another instance, Kimura et al. designed an organogelator 4 (Fig. 3a) comprised of trans-1,2-bis(alkylamide)cyclohexane and thiol moieties and pre-formed GNPs were self-organized in this self-assembled fibrillar network (SAFIN) of the gel matrix to form a stable soft-nanocomposite.27 Externally synthesized GNPs (average diameter 1.7 nm, surface plasmon resonance, SPRmax = 512 nm) were stabilized using octanethiol as the capping agent. In the self-assembled state, dithiol containing amphiphilic molecules acquired a rigid fibrillar morphological structure. The dithiol groups are efficient to replace the thiol ligands of the GNP surface. Simultaneously, the dithiol ligands can bind the GNPs more strongly. Hence, the site exchange reactions facilitated the formation of nanocomposites which were highly stable even after one month devoid of any precipitation (Fig. 3a). The transmission electron microscopic (TEM) images revealed the existence of GNPs in the intertwined fibrillar network with diameters of 10–30 nm (Fig. 3b–d).
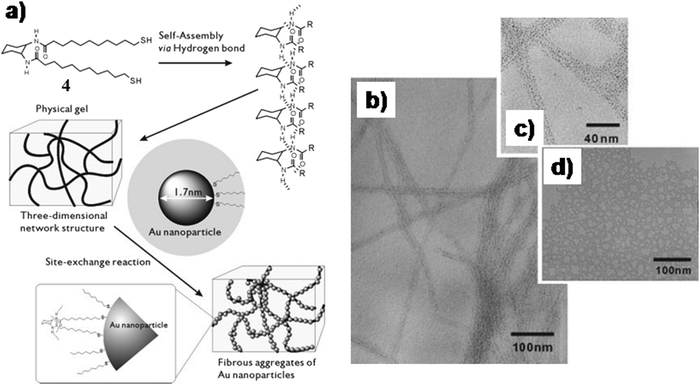 | ||
| Fig. 3 (a) Schematic illustration of organization of GNPs around organic fibers. (b) TEM image and (c) magnified TEM image of fibrous aggregates of GNPs in the presence of 4; (d) TEM image of aggregates of GNPs. Reproduced with permission from ref. 27, Wiley VCH. | ||
Yamaguchi and co-workers developed fluorescent GNP composites from two-component organogels 5a and 5b (Fig. 4a).28 The native gelator molecules exhibited the emission maximum (λem) at 450 nm. The thio-ether residue could efficiently bind with the GNP via favorable S–Au interaction. In the presence of GNPs, the luminogenic gelator molecules got more ordered which facilitated the extent of the intermolecular charge transfer process (5a/5b (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) to the ordered GNPs). Hence, the λem of the soft-nanohybrid was notably red-shifted in the range of 600–800 nm.
1, w/w) to the ordered GNPs). Hence, the λem of the soft-nanohybrid was notably red-shifted in the range of 600–800 nm.
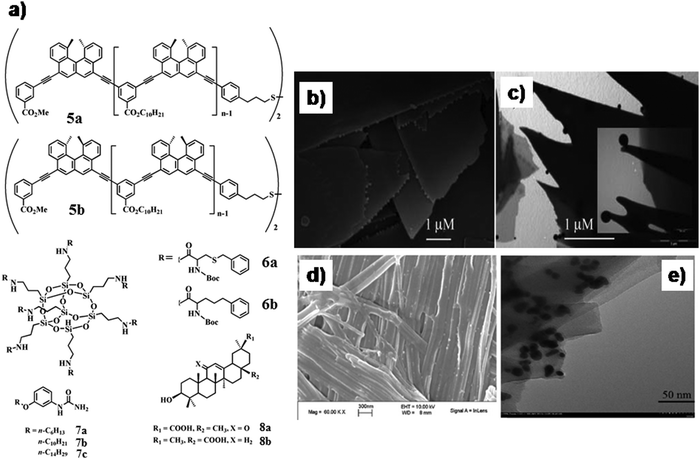 | ||
| Fig. 4 (a) Chemical structure of compounds 5–8; SEM image of xerogels prepared from gels of (b) 7b in water and sheets with GNPs. TEM image of (c) hydrogel of 7a with GNPs (inset, magnified image). (d) The fibrillar network of the GNP–organogel hybrid material by SEM. (e) TEM images of gold nano-crystals of different shapes on the surface of the gel fibers. Reproduced with permission from ref. 30 and 31, The Royal Society of Chemistry. | ||
In another report, Wang and co-workers used polyhedral oligomeric silsesquioxane core-tagged benzylcysteine (6a)- and homo-phenylalanine (6b)-based organogelators for the design of a gel-nanohybrid with GNPs (Fig. 4a).296a and 6b self-assembled in a 3-D loofah-like nano-structure to develop self-standing organogels. Integration of GNPs led to the formation of a composite gel only with 6a. This loofah-like (porous) morphology may engulf spherical GNPs to construct a soft-nanohybrid due to the similar dimension of the pore size and nanoparticles. Moreover, the presence of a thiobenzyl residue in the molecular backbone of 6a facilitated the S–Au interaction to incorporate a maximum amount of GNPs in the gel matrix. The newly formed soft-nanocomposite exhibited 100-fold greater mechanical stiffness (storage modulus G′ > 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Pa for 6a/GNPs = 6%/0.1%).
000 Pa for 6a/GNPs = 6%/0.1%).
Redox reaction which converts HAuCl4 into GNPs could also be achieved in situ within the self-assembled system to develop novel soft-nanocomposites. Several earlier reports described that the structural features of the amphiphiles can serve as effective reducing sites. In this context, Vemula et al. demonstrated the pathways to form soft-nanocomposites where HAuCl4 to GNP transformation occurred in situ with the assistance of gelators cum reducing agents 7a–c (Fig. 4a).30 The initial colour change of the preheated solution (up to 50 °C) of HAuCl4 was observed from yellow to colourless and concurrently to a pink coloured gel at room temperature, which was identified as GNP gel (SPRmax = 553 nm). Scanning electron microscopic (SEM) and TEM images exhibited unaltered gel morphology, regardless of the presence of GNPs which have a dimension in the range of 11–15 nm and interestingly suspended on the peripheries of the gel platelets (Fig. 4b and c). The free NH2 group is responsible for the reduction of Au(III) to Au(0). Similarly, Ju and co-workers synthesized GNPs in the organogel matrix of bis-triterpenoid derivatives 8a and 8b (Fig. 4a).31 Tetrabutylammonium borohydride (reducing agent) was utilized to convert Au(III) to Au(I) and gel–GNP (purple-black) nano-hybrid material was achieved. The existence of both Au-nanocrystal (diameter of 5–30 nm, SPRmax = 538 nm) and fiber was revealed by SEM and TEM images (Fig. 4d and e). Hence, apart from conventional free amine (–NH2) or thiol group (–SH) containing gelators, some natural products such as steroid and terpenoid derivatives could also be utilized as the template to synthesize in situ GNPs.
In another instance, Kawai and co-workers synthesized GNPs in the matrix of hydrogel/organogels derived from N-stearoyl amino acids 9a–e (Fig. 5a) using different reducing agents (LiEt3BH) and methods (photo-irradiation).32 The free amino groups stabilized the GNPs within the SAFIN. Hence homogeneous suspension of GNPs was obtained from the organogels (in toluene) of 9a–c by using LiEt3BH or from the hydrogel of 9c by light irradiation. In another instance Mitra and Das developed GNPs (in situ) of different sizes and shapes by the reduction of HAuCl4 within a tryptophan-based hydrogel.33 The presence of a tryptophan moiety in the gelator molecules facilitated in situ shape-controlled GNP synthesis. Tryptophan reduced the chloroaurate ion to Au(0) within such a structure-directing 3D-network without any additive or foreign element. That could possibly lead to the formation of GNPs of different shapes and sizes, which were immediately stabilized by the other tryptophan moieties within the gel network. Moreover, the difference between the porous network structures of hydrogels was used to develop GNPs of varying shapes. The dimension of the porous network helped to regulate the size and shape of the GNPs (triangular, worm-like, octahedral, decahedral) (Fig. 5b–e). The hydrogelators are able to swell with water and the swollen gels having liquid filled void space are expected to control the growth and organization of the nanoparticles.
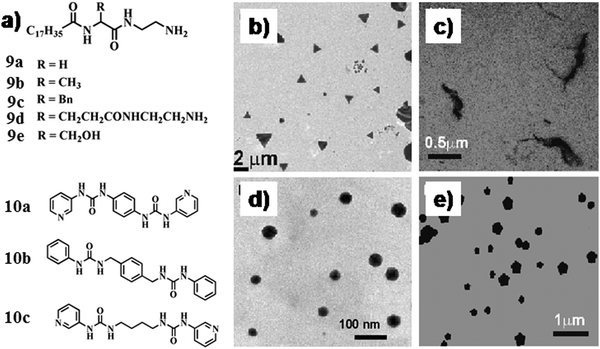 | ||
| Fig. 5 (a) Chemical structure of compounds 9 and 10. (b–e) TEM images of in situ synthesized GNPs of different shapes where the gel-matrix was used as the template. Reproduced with permission from ref. 33, J. Phys. Chem. C, 2008, 112, 8159–8166. Copyright 2008 American Chemical Society. | ||
In another report, Kumar et al. designed bis-pyridyl compounds 10a–c (Fig. 5a) which formed a supramolecular gel in various solvents.34 This gel matrix could efficiently reduce HAuCl4 to form Au nano-clusters/GNPs without adding any exogenous reducing and capping agent. The GNP-based soft-nanocomposites were efficient in catalyzing the reduction of p-nitrophenol to p-aminophenol in the absence of any exogenous reductant. The small Au nano-clusters produced in the organogel were fluorescent and this property has been further utilized to sense Hg(II).
2.2 Silver nanoparticle-based soft-nanocomposites
Inclusion of pre-formed silver nanoparticles (AgNPs) within the soft-materials also has a notable influence on the variation of nanostructure of the soft nano-composites. The morphological aspects of the soft-materials also play a crucial role in decorating the metallic nanoparticles around the self-assembled nanostructures to construct suitable novel soft-nanocomposites. To this end, Sundararajan and co-workers developed biscarbamate derivative 11 (Fig. 6a), which formed a thermo-reversible organogel in benzonitrile exhibiting thin sheet like nano-structures that wrap into hollow fibers.35 The hollow fibers are able to engulf the spherical GNPs within their dimension to construct stable soft-nanocomposite. Presumably, the inner dimension of the hollow fibers could efficiently integrate the spherical metallic nanoparticles to construct a stable soft-nanocomposite. These incorporated AgNPs were homogeneously distributed on the periphery of the fibrillar network (Fig. 6b and c).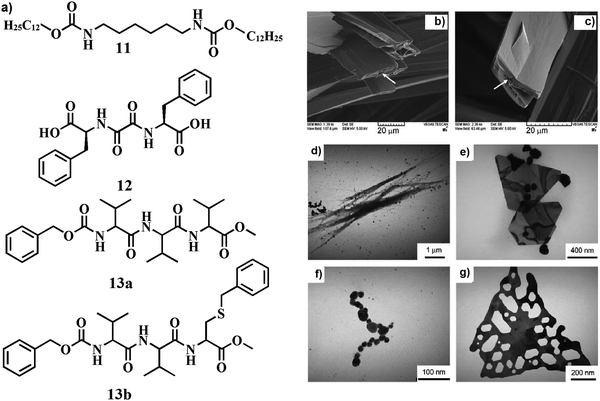 | ||
| Fig. 6 (a) Chemical structure of compounds 11–13. (b and c) SEM images of homogeneously distributed AgNPs on the periphery of gel fibers. The arrows indicate pores in the AgNP-filled solid fibers. Reproduced with permission from ref. 35, Wiley VCH. (d–g) TEM images of peptide–nanoparticle aggregates of different shapes. Reproduced with permission from ref. 38, The Royal Society of Chemistry. | ||
Owing to its high sensitivity surface-enhanced Raman scattering (SERS) has attracted much attention, in particular for chemical sensing and biochemical analysis. Owing to its ability to provide effective, molecularly specific information about a species on a metal surface, it has been applied to detect a single molecule in the atto and zeptomol regime as well as to explore molecular interactions of an adsorbate with a SERS active substrate and complex forming molecules. Žinić and co-workers developed amino acid based hydrogelator 12 (Fig. 6a) that successfully integrated AgNPs within the SAFIN facilitating surface-enhanced Raman scattering (SERS).36 Close interactions between AgNPs and the gelator molecules were exhibited by the steady enhancement of Raman intensity of the respective functional groups. In another report, Pal and co-workers detected SERS by the nanocomposites derived from a polysaccharide alginate gel and gold (Au) and silver (Ag) nano-clusters.37 2-Aminothiophenol and 1,10-phenanthroline were utilized as probes to detect the SERS and in this case, Au-composites showed better response than the corresponding Ag-composites. Hence, SERS is recognized as an analytical tool for providing information on the molecular self-assembly and the associated intermolecular interactions in hydrogels originating from the low molecular weight organic gelator in a silver nanoparticle environment. The described techniques inevitably imply the presence of metal nanoparticles, which can cause different molecular organization in the nanoparticle–hydrogel composite compared to that of pure hydrogel.
In situ synthesis of AgNPs was also associated with different amino acid tethered small molecules. To this end, Taubert and co-workers described L-valine-tagged oligopeptides 13a and 13b (Fig. 6a) to synthesize AgNPs in the gel matrix.38 A stable organogel was formed in n-butanol by mixing 13a and 13b through a β-sheet like structure in the self-assembled state. AgNPs (formed via reduction by DMF) were decorated in the surface of the fibers. Changing the concentration of 13b effectively regulates the shape and size of AgNPs (Fig. 6d–g) through suitable S–Ag interaction.
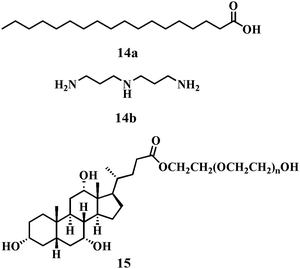
In other reports, the synthesis of gel–AgNP composite materials was accomplished by Bhattacharya and co-workers from a two-component hydrogel comprised of stearic acid, 14a and 3,3′-iminobis(propyl-amine) 14b (Chart 1).39,40 The nanohybrid was prepared by the reduction of AgNO3 by NaBH4 in which the Ag colloids were stabilized in situ by the gel fibers. These newly developed stable soft-nanohybrids exhibited thermo-reversible behaviour. Similarly, Li and co-workers reported suspended AgNPs in the hydrogel matrix from cholic acid based polyethylene glycol tagged polymeric molecule 15 (Chart 1), which acted as a stabilizer preventing the AgNPs from further growth and aggregation as well as a reductant under day light.41 Thus, in situ synthesis of AgNPs through a single step pathway in the gel matrix was utilized to develop this novel soft-nanocomposite.
In situ development of metal nanoparticles often plays a crucial role in the morphological tuning of self-aggregates due to feasible three-dimensional (3D) orientation of the molecules around the nanoparticle surface. In this context, Chatterjee et al. developed a riboflavin (R) and melamine (M) based two-component system to produce AgNPs (Fig. 7).42 Mixing different quantities of AgNO3 powder with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) mixture of riboflavin and melamine in water followed by homogenization at 90 °C produced an AgNP–hydrogel soft-nanocomposite (riboflavin serves as a reductant). Different spectroscopic and microscopic techniques revealed an ordered morphological alteration of the gel. The specific morphology of the hydrogel (hollow tubes) was transformed into either helical fibers, rod-like structures, or spheroidal aggregates, depending on the amount of AgNO3 used. Also, the size of the AgNPs was significantly increased with the mass of AgNO3 (from 12 ± 4 to 52 ± 8 nm and finally to 1000 ± 200 nm). The tubular morphology has originated from the planar sheet structure of the RM-complex due to π−π stacking causing interdigitation of ribityl chains (shown as a tail) and making H-bonds with each other (Fig. 7). On AgNP formation and its stabilization by R, a slight slipping of the R plane from that of the M plane occurs due to the mechanical force exerted by the AgNPs. The size of AgNPs dictates the amount of slipping of the R and M planes causing a change in the morphology. In the case of the smallest AgNPs (12 ± 4 nm), the effect of AgNP size on the R side is low but sufficient to disturb the orientation of the chiral ribityl chain to bend the sheet, preventing tube formation and forming a rod morphology from stacking of the sheets. With the increasing size of AgNPs (52 ± 8 nm), the slipping of the R plane from the M plane is relatively large, inhibiting the growth of the sheet laterally but promoting its longitudinal growth due to a strong π–π stacking force among the twisted supramolecular complexes (Fig. 7). This gives rise to the helical fibrillar morphology of the RM-complex which further self assembles to produce superhelical fibers. However, in the case of bigger AgNPs (1000 ± 200 nm), destabilization of the supramolecular complex formation took place due to the large mechanical force. Thus by increasing the dimension of the AgNPs one could efficiently regulate the hierarchical change of morphology of two component self-assembled systems.
1 (w/w) mixture of riboflavin and melamine in water followed by homogenization at 90 °C produced an AgNP–hydrogel soft-nanocomposite (riboflavin serves as a reductant). Different spectroscopic and microscopic techniques revealed an ordered morphological alteration of the gel. The specific morphology of the hydrogel (hollow tubes) was transformed into either helical fibers, rod-like structures, or spheroidal aggregates, depending on the amount of AgNO3 used. Also, the size of the AgNPs was significantly increased with the mass of AgNO3 (from 12 ± 4 to 52 ± 8 nm and finally to 1000 ± 200 nm). The tubular morphology has originated from the planar sheet structure of the RM-complex due to π−π stacking causing interdigitation of ribityl chains (shown as a tail) and making H-bonds with each other (Fig. 7). On AgNP formation and its stabilization by R, a slight slipping of the R plane from that of the M plane occurs due to the mechanical force exerted by the AgNPs. The size of AgNPs dictates the amount of slipping of the R and M planes causing a change in the morphology. In the case of the smallest AgNPs (12 ± 4 nm), the effect of AgNP size on the R side is low but sufficient to disturb the orientation of the chiral ribityl chain to bend the sheet, preventing tube formation and forming a rod morphology from stacking of the sheets. With the increasing size of AgNPs (52 ± 8 nm), the slipping of the R plane from the M plane is relatively large, inhibiting the growth of the sheet laterally but promoting its longitudinal growth due to a strong π–π stacking force among the twisted supramolecular complexes (Fig. 7). This gives rise to the helical fibrillar morphology of the RM-complex which further self assembles to produce superhelical fibers. However, in the case of bigger AgNPs (1000 ± 200 nm), destabilization of the supramolecular complex formation took place due to the large mechanical force. Thus by increasing the dimension of the AgNPs one could efficiently regulate the hierarchical change of morphology of two component self-assembled systems.
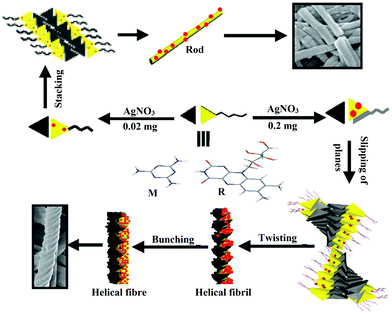 | ||
Fig. 7 Schematic representation of the formation of different morphologies in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) R/M system from mechanical effects by in situ formation and stabilization of AgNPs by riboflavin (red balls). Reproduced with permission from ref. 42, The Royal Society of Chemistry. 1 (w/w) R/M system from mechanical effects by in situ formation and stabilization of AgNPs by riboflavin (red balls). Reproduced with permission from ref. 42, The Royal Society of Chemistry. | ||
In a separate work, Steed et al. reported an interesting observation regarding the development of pyridyl bis(urea) gelator, 16 and 17 (Fig. 8a).43 In this gel matrix, the AgNPs were synthesized by the addition of AgBF4 to a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (v/v) THF
3 (v/v) THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solution of gelator via sonication. In the presence of light, the material transformed into a AgNP composite in 7–14 days. By varying the ratios of AgBF4, the gelator molecule, the THF–water solvent mixture and the exposure time of the ambient light the size and distribution of AgNPs in the gel matrix can be regulated. AgBF4
water solution of gelator via sonication. In the presence of light, the material transformed into a AgNP composite in 7–14 days. By varying the ratios of AgBF4, the gelator molecule, the THF–water solvent mixture and the exposure time of the ambient light the size and distribution of AgNPs in the gel matrix can be regulated. AgBF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) gelator at a 1
gelator at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) ratio produced small AgNPs (2–6 nm, SPRmax = 430 nm) bound to SAFINs of 16 (Fig. 8b). With the increase in the concentration of AgBF4, bigger AgNPs (5–50 nm up to 100 nm) were formed. The other compound 17 without any N-donor was inefficient in forming any gel under an identical environment. This observation significantly depicts the key role of pyridyl rings in the molecular backbone in the formation of AgNPs.
1 (w/w) ratio produced small AgNPs (2–6 nm, SPRmax = 430 nm) bound to SAFINs of 16 (Fig. 8b). With the increase in the concentration of AgBF4, bigger AgNPs (5–50 nm up to 100 nm) were formed. The other compound 17 without any N-donor was inefficient in forming any gel under an identical environment. This observation significantly depicts the key role of pyridyl rings in the molecular backbone in the formation of AgNPs.
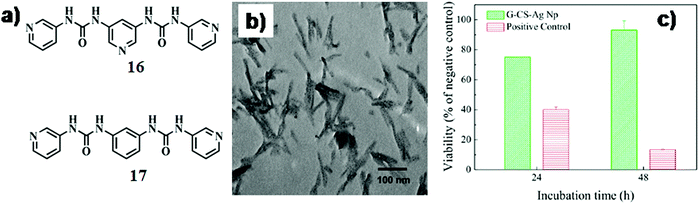 | ||
| Fig. 8 (a) Chemical structure of compounds 16 and 17. (b) TEM image of Ag-nanoparticles in the gel of 16 with 1 equiv. of AgBF4. Reproduced with permission from ref. 43, The Royal Society of Chemistry. (c) Cell viability of L-929 murine fibroblasts cultured with an extracted medium obtained from the polyvinyl chloride (positive control) and AgNP–hydrogel soft-nanocomposite with respect to that obtained from high-density polyethylene (negative control). Reproduced with permission from ref. 45, Biomacromolecules, 2015, 16, 1301–1310. Copyright 2015 American Chemical Society. | ||
Recently, Kumar and co-workers developed agarose- and chitosan-based gels in an ionic liquid (1-butyl-3-methylimidazolium chloride) and silver oxide (Ag2O) nanoparticles were synthesized in situ to form soft-nanocomposites in a single step.44 Inclusion of AgNPs in ionogels reduced the gelling temperature and increased the melting temperature, thus increasing the thermal hysteresis. Moreover, the nanocomposite ionogel showed reasonably good gel strength possibly due to the bridging role of AgNPs between polymer chains and formation of a network structure along with the usual electrostatic and hydrogen bond interactions between polymer groups and the ionic liquid.
AgNPs are known to exhibit huge advantages over others due to their striking bactericidal effects as well as non-toxic effects towards eukaryotic cells. In this regard, Gabilondo and co-workers developed bio-nanocomposite hydrogels based on furan-appended gelatin and chondroitin sulfate cross-linked (Diels–Alder cyclo-addition) with benzotriazole maleimide functionalized AgNPs.45 In addition to this, amide coupling between free ε-amino groups of furfuryl gelatin and carboxylic groups of chondroitin sulfate led to the formation of a stable hydrogel. The gel-nanohybrid exhibited higher (78%) controlled release of the model drug chloramphenicol in contrast to the control hydrogel without AgNPs (only 27% release). They further performed drug release and biocompatibility studies with the as-prepared bio-nanocomposite hydrogel which showed low toxicity (Fig. 8c).
In another report, Mandal et al. designed a L-lysine-based amphiphilic hydrogelator consisting of a naphthalene moiety at the N terminus and an ethyleneoxy unit with free primary amine at the C terminus (18, Fig. 9a).46 The amphiphile showed good gelation ability with a minimum gelation concentration of 6.0 mg mL−1 in binary mixtures of dimethyl sulfoxide/phosphate buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v, pH 7.4). Subsequently, AgNPs with an average diameter of 35–40 nm (SPRmax = 430 nm) were synthesized within the hydrogel by in situ photo-reduction of AgNO3 under sunlight where the gelators act as reducing/stabilizing agents. This novel soft-nanocomposite showed significant mechanical strength (∼29-fold higher than that of the native gel at MGC) and thixotropic self-recovery properties, which made the composite suitable to be used as a syringe-injectable hydrogel. The IC50 value of this soft-nanocomposite against both Gram-positive and Gram-negative bacteria was found to be 32.0 μg mL−1 and 45.0 μg mL−1, respectively (Fig. 9b and c). The mechanism behind the antibacterial action of the AgNP-incorporated soft nanocomposite is still not completely understood. Presumably, AgNPs come into contact with the bacterial cell membrane by their favourable interaction with thiol (–SH)-containing proteins and damage the cell wall thereby leading to bacterial death. Additionally, AgNPs might penetrate into the bacterial cell and destroy DNA or mitochondria resulting in bacterial killing. Notably, the IC50 value of the AgNP-infused soft nanocomposite was higher for Gram-negative bacteria than for Gram-positive bacteria. This is possibly owing to the additional presence of a lipopolysaccharide and phospholipid layer for the Gram-negative bacteria, which is not present in Gram-positive bacteria. The additional layer in the cell membrane of Gram-negative bacteria might have provided extra protection, due to which a slightly higher concentration of AgNPs was required to destroy the membrane integrity. In addition, they showed low hemolytic activity and high cytocompatibility to mammalian cells.
4 v/v, pH 7.4). Subsequently, AgNPs with an average diameter of 35–40 nm (SPRmax = 430 nm) were synthesized within the hydrogel by in situ photo-reduction of AgNO3 under sunlight where the gelators act as reducing/stabilizing agents. This novel soft-nanocomposite showed significant mechanical strength (∼29-fold higher than that of the native gel at MGC) and thixotropic self-recovery properties, which made the composite suitable to be used as a syringe-injectable hydrogel. The IC50 value of this soft-nanocomposite against both Gram-positive and Gram-negative bacteria was found to be 32.0 μg mL−1 and 45.0 μg mL−1, respectively (Fig. 9b and c). The mechanism behind the antibacterial action of the AgNP-incorporated soft nanocomposite is still not completely understood. Presumably, AgNPs come into contact with the bacterial cell membrane by their favourable interaction with thiol (–SH)-containing proteins and damage the cell wall thereby leading to bacterial death. Additionally, AgNPs might penetrate into the bacterial cell and destroy DNA or mitochondria resulting in bacterial killing. Notably, the IC50 value of the AgNP-infused soft nanocomposite was higher for Gram-negative bacteria than for Gram-positive bacteria. This is possibly owing to the additional presence of a lipopolysaccharide and phospholipid layer for the Gram-negative bacteria, which is not present in Gram-positive bacteria. The additional layer in the cell membrane of Gram-negative bacteria might have provided extra protection, due to which a slightly higher concentration of AgNPs was required to destroy the membrane integrity. In addition, they showed low hemolytic activity and high cytocompatibility to mammalian cells.
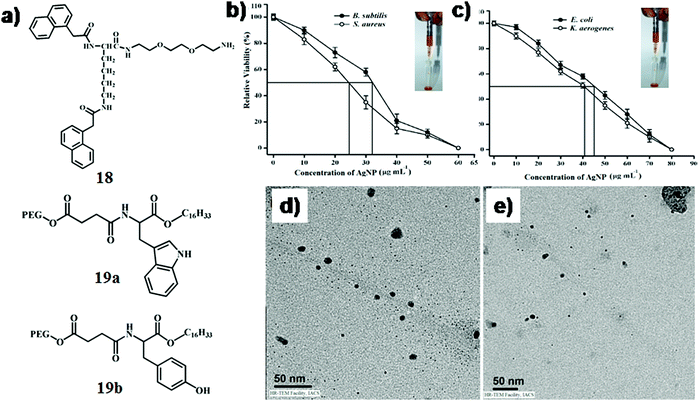 | ||
| Fig. 9 (a) Chemical structure of compounds 18 and 19. Relative viability of (b) B. subtilis and S. aureus, and (c) E. coli and K. aerogenes after 3 h of incubation on the AgNP-incorporated gel nanocomposite with varying AgNP concentration while keeping the hydrogelator (18) concentration fixed at 0.6% w/v. Determined using the colony count method. The rectangles indicate the respective IC50 values (inset: injectable hydrogel). Reproduced with permission from ref. 46, Wiley VCH. TEM images of (d) SWNT-19a-AgNP and (e) SWNT-19b-AgNP. Reproduced with permission from ref. 47, PLOS. | ||
In another instance, Brahmachari et al. developed a new class of trihybrid soft-nanocomposites where AgNPs were decorated on single walled carbon nanotubes (SWNTs), which were dispersed in water by L-tryptophan/tyrosine based amphiphiles, 19a and b (Fig. 9a).47 Both neutral amphiphiles (comprised of a long alkyl chain (C-16) and poly ethylene glycol (PEG550) in the hydrophobic as well as hydrophilic end, respectively) dispersed SWNTs with an efficiency of 75% and 78%, respectively, for 19a and 19b. Presumably, the steric bulk of PEG550 in the molecular backbone facilitated the dispersion of SWNTs in water. In addition to this, the water molecules connected with the glycol chain via H-bonding further aided the dissolution of SWNTs possibly by preventing the coalescence of nanotubes. A green methodology was considered where AgNPs were synthesized in situ under sunlight from AgNO3 at physiological pH on the surface of the dispersed SWNTs. The ratio of AgNO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) capping agent (19a and 19b) was maintained at 1
capping agent (19a and 19b) was maintained at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/w) and the dispersion was exposed to sunlight (15 min). These in situ synthesized AgNPs (diameter ∼8–10 nm) were observed on the edges of the SWNTs in the TEM images of SWNT-19a-AgNP and SWNT-19b-AgNP (Fig. 9d and e). The antibacterial activity of the nanohybrids was studied against Gram-positive (Bacillus subtilis and Micrococcus luteus) and Gram-negative bacteria (Escherichia coli and Klebsiella aerogenes). The SWNT suspensions exhibited moderate killing efficacy (40–60%) against Gram-positive bacteria; however no bactericidal effect was observed against Gram-negative bacteria. The newly developed SWNT–amphiphile–AgNP nanocomposite had superior killing efficacy (∼90%) against all bacteria due to the enhancement of the local concentration of AgNPs around the SWNT surface and better penetration ability of AgNP decorated SWNTs.
10 (w/w) and the dispersion was exposed to sunlight (15 min). These in situ synthesized AgNPs (diameter ∼8–10 nm) were observed on the edges of the SWNTs in the TEM images of SWNT-19a-AgNP and SWNT-19b-AgNP (Fig. 9d and e). The antibacterial activity of the nanohybrids was studied against Gram-positive (Bacillus subtilis and Micrococcus luteus) and Gram-negative bacteria (Escherichia coli and Klebsiella aerogenes). The SWNT suspensions exhibited moderate killing efficacy (40–60%) against Gram-positive bacteria; however no bactericidal effect was observed against Gram-negative bacteria. The newly developed SWNT–amphiphile–AgNP nanocomposite had superior killing efficacy (∼90%) against all bacteria due to the enhancement of the local concentration of AgNPs around the SWNT surface and better penetration ability of AgNP decorated SWNTs.
2.3 CdS/CdSe nanoparticle-based soft-nanocomposites
Cd-Based nanoparticles and quantum dots (QDs) are well-recognized due to their optoelectronic behaviours. At the early stage, Simmons et al. described the inclusion of CdS-nanoparticles into an organogel matrix derived from sodium bis(2-ethylhexyl)sulfosuccinate, with the assistance of p-chlorophenol in isooctane medium.48,49 Water droplets serve as micro-reactors to produce nanoparticles of different size in water-in-oil microemulsions (Fig. 10a). Therefore, when the water-in-oil microemulsion-containing nanoparticles got converted to an organogel, the particles took an intrinsic part of the gel as opposed to a seeded system where the particles may be randomly located throughout the system. Gels became luminescent due to the inclusion of CdS-QDs. Furthermore, incorporation of paramagnetic ferrites in the gel phase induced magnetic properties in the resulting soft-nanocomposites.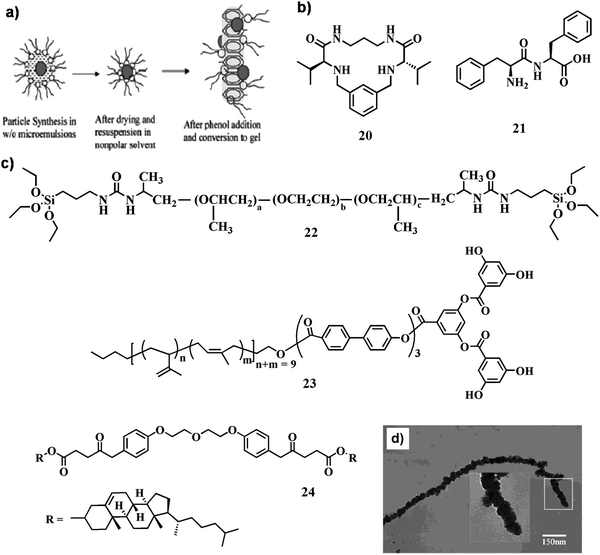 | ||
| Fig. 10 (a) Pictorial representation of the nanoparticle incorporated gel formation mechanism. Reproduced with permission from ref. 48, Nano Lett., 2002, 2, 1037–1042. Copyright 2002 American Chemical Society. (b and c) Chemical structure of compounds 20–24. (d) TEM image of the pearl-necklace CdS nanofibers; the inset shows an enlargement of the boxed section. Reproduced with permission from ref. 55, Langmuir, 2004, 20, 6470–6475. Copyright 2002 American Chemical Society. | ||
Luminescent organogels based on QDs have potential applications as advanced hybrid materials in different fields. Several factors need to be considered when selecting the components to prepare hybrid organogels with suitable optical properties: (i) the inorganic nanoparticles and the gelator need to be dispersed in a compatible solvent in order to obtain a homogeneous solution of the system, (ii) the components must be stable under the experimental conditions, and (iii) the resulting QD–organogel soft-nanohybrid must exhibit excellent transparency for optical characterization. In this context, Luis and co-workers described the inclusion of various CdSe nanoparticles such as CdSe core and CdSe/ZnS core–shell nanoparticles capped with trioctylphosphine oxide and n-octadecylamine ligands in a preformed organogel obtained from a pseudo peptidic macrocyclic gelator, 20 (Fig. 10b).50 A stable and thermo-reversible soft-nanocomposite was obtained upon integration of CdSe QDs within the gel matrix. The presence of QDs not only disrupts the supramolecular organization of the internal fibrillar network of the organogel to a significant extent, but also decreases the critical concentration of the gelator needed to form stable and thermoreversible organogels. Notably, the intrinsic optical properties as well as luminescence intensity of the CdSe nanoparticles remained intact in the hybrid gel.
In a separate work, Li and co-workers reported the inclusion of multicolored CdSe QDs (green, yellow, orange, and red) inside a dipeptide gel, 21 (Fig. 10b).51 Being one of the smallest dipeptide gelators, diphenylalanine forms a gel in organic solvents through hydrogen bonds of peptide main chains and the π–π interactions between the aromatic residues of the peptide. The supramolecular gel-matrix could entrap hydrophobic QDs which improves its stability and prevents it from oxidation. Moreover, the maxima of the emission spectra after encapsulating the QDs in the gels are blue-shifted (approximately 2–10 nm for the respective QDs) compared to those of the free QDs in toluene. This blue shift in the emission spectra of the encapsulated QDs within gels was observed possibly due to the attachment of QDs with the fibrillar network. This result suggests that some nanocrystals may be attached to the fibrils likely because of the ligand interaction between the dipeptide molecule and the nanocrystal, while others are dispersed in the interspace of the fibrillar network.
Brock and co-workers demonstrated the production of CdSe (ZnS) semiconductor QDs in micron thick xerogel films with high transparency and luminescence by using sol–gel methods.52 The xerogel films were prepared by dipping glass substrates horizontally into a sol of 11-mercaptoundecanoic acid-capped CdSe (ZnS) followed by gelling and drying under ambient conditions. The xerogel films thus obtained showed conductivity in the order of 10−3 S cm−1 possibly due to the formation of an electrically connected network of CdSe (ZnS) within the xerogel film.
Recently, Goncalves et al. focused on the advancement of a one-pot synthesis of CdSe NPs in the organic–inorganic gel matrix where ureasilicate (22, Fig. 10c) was used as the precusor.53 The synthetic approach uses the CdSe precursors (Cd2+ and SeSO32− ions) in the form of aqueous solutions, which are directly added to the precursors of the urea silicate matrix and 3-mercaptopropyltrimethoxysilane as a stabilizing agent. The main advantage of using precursors in the form of aqueous solution is the easy control of the molar ratio between the precursor ions. During the sol–gel process a highly transparent solid gel is produced with the formation of CdSe nanoparticles that showed quantum size effect and near band-edge photoluminescence emission.
CdS nanoparticles can be synthesized in situ within the self-assembled system without the addition of any exogenous materials. To this end, Sone et al. reported the synthesis of CdS nanoparticles of nanoscale helical morphology using a self-assembled organogel (23, Fig. 10c) as a template.54 Self-assembled helical nanoribbons were generated first in ethyl methacrylate. Addition of Cd2+ ions concentrated adjacent to the hydrophilic domain in the nanoribbon followed by reduction (H2S) led to the in situ production of CdS NPs. Separately, Zhao and co-workers designed a cholesterol-tagged organogelator (24, Fig. 10c) to synthesize CdS NPs.55 Depending on time, the gel fibers were either fabricated with CdS NPs or fully covered to form pearl-necklace CdS nanofibers (Fig. 10d). The arrangement of the organogelator changed due to the interaction between the inorganic precursor and the organogelator before the transcription process of organogel fibers. The further growth of CdS occurred on the nucleation sites along the organogel fibers into pearl-necklace nanofibers, in which the pores were related to the absorption of free molecule 24 in solution on the primary CdS particles.
2.4 TiO2 nanoparticle-based soft-nanocomposites
Porous, nano-structured materials have attracted much attention due to their diversified applications as sorption media, molecular sieves, supports, and catalysts.56–59 In particular, nano-structured TiO2 materials are of great interest for possible applications in photovoltaic cells, catalysts and semiconductor devices.60–62 Over the last few decades, a number of processes have been documented for the fabrication of hollow fibers of metal oxides with diameters ranging from nanometers to micrometers.63 The formation of such hollow titanium dioxide fibers leads to increased surface areas compared to filled fibers and hence should enhance the effectiveness of the material. The use of polymer fibers as templates that impart a secondary structure to the tubes will further enhance the surface area of the inorganic structure. In this context, Caruso et al. developed a sol–gel procedure for the metal oxide coating of polymer fibers, which were formed using an electro-spinning technique. Such fibers have previously been coated with polymers (poly(p-xylylene)) and metals (aluminum and gold) using chemical vapour deposition and physical vapour deposition, respectively.64 During the electro-spinning process, the polymer is overlaid to form a mat of polymer fibers. The fibers studied for the sol–gel coating technique were fabricated by the electro-spinning of a poly(L-lactide)/dichloromethane solution. According to the SEM images (Fig. 11a–c), the poly(L-lactide) fibers have a range of diameters from 0.3 to 3.5 μm, with most having diameters between 1.0 and 1.5 μm. The sol–gel coating could mimic the fiber and further transform them to nodules on the inner surface of the tubes.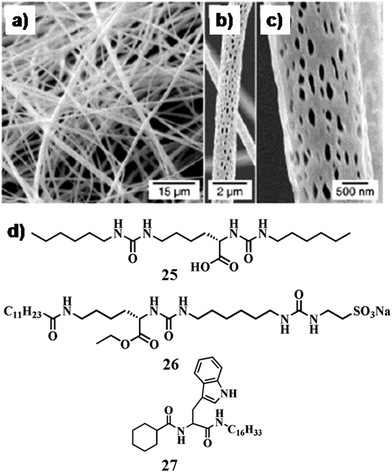 | ||
| Fig. 11 SEM images of the poly(L-lactide) electrospun fibers: (a) overview of the fibrous mat produced using the electrospinning technique, (b and c) surface structure of the polymer fiber observed at higher magnification. (d) Chemical structure of compounds 25–27. Reproduced with permission from ref. 64, Wiley VCH. | ||
In another report, Suzuki and co-workers introduced gelators 25 and 26 (Fig. 11d) for template mediated synthesis of different superstructures (e.g., nanofibers, nanoribbons, nanorods) of TiO2 nanoparticles.65 Depending on the charge and functionality of the gelators, the nanostructures were judiciously modified. In the process of formation of superstructures, propylamine served as a catalyst for the sol–gel polymerization through partial reaction with the –COOH groups of 25. Moreover, the electrostatic attraction between the charged gel fibers and the TiO2 precursor [Ti(OiPr)4] facilitated the formation of a shape regulated novel soft-nanohybrid. In contrast, nanoparticles were generated from 26, acquired negative charges and the polymerization adjacent to the nanofibers was restricted due to electrostatic repulsion.
In recent times, Dutta et al. from our group designed a series of small amphiphiles using a common precursor, able to form a gel in different solvents as well as in ionic liquids (ILs).66 In this regard, ionogels (IGs) of 27 (Fig. 11d) were efficient in preparing spherical TiO2 nanoparticles, which were produced by the addition of titanium tetra-isopropoxide to IGs in aqueous solution (10% H2O) of BMIMBr followed by equimolar water through calcination. Generation of uniform-shaped anatase TiO2 nanoparticles (diameter ∼25–30 nm) was found in the SAFIN of the IG matrix indicating successful formation of soft-nanomaterials.
2.5 Other metallic and inorganic nanoparticle-based soft-nanocomposites
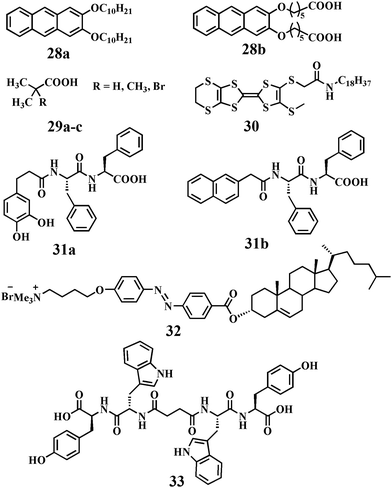
Various other nanoparticles were incorporated in self-assembled systems especially in the gel matrix. Guerzo and co-workers demonstrated inclusion of ZnO NPs within an organogel formed by 28a,b (Chart 2).67 The ZnO capping agent 28b was chosen to achieve an optimal gelator–nanoparticle interaction. 2,3-Didecyloxyanthracene-based capping ligand 28b showed improved dispersibility of the nanoparticles in the organogel matrix due to the complementary interaction between the ZnO nanoparticles and the structurally similar 2,3-didecyloxyanthracene molecules. In addition, photo-responsive 28a was also utilized to develop a light-harvesting matrix at very low concentrations of ZnO nanoparticles. This new photoactive soft-nanocomposite has revealed the synergistic effect between the two constituents (i.e., gelator and ZnO NPs) in the construction of the 3D network. Indeed, the organogels 28a and b serve as a scaffold to stabilize the dispersion of ZnO NPs while the nucleation process inducing the gelation is assisted by the organic capped ZnO NPs.In another instance, Mandal and co-workers developed an ultrasound-induced soft-nanocomposite with the combination of an organogel derived from multifarious isobutyric acids (including 2-methylisobutyric acid and 2-bromoisobutyric acid, 29a–c, Chart 2) and ZnO nanoparticles.68 ZnO nanoparticles were readily soluble by isobutyric acids upon ultrasound irradiation. This technique facilitated in situ generation of the Zn–isobutyrate complexes by sono-crystallization into gel fibers. Formation of well defined networks of fibers of a long aspect ratio due to lamellar organization was observed and the average diameter of the fiber ranged from 30 to 65 nm depending on the nature of the isobutyric acids used.
Electrically conductive or magnetic nanoparticles have immense importance for the preparation of gel-nanocomposites. Puigmartí-Luis and co-workers developed a soft-nanocomposite derived from oleate-stabilized iron oxide nanoparticles and a tetrathiofulvalene (TTF)-based organogelator, 30 (Chart 2).69 The presence of a long alkyl chain (n-octadecyl) on the nanoparticle surface facilitated dissolution in n-hexane to enhance the local concentration of nanoparticles within the gel. However, at low concentration (<10% in weight) of the precursor of iron oxide nanoparticles, the nanoparticles were systematically arranged throughout the fibrillar network. The electrical conductivity of these composites decreased with the increasing amount of nanoparticle loading due to the interruption of conducting pathways by the inorganic domain.
Yang et al. demonstrated a magneto-rheological hydrogel assembly by the addition of a minute quantity of magnetic nanoparticles coated with 31a (Chart 2), similar to the host hydrogelator (31b, Chart 2).70 The presence of an external magnetic field could efficiently deflect the magnetic NPs leading to the breakdown of the gel-nanocomposite to the sol state. Therefore, this new approach could be utilized for drug delivery purpose in the presence of a magnet field as stimuli. In this regard, Lee et al. developed a chitosan based polymeric gel which was formulated with iron oxide magnetic nanoparticles (IMN).71 An anticancer drug (doxorubicin) was successfully encapsulated in this novel soft-nanocomposite. Upon triggering this drug entrapped IMN-based soft-nanocomposite with an alternating magnetic field, the drug was successfully released and showed efficient killing of cancer cells.
Ono et al. first reported a cholesterol-based organogelator (32, Chart 2) for the production of silica nano-tubular structures (tetraethoxysilane as the precursor).72 Co-assembly between surfactants and inorganic nanoparticles is an appealing research field which has been proved to be an effective approach to create hybrid materials. Recently, Wei et al. reported a new type of co-assembly of an anionic surfactant and nanoparticles.73 The hydrogel formation can be achieved by mixing the anionic surfactant sodium laurate with silica nanoparticles (silica NPs) in an aqueous solution with dissolved potassium chloride. The co-assembled fibers were 50–100 nm in width and a few micrometers in length. The viscosity η0 of the 50 mM sodium laurate–400 mM KCl solution is about 0.1 Pa s. As the concentration of silica nanoparticles increased to 1 wt%, the zero-shear viscosity η0 of the sample showed a striking increase to about 3 kPa (∼104 times enhancement). Such a unique phenomenon of the nanostructures provides a new insight into the NP–surfactant co-assembly systems and their potential applications in industry.
In a separate work, Maity et al. synthesized a bola amphiphile (33, Chart 2) comprised of tryptophan and tyrosine moieties which formed a hydrogel on sonication.74In situ synthesis of Pd NPs in the gel matrix was obtained from PdCl2. The Pd NPs became stable in the gel medium either due to the interaction with COO− of the bola amphiphile or through the interaction with amide bonds. Interestingly, the peptide-nanofiber-supported Pd NPs showed effective catalytic activity toward the deprotection of different types of N-terminal (Boc-, Cbz-, Fmoc-, and Nmoc) amino acids and peptides. Recently, Zhao and co-workers included NPs in photodegradable hydrogels to achieve NIR-light triggered release of bio-molecules.75 The hydrogel is composed of a cross-linked hybrid polyacrylamide–poly(ethylene glycol) structure containing photo-responsive o-nitrobenzyl groups incorporated with core–shell NaYF4:TmYb nanoparticles and different proteins. NIR-light was absorbed by the NPs which in turn produced UV light which was responsible for the breaking of o-nitrobenzyl groups. This NIR-light triggered conversion of the gel-to-sol could efficiently release the entrapped protein (Fig. 12).
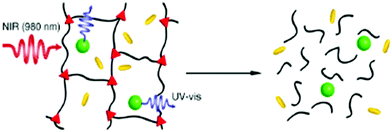 | ||
| Fig. 12 Schematic illustration of the NIR-light-triggered degradation of a photosensitive hydrogel using the UV-light generated by encapsulated up-conversion nanoparticles (UCNPs). The polymeric components are depicted as black lines, the photo-cleavable cross-links as red triangles, the UCNPs as green spheres, and the trapped biomacromolecules as yellow rods. Reproduced with permission from ref. 75, J. Am. Chem. Soc., 2012, 134, 16558–16561. Copyright 2012 American Chemical Society. | ||
3 Soft-nanocomposites with different carbon nanomaterials (CNMs)
Various allotropes of carbon such as three-dimensional fullerenes or quasi-fullerenes, two-dimensional nanosheets (graphene), pseudo one-dimensional carbon nanotubes (CNTs) and zero-dimensional carbon dots (CDs) represent a group of striking nano-architectures with excellent material properties and significant applications.76 Considering the physical features and outstanding properties of CNMs, researchers dream to engineer new functional materials combining the CNMs and organic materials. In comparison to the other metallic/non-metallic nanoparticles, CNMs find superiority in applications due to their ease of functionalization, which is the ultimate key to the exploration of their huge potential. As an example, functionalization increases solubility in common solvents, which often leads to the construction of new hybrid materials having superior properties compared to that of their individual constituents. The outstanding sensitivity of CNMs to changes in surface conductivity due to the presence of adsorbates broadens their application as highly sensitive nanoscale sensors. Thus, the development of strategies that involve covalent/non-covalent modification of CNMs as a suitable guest is highly desirable.3.1 Fullerene-based soft-nanocomposites
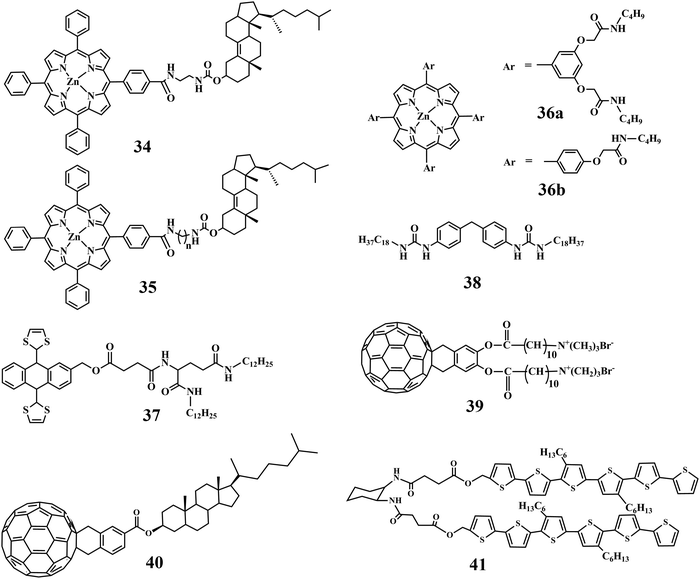
The unique physico-chemical properties of fullerenes are governed mainly by their unusual shape. The investigation of molecular receptors that can form stable associates in solution phase is one of the most active fields in the area of fullerene chemistry. To this end, host–guest complexes based on fullerenes are widely studied with small amphiphiles, dendrimers, supramolecular polymers and others. The first report on gel–fullerene composites was published by Ishi et al. where the gel is formed by a porphyrin-based organogelator, 34 (Chart 3).77 The fullerene gets attached mainly in the hydrophobic organogel matrix. The gel matrix was more stabilized due to the incorporation of fullerene via the formation of a host–guest (sandwich type) complex (ligand![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fullerene = 2
fullerene = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Thus, the intermolecular interaction between the porphyrin unit and the fullerene plays a crucial role in enhancing the gelation ability of the porphyrin-appended gelator by the addition of fullerene. In another report, Shinkai and co-workers showed the sole influence of fullerene on the gelation power of Zn(II) porphyrin appended cholesterol based gelators (35, Chart 3) with –CH2– spacer that gelated the common aromatic solvents.78 The gelation abilities are markedly dependent on the odd or even carbon numbers in the spacer (–CH2–)n moiety connecting the Zn(II) porphyrin moiety with the cholesterol unit. Moreover, in the presence of fullerene the less ordered Zn(II) porphyrin chromophores are rearranged in a highly ordered aggregation mode. To the similar end, Shirakawa et al. found that the porphyrin-based compounds 36a and b (Chart 3) have a unique ability to form a 1-D multicapsular structure in the presence of fullerene while they get aggregated into a 2-D sheet like morphology in the absence of fullerene.79 These newly developed fullerene-induced morphological changes in superstructures were observed because of the feasible porphyrin–fullerene interaction as well as the skillful programming of hydrogen bonding sites.
1). Thus, the intermolecular interaction between the porphyrin unit and the fullerene plays a crucial role in enhancing the gelation ability of the porphyrin-appended gelator by the addition of fullerene. In another report, Shinkai and co-workers showed the sole influence of fullerene on the gelation power of Zn(II) porphyrin appended cholesterol based gelators (35, Chart 3) with –CH2– spacer that gelated the common aromatic solvents.78 The gelation abilities are markedly dependent on the odd or even carbon numbers in the spacer (–CH2–)n moiety connecting the Zn(II) porphyrin moiety with the cholesterol unit. Moreover, in the presence of fullerene the less ordered Zn(II) porphyrin chromophores are rearranged in a highly ordered aggregation mode. To the similar end, Shirakawa et al. found that the porphyrin-based compounds 36a and b (Chart 3) have a unique ability to form a 1-D multicapsular structure in the presence of fullerene while they get aggregated into a 2-D sheet like morphology in the absence of fullerene.79 These newly developed fullerene-induced morphological changes in superstructures were observed because of the feasible porphyrin–fullerene interaction as well as the skillful programming of hydrogen bonding sites.
The gelation ability of an ex-tetrathiafulvalene-based organogelator (37, Chart 3) in an organic medium, e.g., toluene, DMSO and EtOH, has been found to be increased markedly upon incorporation of fullerene within it.80 The effective π–π stacking between the ex-tetrathiafulvalene moiety and the fullerene resulted in successful inclusion of the 3D-allotrope of carbon. In addition, suitable solvophobic and concave–convex interactions also play a key role in forming this soft-nanohybrid. This has improved the gel melting temperature significantly.
A Bis-urea derived gelator (38, Chart 3) was developed for the self-assembly of fullerene in different organic solvents.81 The native molecule formed a helical nanobelt during self-assembly. In this report, fullerene induced morphological transformation from nanobelt to nanorod has been observed. As mechanistic understanding, the suspended fullerenes within the gel got crystallized in an ordered arrangement via specific non-covalent interactions (hydrophobic, hydrophilic, and other weak interactions) leading to the development of a new nano-architecture, nanorods.
Till now, we discussed the inclusion of non-functionalized fullerene directly into the self-assembled systems. However, functionalized fullerene has also been attached covalently with the amphiphilic molecules. A fullerene-appended amphiphile can act as a new organic gelator (39, Chart 3) and the aggregation properties are profoundly influenced by the presence of covalently attached fullerene.82 The alteration in molecular arrangement led to the transformation of the nano-structure from globular to fibers probably due to alteration from a less-ordered to a highly ordered structure. The change in the molecular level aggregation pattern is the key factor for the macroscopic change from solution state to gel state. Separately, a fullerene-coupled cholesterol based amphiphilic molecule, 40 (Chart 3), was transformed to chirally ordered self-assemblies.83 The cooperative and cohesive interaction between fullerene and cholesterol initiated to form 1D-columnar packing. Hence, self-assembled fullerene was produced within the matrix of supramolecular self-assemblies. Similarly, fullerene coupled with an L-glutamide-derivative exhibited an increase in suspensibility in different organic media to form gel.84
In another article, Xue et al. deigned an electron donating π-gelator (PCQ) that can generate a complex with a fullerene derivative (C12C60COOH, electron acceptor) (Fig. 13).85 The complex self-assembled into nano-fibers where fullerene and gelator are packed into a 1-D interdigitated nano-structure. This type of ordered nano-structure ensured efficient electron transport so that huge photocurrents can be produced. These soft-nanocomposites comprised of donor–acceptor-based small molecules or polymers blended with fullerene or its derivatives were mainly used as active materials in the bulk hetero-junction organic solar cells.
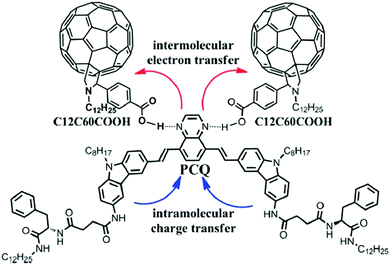 | ||
| Fig. 13 PCQ and C12C60COOH structures and the intermolecular hydrogen bonds between them. Reproduced with permission from ref. 85, The Royal Society of Chemistry. | ||
On the other hand, Tevis et al. described a hairpin-shaped sexithiophene molecule, 41 (Chart 3), for the construction of self-aggregated nanowires via suitable molecular recognition of fullerene within the grooves of the fibers.86 This hairpin-shaped molecule formed long nanowires and then converted to an interpenetrated self-supporting gel in organic solvents. The distinct grooved nature had the ability to form both H-aggregated π–π stacks along the principal axis of the nanowire and J-aggregates, which must result in bundling due to edge-to-edge interactions among the arms of the hairpin molecules. This successful complexation between fullerene and gelators prohibited excess phase separation and promoted high quality interfaces for exciton splitting. Therefore, the self-aggregated ordered structure derived via soft-nanohybrid formation provided better efficiency in device performance.
3.2 Graphene/graphene oxide-based soft-nanocomposites
The family of two-dimensional allotrope of carbon, graphene, includes single and multilayered graphene, graphene oxide (GO) and reduced graphene oxide (RGO). Graphene exhibits high Young's modulus, excellent thermal conductivity and high charge-carrier mobility under ambient conditions.87–89 The zero band gap with long-range ballistic transport of graphene facilitates this for potential application in developing electronic devices. Hence, the composites of graphene with small molecule or polymeric self-assemblies through interaction at the molecular level could enable effective harnessing of such properties. Pristine graphene is highly hydrophobic in nature and thus insoluble in aqueous medium or in any other common solvent. The oxidized form of graphene, i.e. graphene oxide (GO), includes various polar functional groups such as –OH, –COOH, and –CO– and thus is soluble in aqueous medium. RGO is prepared through mild reduction of GO using different reducing agents. Researchers focused on new strategies involving physical association (non-covalent modification) of graphene with a suitable host to exploit its intrinsic properties. In this context, supramolecular self-assemblies can serve as a host for inclusion of graphene via non-covalent interactions. And it becomes the best possible way to sustain the integrity of the graphene frameworks within the organic matrix.To this aim, Rao and co-workers described the successful incorporation of graphene within an organogel matrix (42, 43Fig. 14a).90 Moreover it was observed that an aromatic gelator (42) has a more pronounced interaction with graphene than a non-aromatic gelator. 42 provides H-bond donor (–OH) and acceptor (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) sites through the oxime terminal moiety, van der Waals interactions among the long aliphatic chains, and π–π stacking among conjugated aromatic moieties to allow suitable interactions that lead to gelation. On the other hand, graphenes contain extended π-conjugated 2D aromatic surfaces throughout the nanosheet that could further stabilize the assembly of 42 through π–π stacking interactions. Interestingly, functionalization of graphene provides a suitable path to improve its dispersion in solvents of varying polarities and opens up the possibility of its inclusion within self-aggregated structures. Hence, soft-nanohybrids with pristine graphene/functionalized graphene (GO, RGO, etc.) have beneficial effects and limitations as well including their influence in electronic applications or modulating the mechanical rigidity. The intrinsic solubility of GO is one of the important features to form homogeneous composites and therefore soft-nanocomposites based on GO (and RGO) were developed predominantly compared to that based on pristine graphene. In this regard, Cheng et al. demonstrated the construction of a GO doped soft-nanocomposite via the self-aggregation of a library of amphiphiles having carbohydrate as a polar head group which was linked to a non-polar pyrene group (44, Fig. 15).91 The gelation occurs in both aqueous and organic solvents and is influenced by the molecular structure of gelators. The main driving forces for the gelation were π–π stacking and hydrogen bonding interactions between GO and gelators.
N–) sites through the oxime terminal moiety, van der Waals interactions among the long aliphatic chains, and π–π stacking among conjugated aromatic moieties to allow suitable interactions that lead to gelation. On the other hand, graphenes contain extended π-conjugated 2D aromatic surfaces throughout the nanosheet that could further stabilize the assembly of 42 through π–π stacking interactions. Interestingly, functionalization of graphene provides a suitable path to improve its dispersion in solvents of varying polarities and opens up the possibility of its inclusion within self-aggregated structures. Hence, soft-nanohybrids with pristine graphene/functionalized graphene (GO, RGO, etc.) have beneficial effects and limitations as well including their influence in electronic applications or modulating the mechanical rigidity. The intrinsic solubility of GO is one of the important features to form homogeneous composites and therefore soft-nanocomposites based on GO (and RGO) were developed predominantly compared to that based on pristine graphene. In this regard, Cheng et al. demonstrated the construction of a GO doped soft-nanocomposite via the self-aggregation of a library of amphiphiles having carbohydrate as a polar head group which was linked to a non-polar pyrene group (44, Fig. 15).91 The gelation occurs in both aqueous and organic solvents and is influenced by the molecular structure of gelators. The main driving forces for the gelation were π–π stacking and hydrogen bonding interactions between GO and gelators.
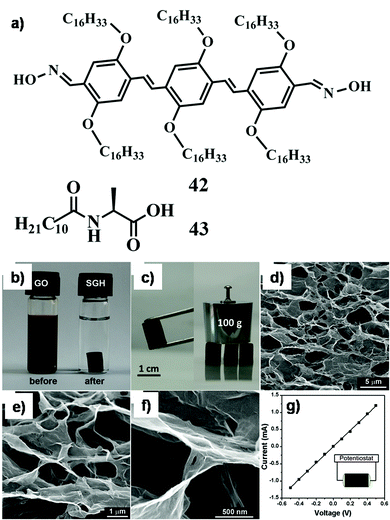 | ||
| Fig. 14 (a) Chemical structure of compounds 42 and 43; (b) photographs of a 2 mg mL−1 homogeneous GO aqueous dispersion before and after hydrothermal reduction at 180 °C for 12 h; (c) photographs of a strong SGH allowing easy handling and supporting weight; (d–f) SEM images with different magnifications of the SGH interior microstructures; (g) room temperature I–V curve of the SGH exhibiting the ohmic characteristic, the inset shows the two-probe method for conductivity measurements. Reproduced with permission from ref. 92, ACS Nano, 2010, 4, 4324–4330. Copyright 2010 American Chemical Society. | ||
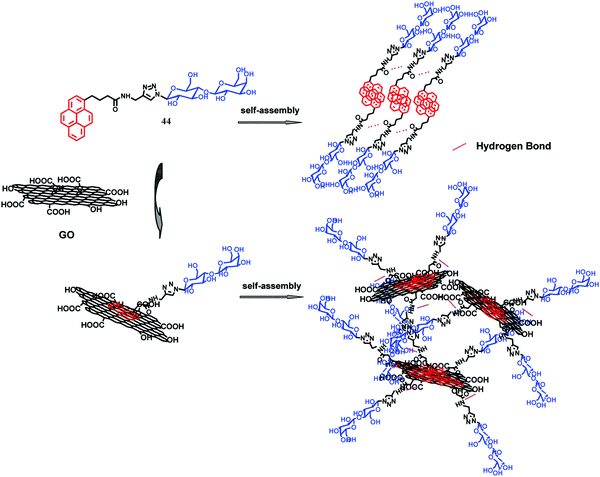 | ||
| Fig. 15 Schematic representation of GO hybrid gel formation. Reproduced with permission from ref. 91, Langmuir, 2012, 28, 3005–3010. Copyright 2012 American Chemical Society. | ||
The extended π-moieties (e.g., pyrene) and different functionalities (e.g., amide, triazole linkage and carbohydrate groups) in the molecular backbone facilitate the non-covalent interactions during gelation. Shi and co-workers developed a one-step hydrothermal process for self-assembled graphene hydrogels (SGHs).92 A minute amount of GO dispersed homogeneously (2 mg mL−1) formed a hydrogel under hydrothermal reduction (180 °C) and the resulting hydrogel displayed excellent mechanical stiffness (∼11.7 kPa) (Fig. 14b and c). Three such monoliths with 0.8 cm diameter can support 100 g of weight with little deformation and the corresponding GO concentration is significantly lower than that reported by Qin et al. (Fig. 14c).93 The overlapping or coalescing ability of flexible graphene sheets resulted in the development of physical cross-linking sites of the framework of the SGH. Thus, the inherent flexibility of graphene sheets is a crucial property for constructing 3D macrostructures. A rheological study revealed that this hydrothermally obtained hydrogel possessed a storage or elastic modulus (G′) of 470 kPa at 10 rad s−1 which was 1–3 orders of magnitude higher than that of other conventional self-assembled hydrogels. Such extraordinarily high mechanical rigidity is mainly attributed to the excellent mechanical properties of the graphene building blocks as well as the dense and strong cross-linking of graphene. The SGH has an interconnected 3D network as imaged by SEM of its freeze-dried samples (Fig. 14d–f). This mechanically stable SGH showed an electrical conductivity of ∼5 × 10−3 S cm−1 (Fig. 14g) which prompted its applications in diversified fields.
Also, hydrophobic interactions between basal planes and H-bond interactions between the functional groups of GO contributed to the self-aggregation. For example, Qin et al. demonstrated a new pathway to develop GO hydrogels by dispersing GO in water without further processing steps.93 The pH-responsive nature, mechanical properties, thermal stability and absorption capacity of the GO hydrogel were investigated. Here, different drying approaches were further developed to investigate the micro-architecture of the obtained graphene hydrogel. Interestingly, this unique self-assembled hydrogel exhibited pH responsiveness at lower GO concentration (3 wt%). This finding can be attributed to, at concentrations of GO exceeding 5 wt%, the strong π–π stacking and hydrophobic interactions surpassing the repulsion interactions, leading to inactivation of the pH-response. In another instance, Shi and co-workers showed that GO sheets were capable of forming polymeric soft-nanocomposites in the presence of poly(vinyl alcohol) (PVA).94 Presumably, the co-assembly of the planar GO sheet and cross-linked PVA chains led to the formation of a hydrogel. More importantly, this GO/PVA hydrogel composite exhibited pH-induced gel–sol transition. It was able to form a gel in acidic media while it underwent gel–sol transition in alkaline conditions. This unique property had been utilized for loading and selective release of drugs at physiological pH.
Till now we have discussed the inclusion of GO/RGO in mainly hydrogel medium. However, there are reports on stabilizing and dispersing GO in apolar solvents by stimuli response. To this end, Xing et al. developed a urea-based organogelator 1-octadecylureido-naphthalene (OUN) that can form a gel in toluene and xylene (Fig. 16).95 GO having a huge π-conjugated surface with rich hydroxyls and carboxyls can be bound by OUN via π–π stacking and H-bonds. This gel showed dual stimuli responsiveness (heat and anion) resulting in phase transition and changes in luminescence properties. Integration of the 2D allotrope of carbon in the ambidextrous gel matrix is one of the challenging tasks for multifarious applications.
Interestingly, Mandal et al. reported the development of an L-lysine-appended low-molecular-weight ambidextrous gelator (45, Chart 4).96 This gelator exhibited excellent hydrogelation as well as organogelation abilities in DMSO/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v) mixtures and in nitrobenzene. Importantly, this gelator was capable of including both GO and RGO in organic as well as aqueous medium. Inclusion of GO/RGO within 45 reinforced the mechanical rigidity (∼13.9 kPa and ∼40 kPa, respectively) of this novel soft-nanohybrid. Hence, the above examples successfully portray the favourable interaction of 2D carbon allotropes (GO/RGO) with π-gelators leading to the genesis of versatile soft-nanohybrids.
4 v/v) mixtures and in nitrobenzene. Importantly, this gelator was capable of including both GO and RGO in organic as well as aqueous medium. Inclusion of GO/RGO within 45 reinforced the mechanical rigidity (∼13.9 kPa and ∼40 kPa, respectively) of this novel soft-nanohybrid. Hence, the above examples successfully portray the favourable interaction of 2D carbon allotropes (GO/RGO) with π-gelators leading to the genesis of versatile soft-nanohybrids.
Apart from the utilization of graphene for increasing the mechanical strength of self-assembled soft-materials there are still other applications regarding the inclusion of the same within soft-materials. Hao and co-workers reported the tuning of the aggregation induced emission effects of the gel derived from the self-assembly of an ultralow-molecular-weight gelator (46a–c, Chart 4) by controlling the addition of GO within the gel matrix.97 The self-assembly of gelators exhibited ∼3-fold enhancement of the fluorescence intensity whereas the introduction of GO quenched the emission intensity of the gel by a factor of 2500. The effect of GO on the mechanical strength of hybrid gels might be attributed to two factors: firstly, the large amount of π–π interaction, which definitely enhances the rigidity or viscosity of the gel, owing to the excellent mechanical properties and good dispersity of GO sheets in polar solvents. Secondly, GO sheets could associate with the individual nanofibers, which may increase the cross-links between fibers. Instead of considering the gel matrix as a host for soft-nanocomposites there are other manifestations of supramolecular self-assemblies that can hold the GO sheets without compromising the stability. To this end, Das and co-workers reported the inclusion of GO within the reverse micelle of cetyltrimethylammonium bromide (CTAB) toward the development of soft-nanocomposites.98 Importantly, Chromobacterium viscosum (CV) lipase encapsulated within CTAB reverse micelles exhibited a 3.8-fold increment in its catalytic activity in the presence of GO in contrast to that obtained in native reverse micelles. In fact, water soluble GO mostly tends to reside at the reverse micellar interface probably because of its intrinsic amphiphilic character (hydrophobic carbon backbone) and the hydrophilic functionalities (negative surface charges from different functional groups like –COOH, –OH, epoxide, etc.). The “space” in the immediate vicinity of the surface-active or interfacially solubilized enzyme in reverse micelles is one of the vital factors for enhancing the catalytic efficacy of the enzyme (Fig. 17). GO incorporated reverse micelles also serve as a better host for different surface-active enzymes (e.g., horseradish peroxidase (HRP), soybean peroxidase (SBP)). In the presence of GO, the catalytic activity of HRP and SBP enhanced by 2.6 and 2.3 times, respectively, compared to that in the native reverse micelle. Electrostatic attraction between negatively charged GO and the cationic polar head group of CTAB presumably facilitated localizing the GO at the interface augmenting the interfacial area for enzyme activity (Fig. 17).
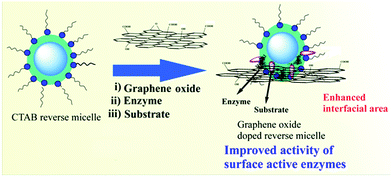 | ||
| Fig. 17 Pictorial representation of graphene oxide (GO) included reverse micelles comprising enzyme and substrate. Reproduced with permission from ref. 98, Elsevier. | ||
3.3 Carbon nanotube-based soft-nanocomposites
The CNT is the pseudo one-dimensional allotrope of carbon which has been broadly explored in various scientific domains.8,99,100 CNTs are generally categorized into two types: (i) single-walled carbon nanotubes (SWNTs) and (ii) multi-walled carbon nanotubes (MWNTs). Both types of CNTs have attracted a great deal of interest because of their large aspect ratio, which makes them useful from optoelectronics to biomedicine. Therefore, reports on the integration of CNTs in the self-assembled soft materials are significantly higher compared to those related to the other allotropes of carbon. To this aim, numerous aliphatic and aromatic gelators were shown to interact with pristine/functionalized-CNTs in both organic medium and water. Gel-nanocomposite formation generally takes place mostly via the non-covalent interaction at the molecular level. There were also reports where CNT based soft-nanocomposites were prepared through the formation of covalent bonds. Pal et al. demonstrated the production of an organogel–CNT nano-hybrid where the L-alanine appended organogelator (47, Fig. 18a) could successfully include SWNTs, MWNTs and functionalized SWNTs within the SAFIN.101 Rheological studies revealed that the mechanical stiffness of the nanocomposites was much higher in contrast to the gel itself (Fig. 18b). Thermo-reversible phase transformation behaviours of such low molecular weight CNT-based soft-nanohybrids by short NIR exposure had also been demonstrated for the first time. Interestingly, though UV-vis radiation is known to be harmful for living systems, NIR radiation is transparent and unreactive towards biological systems. Hence, the use of NIR light is highly effective for the construction of smart materials. In another instance, Salvetat and co-workers have reported an interesting strategy for suspension of SWNTs in hyaluronic acid (HA) solution, and induced the formation of an unbreakable hydrogel by the assistance of cross-linked divinyl sulfone (Fig. 18c).102 Almost 4-times enhancement in the G′ value of the hydrogel was reported by inclusion of a minute amount of SWNTs (0.06 wt%) without alteration in the water entrapment capability and shear thinning property of the hydrogel itself. In fact, the elasticity of hydrogels is reinforced by the presence of CNTs.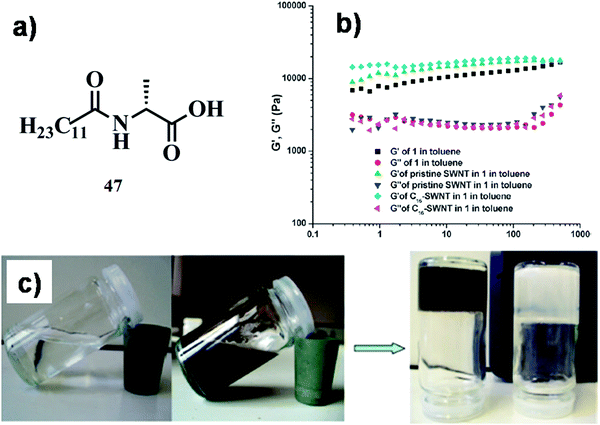 | ||
| Fig. 18 (a) Chemical structure of compound 47. (b) Frequency sweep experiments of the organogels (10 mg mL−1) alone, the 0.24 wt% pristine SWNT–gel nanocomposite and the 0.24 wt% gel nanocomposite. Reproduced with permission from ref. 101, The Royal Society of Chemistry. (c) Photograph of hyaluronic acid solution (transparent, on the left) and dispersion of 0.06% SWNTs by mass within hyaluronic acid solution (dark, on the right). The arrow leads to the photograph showing invertible hyaluronic acid gels, native (white) and hybridized (dark), formed after 30 min of reaction with divinyl sulfone. Reproduced with permission from ref. 102, Biomacromolecules, 2008, 9, 505–509. Copyright 2008 American Chemical Society. | ||
Wang et al. reported a facile strategy to introduce SWNTs into the supramolecular hydrogel formed by host–guest interactions.103 The resulted hybrid hydrogel retained the basic characters of the supramolecular hydrogel and pristine SWNTs, especially the shear-thinning and thermal stimulus responsive properties, which are important in drug delivery and controlled release of the entrapped cargo. In spite of accelerating the gel formation by SWNTs, the soft-nanohybrid became weaker in mechanical strength compared to the native hydrogel. A solution-based approach to decorating an ultra-light freestanding MWNT aerogel monolith was reported by Zhai and co-workers.104 This idea was based on the percolation model, i.e. the increasing interaction potential between CNTs decreases their critical concentration to form an infinite percolation network, which is responsible for the gelation of a fluid. Hence, introducing strong interactions between CNTs reproduces a potential solution to encourage the gelation of MWNT suspension at a very low concentration of CNTs that can be further extended to ultra-light CNT aerogels. MWNTs were functionalized with poly(3-(trimethoxysilyl)propyl methacrylate), which can efficiently disperse and stabilize MWNTs in solution as well as form permanent chemical bonds between MWNTs by the construction of cross-linked polysilsesquioxane (Fig. 19). The removal of the solvent from wet gels resulted in free-standing MWNT aerogels with a surface area of 580 m2 g−1. This excellent compression recoverable property and the high electrical conductivity of these novel soft-nanocomposites made them interesting towards the chemical vapour sensing materials.104
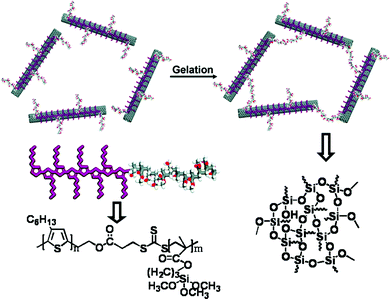 | ||
| Fig. 19 Schematic illustration of the gelation process of polymeric compound dispersed MWNTs. Reproduced with permission from ref. 104, ACS Nano, 2010, 4, 7293–7302. Copyright 2010 American Chemical Society. | ||
In another instance, Mandal et al. from our group have also developed a series of amino acid/dipeptide tagged hydrogelators comprised of a quaternary ammonium/imidazolium group as the hydrophilic head and a C16 alkyl chain as the hydrophobic unit (48a,b, Chart 5).105 Interestingly, the imidazolium based hydrogelators (48a,b, Chart 5) were capable of accommodating a large amount of SWNTs (2–3.5% w/v) at their MGC without compromising their individual properties. The additional H-bonding and π–π interactions between the imidazolium residues probably play a crucial role in improving the gelation efficiency. These newly developed hydrogels are highly effective in incorporating SWNTs in the SAFIN resulting in 85 times enhancement in mechanical rigidity.
Till now we have described the formation of CNT based/induced soft-nanocomposites with a prime focus to increase the mechanical stiffness of soft-materials. Alongside, the CNT was also utilized as an elegant cellular transporter because of its extraordinary ability to translocate through the cell membrane.
To this aim, various small amphiphilic molecules (termed as the dispersing agent) were also designed to wrap and then disperse CNTs (via the formation of hemi-micellar aggregates, Fig. 20) in pure aqueous medium to explore their possible application in biomedicine. The involvement of non-covalent interactions to prepare suitable CNT dispersion is preferred compared to the covalent functionalization of CNTs since the intrinsic properties of CNTs would not be compromised in the case of the former method. In this regard, a library of ionic and non-ionic surfactants and polymers have been tested by Smalley and co-workers to check their ability to disperse SWNTs.106 The large size of the hydrophilic head group as well as the long polymeric chain of non-ionic surfactants/polymers helps to disperse SWNTs successfully. Judicious modification of the head and tail groups in the molecular backbone facilitates the dispersibility of SWNTs. Thus, one can tailor the suspendability of CNTs and the different properties of those suspensions by altering the surfactant or polymer's head and tail groups to fit them in specific applications. The ability to disperse SWNTs with different surfactants in aqueous medium opens the door for material engineers, biomedical researchers, and others. Bianco and co-workers reported a strategy for the introduction of two orthogonally protected amino groups on the sidewalls of CNTs and subsequent attachment of fluorescein isothiocyanate (FITC) and methotrexate (MTX).20 MTX which is widely used as an anti-cancer drug suffers from low cellular uptake.107 Its conjugation to CNTs represents a promising approach to overcome its limited cellular uptake by enhancing its internalization via the functionalized CNT. Thus the doubly functionalized CNT certainly showed new perspectives in the area of biomedicine. The attachment of molecules targeting the specific receptors on tumor cells will help to improve the response to anticancer agents. Ulijn and Co-workers demonstrated the ability of Fmoc [N-(fluorenyl-9-methoxycarbonyl)] protected amino acids (49a,b, Chart 5) to disperse CNTs.108 The CNT-surface is hydrophobic in nature and when Fmoc amino acids bind to the surface, polar groups (–OH and –COOH) on the amide backbone give rise to surfactant-like properties. Additional interactions also take place between surface-bound and non-bound Fmoc amino acids via π–π stacking and H-bonding, which might contribute to the overall formation of a stable dispersion of CNTs in aqueous media. Moreover, they have reported enzymatically activated SWNT surfactants that produce homogeneous aqueous nanotube suspensions under physiological conditions. Thus, the switchable, versatile and low cost surfactants provided a step forward for biological applications of self-assembled soft-nanocomposites.
In another study, Brahmachari et al. from our group developed different amino acid based cationic and anionic amphiphiles (50a,b, Chart 5) as cytocompatible dispersing materials that showed excellent SWNT dispersion efficiency in aqueous medium.109 It has also been noticed that a larger surfactant often finds it more difficult to navigate into these intertube spaces. Interestingly, in the case of the present amphiphiles, the higher charge density in a smaller head sized amphiphile enhances the electrostatic repulsion among the wrapped nanotubes and minimizes the chances of rebundling of CNTs resulting in an increase in the dispersion efficiency. Moreover, these novel soft-nanohybrids exhibited extraordinary media stability and cytocompatibility against mammalian cells and were explored as efficient cellular transporters. Again, we developed a new class of amphiphilic biocompatible nanohybrids comprising CNT and amino acid based small amphiphilic molecules (51a,b, Chart 5) that exhibited an outstanding suspension ability and remarkable stability in biological media, and most interestingly they were highly efficient in transporting proteins through cell membranes.21
We have further developed a SWNT dispersing agent by incorporation of the biotin unit into the amphiphilic backbone to develop a multifarious delivery vehicle with greater suspension stability, substantial cell viability and most importantly with target specificity towards cancer cells.110 This biotin tagged amphiphile–SWNT dispersion (soft-nanocomposite) effectively transported the Cy3-oligoneucleotide (loaded on the CNT-surface) selectively inside the cancerous HeLa and HepG2 cells (with overexpressed biotin receptors) compared to non-cancerous CHO and HEK-293 cells (devoid of overexpressed biotin receptors, Fig. 21). The presence of a biotin moiety in the cellular transporters facilitated the internalization of the cargo due to the over-expressed biotin receptors in the cancer cells. Significantly, this nanocomposite was also capable of targeted delivery of doxorubicin inside cancer cells, which resulted in efficient killing of cancer cells (HeLa) compared to healthy cells (CHO) (Fig. 21).
 | ||
| Fig. 21 Pictorial representation of the specific delivery of the drug and biomolecule loaded SWNT-biotinylated amphiphile nanoconjugate into cancer cells. Reproduced with permission from ref. 110, The Royal Society of Chemistry. | ||
In another report, Ghosh et al. from our laboratory modified the structure of the dispersing agent in such a way that precipitation of the SWNT dispersion should take place at tumorogenic environmental pH (6.15–6.5).111 In fact, it was utilized in the pH-responsive release of doxorubicin in the microenvironment of cancer cells. The cholesterol-based dityrosine carboxylate amphiphile (52, Fig. 22a) efficiently dispersed the SWNT in aqueous medium with pH-responsive precipitation and solubilization at pH ∼ 6.0–6.5. In fact, at pH 6.5 or below, protonation of the amphiphilic dispersing agent led to destabilization of the dispersion resulting in precipitation of the SWNT and detachment of DOX from the SWNT surface. This nanohybrid provided the platform for loading of the sparingly soluble drug (doxorubicin) on the SWNT surface as well as helped to increase the local concentration of the drug in the vicinity of cancer cells compared to normal cells resulting in pH-responsive selective killing of cancer cells.
 | ||
| Fig. 22 (a) Chemical structure of compounds 52 and 53. Bright field and fluorescence microscopic images of cells after 6 h incubation with SWNT-53b-doxorubicin, (b and c) MCF7 cells, (d and e) MDA-MB-231 cells, (f and g) A549 cells, and (h and i) HeLa cells. Reproduced with permission from ref. 112, Elsevier. | ||
Difference between cancer cells and non-cancerous cells was also achieved by using an estrogen receptor (ER) binding amphiphilic dispersing agent.112 ER, a class of nuclear hormone receptor, is overexpressed mostly in estrogen responsive organs such as ovary, uterus, mammary glands, etc. 17β-Estradiol is well recognized to bind with estrogen receptors resulting in their conformational changes. Keeping this in mind, we demonstrated the synthesis of a 17β-estradiol based dispersing agent (53a,b, Fig. 22a) comprising a polyethylene glycol (PEG) moiety linked through succinic acid that efficiently dispersed the SWNTs in aqueous medium. Doxorubicin loaded on these nanohybrids was selectively delivered within estrogen receptor rich cancer cells, MCF7 and A549 (Fig. 22b, c, f and g). Both these ER positive cancer cells were killed with ∼3-fold higher efficacy compared to MDA-MB-231 and HeLa cells (Fig. 22d, e, h and i) that are deprived of estrogen receptors. Later Das et al. from our group synthesized an α5β1 integrin receptor specific RGDK (Arg-Gly-Asp-Lys) tagged lipopeptide (54, Fig. 23a) that successfully dispersed SWNTs in aqueous medium.113 This newly developed cytocompatible RGDK–lipopeptide–SWNT nanocomposite delivered the cargo (Cy3 tagged oligonucleotide) to tumor (B16F10) cells selectively via the α5β1 integrin receptor. More importantly, this soft-nanohybrid also successfully delivered the anti-cancer curcumin within B16F10 cells in contrast to non-cancerous NIH3T3 cells leading to the selective killing of B16F10 cells through the late apoptotic pathway. Importantly, in vivo bio-distribution studies in melanoma (B16F10) bearing C57BL/6J mice revealed tumor selective accumulation of intravenously administered RGDK–lipopeptide–SWNT nano-vector associated curcumin.
 | ||
Fig. 23 (a) Chemical structure of compounds 54–56; TEM image of (b) 55a prepared in DMSO–water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), (c) dispersed CNT in water by 56, (d) vesicle–CNT conjugate; (e) percentage killing of B16F10 cells incubated with doxorubicin-loaded vesicle, dispersed CNT, and vesicle–CNT conjugate for 12 h with varying doxorubicin concentration. Percent errors are within ±5% in triplicate experiments. Reproduced with permission from ref. 22, Wiley VCH. 1, v/v), (c) dispersed CNT in water by 56, (d) vesicle–CNT conjugate; (e) percentage killing of B16F10 cells incubated with doxorubicin-loaded vesicle, dispersed CNT, and vesicle–CNT conjugate for 12 h with varying doxorubicin concentration. Percent errors are within ±5% in triplicate experiments. Reproduced with permission from ref. 22, Wiley VCH. | ||
Recently, we reported the development of new soft-nanocomposites, vesicle–SWNT conjugate, with the aim of a better delivery vector by avoiding their individual limitations. Dinda et al. prepared a vesicle–CNT conjugate by a simple boronic acid–diol adduct between a self-assembled monolayer vesicle and aqueous suspension of SWNT.22 Trimesic acid based phenylboronic acid appended triskelion amphiphiles (55a,b, Fig. 23a) were utilized for the development of the vesicle (Fig. 23b) and the aqueous suspension of SWNT (Fig. 23c) was prepared with a cholesterol-tagged glucose-based amphiphile (56, Fig. 23a). These two combined through covalent linkage by using a boronic acid–diol interaction to construct this boronate–diol adduct which can simultaneously hold both vesicle and SWNT leading to the formation of new soft-nanocomposites (Fig. 23d). Doxorubicin was loaded on this conjugate with a higher capacity compared to the individual cargo transporter (vesicle or CNT). This cytocompatible soft-nanocomposite exhibited an efficient cellular transportation ability of the loaded doxorubicin as well as a better killing efficacy of cancer cells compared to the drug-loaded only vesicle or CNT (Fig. 23e). The improved efficiency in the cellular transportation of this soft-nanocomposite with a regulated dose of loaded drug paved an innovative pathway for developing smart delivery vehicles.
3.4 Carbon dot-based soft-nanocomposites
Intrinsically fluorescent carbon dots (CDs) are newly developed ‘zero-dimensional’ green nanomaterials, which represent a special class of carbon allotropes with a size less than 10 nm. These highly potential CDs are used in energy storage, bioimaging, cargo delivery, sensors, and diagnostics.114–116 Numerous advantages of CDs such as stable emission properties, high aqueous stability, versatile surface chemistry and high cytocompatibility made them superior compared to environmentally hazardous and toxic nanomaterials including lead, cadmium, etc. Thus, the development of soft-nanocomposites comprised of carbon dots/carbonaceous quantum dots is one of the primary emerging domains across the discipline. In this regard, Nandi et al. reported that graphene quantum dots (GQDs) surface-functionalized with hydrocarbon chains self-aggregated to form vesicles in pure water (Fig. 24a).117 The amphiphilic GQD vesicles exhibited multicolor luminescence that can be readily explored for membrane studies. The GQD vesicles were utilized for microscopic study of membrane interactions and disruption by the peptide beta-amyloid. More recently, Dash and co-workers developed CDs (G-dots) synthesized from guanosine 5′-monophosphate (5′-GMP) and explored the self-assembly character of guanosine to form CD based hydrogels without the addition of any external agent (Fig. 24b).118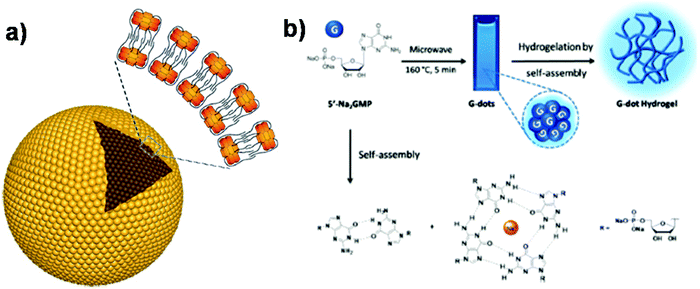 | ||
| Fig. 24 (a) Schematic model of the unilamellar amphiphilic GQD vesicles. The graphene cores of the individual GQDs (orange) form the external bilayer surface, while the hydrophobic acyl chains, shown in black, form the hydrophobic internal layer. Reproduced with permission from ref. 117, Wiley VCH. (b) Formation of 50-GMP carbon dots (G-dots). Reproduced with permission from ref. 118, The Royal Society of Chemistry. | ||
To this end, Sarkar et al. from our group reported the effect of surface modified carbon dots (CDs) toward the nanostructure of the CTAB reverse micelle.119 We designed various hydrophilic/hydrophobic surface functionalized CDs from citric acid. Surface functionalization was varied by changing different amino acids to long alkyl chains. The dimension of the CDs was found to be ∼5–7 nm whereas the diameter of the reverse micelle was ∼20 nm. Most fascinatingly, the size of the reverse micelles noticeably amplified up to 120–200 nm by incorporating hydrophobic CDs whereas no change in the size of reverse micelles was found in the presence of hydrophilic CDs. Inclusion of hydrophobically tailored carbon dots plays an important role in the reorganization of the molecular packing of the aggregates. CDs comprising a hydrophobic long chain get accommodated at the interfacial area of reverse micelles through the hydrophobic interaction between the surfactant tail and the surface modifier of carbon dots leading to an increase in the size of the microemulsions. The influence of CDs on the interfacial properties of the aggregates as well as on the activity of CV lipase was investigated. Lipase showed remarkable improvement (3.7-fold) in its activity within a significantly large CTAB reverse micelle doped with hydrophobically functionalized CDs (Fig. 25).
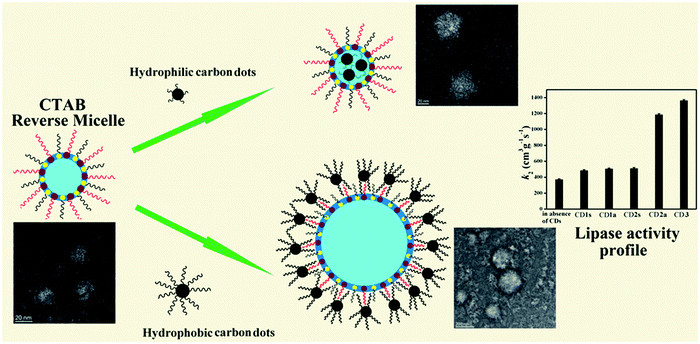 | ||
| Fig. 25 Pictorial representation of surface functionalized carbon dot (CD) included reverse micelles. Reproduced with permission from ref. 119, Langmuir, 2016, 32, 3890–3900. Copyright 2016 American Chemical Society. | ||
4 Summary and outlook
In conclusion, amalgamation of different supramolecular self-assemblies with nanomaterials can give rise to new characteristics due to the synergistic contribution of both materials. After the initial works intended to use molecular gels as media for the preparation and stabilization of nanoparticles, recent reports have suggested that soft-nanocomposites are advantageous for both constituents. These soft-nanocomposites have interesting properties including electrical conductivity, viscoelasticity, thermal robustness, magnetic, phase-selective, redox and near-infrared radiation sensitive properties and so on. Thus, various design strategies for synthesis as well as composite preparation with interesting applications in the area of supercapacitors, nanoelectronics, photovoltaic devices, chemical and biosensors, biomedicine and so on have been discussed here.In spite of there being lots of research work on soft-nanocomposites, there are still many challenges to overcome before functional soft-nanocomposites may be employed in practical applications. Further development of new approaches for synthesis as well as composite preparation with interesting applications is also needed. Similar benefits may be envisioned with new avenues of application involving chemistry at the hetero-junction of synthetic soft materials derived from various functional molecules and advanced nanomaterials. Finally, a possible outlook is planned for the development of next generation multifunctional soft-nanocomposites, which could be achieved by amalgamation of nanomaterials and functional molecules in order to take advantage of their individual properties.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
P. K. D. is thankful to the Science and Engineering Research Board (SERB), Department of Science and Technology (DST), India, for financial assistance (No. EMR/2017/000656). P. C. and S. D. acknowledge CSIR, India, for their Research Fellowships. All biological samples and cell lines (HeLa, NIH3T3, HepG2, HEK-293, CHO, MCF7, MDA-MB-231, A549 and B16F10 cells) were procured from NCCS, Pune, India.References
- A. Ajayaghosh, V. K. Praveen and C. Vijayakumar, Chem. Soc. Rev., 2008, 37, 109–122 RSC.
- E. Carretti, M. Bonini, L. Dei, B. H. Berrie, L. V. Angelova, P. Baglioni and R. G. Weiss, Acc. Chem. Res., 2010, 43, 751–760 CrossRef CAS.
- B. Escuder, F. Rodriguez-Llansola and J. F. Miravet, New J. Chem., 2010, 34, 1044–1054 RSC.
- S. I. Stupp, Nano Lett., 2010, 10, 4783–4786 CrossRef CAS.
- B. Xu, Langmuir, 2009, 25, 8375–8377 CrossRef CAS.
- A. Vintiloiu and J. C. Leroux, J. Controlled Release, 2008, 125, 179–192 CrossRef CAS.
- I. Tomatsu, K. Peng and A. Kros, Adv. Drug Delivery Rev., 2011, 63, 1257–1266 CrossRef CAS PubMed.
- E. Miyako, K. Kono, E. Yuba, C. Hosokawa, H. Nagai and Y. Hagihara, Nat. Commun., 2012, 3, 1–9 Search PubMed.
- F. Karchemski, D. Zucker, Y. Barenholz and O. Regev, J. Controlled Release, 2012, 160, 339–345 CrossRef CAS PubMed.
- T. Kim, C. Do, S. Kang, M. Lee, S. Lim and S. Choi, Soft Matter, 2012, 8, 9073–9078 RSC.
- M. Mandal, S. K. Ghosh, S. Kundu, K. Esumi and T. Pal, Langmuir, 2002, 18, 7792–7797 CrossRef CAS.
- A. P. Herrera, O. Resto, J. G. Briano and C. Rinaldi, Nanotechnology, 2005, 16, S618–S625 CrossRef PubMed.
- S. Maiti, K. Das, S. Dutta and P. K. Das, Chem. – Eur. J., 2012, 18, 15021–15030 CrossRef CAS.
- A. Shome, T. Kar and P. K. Das, ChemPhysChem, 2011, 12, 369–378 CrossRef CAS.
- J. Song, J. Zhou and H. Duan, J. Am. Chem. Soc., 2012, 134, 13458–13469 CrossRef CAS.
- P. Kumar Vemula, U. Aslam, V. Ajay Mallia and G. John, Chem. Mater., 2007, 19, 138–140 CrossRef.
- M. Cametti and Z. Dzolic, Chem. Commun., 2014, 50, 8273–8286 RSC.
- S. Bhattacharya and S. K. Samanta, Chem. Rev., 2016, 116, 11967–12028 CrossRef CAS.
- D. Das, T. Kar and P. K. Das, Soft Matter, 2012, 8, 2348–2365 RSC.
- G. Pastorin, W. Wu, S. Wieckowski, J. P. Briand, K. Kostarelos, M. Prato and A. Bianco, Chem. Commun., 2006, 1182–1184 RSC.
- S. Brahmachari, D. Das, A. Shome and P. K. Das, Angew. Chem., Int. Ed., 2011, 50, 11243–11247 CrossRef CAS.
- S. Dinda, D. Mandal, S. Sarkar and P. K. Das, Chem. – Eur. J., 2017, 23, 15194–15202 CrossRef CAS.
- C. N. R. Rao, G. U. Kulkarni, P. J. Thomas and P. P. Edwards, Chem. Soc. Rev., 2000, 29, 27–35 RSC.
- P. K. Jain, X. Huang, I. H. El-Sayed and M. A. El-Sayed, Acc. Chem. Res., 2008, 41, 1578–1586 CrossRef CAS PubMed.
- S. Bhattacharya, A. Srivastava and A. Pal, Angew. Chem., Int. Ed., 2006, 45, 2934–2937 CrossRef CAS PubMed.
- I. A. Coates and D. K. Smith, J. Mater. Chem., 2010, 20, 6696–6702 RSC.
- M. Kimura, S. Kobayashi, T. Kuroda, K. Hanabusa and H. Shirai, Adv. Mater., 2004, 16, 335–338 CrossRef CAS.
- K. Yamamoto, Z. An, N. Saito and M. Yamaguchi, Chem. – Eur. J., 2013, 19, 10580–10588 CrossRef CAS.
- H. He, S. Chen, X. Tong, Y. Chen, B. Wu, M. Ma, X. Wang and X. Wang, Soft Matter, 2016, 12, 957–964 RSC.
- P. K. Vemula and G. John, Chem. Commun., 2006, 2218–2220 RSC.
- J. Lu, J. Wu and Y. Ju, New J. Chem., 2014, 38, 6050–6056 RSC.
- F. Delbecq, K. Tsujimoto, Y. Ogue, H. Endo and T. Kawai, J. Colloid Interface Sci., 2013, 390, 17–24 CrossRef CAS.
- R. N. Mitra and P. K. Das, J. Phys. Chem. C, 2008, 112, 8159–8166 CrossRef CAS.
- D. P. Kumar, RSC Adv., 2014, 4, 45449–45457 RSC.
- M. K. Khan and P. Sundararajan, Chem. – Eur. J., 2011, 17, 1184–1192 CrossRef CAS.
- S. Miljanić, L. Frkanec, T. Biljan, Z. Meić and M. Žinić, Langmuir, 2006, 22, 9079–9081 CrossRef.
- S. Saha, A. Pal, S. Pande, S. Sarkar, S. Panigrahi and T. Pal, J. Phys. Chem. C, 2009, 113, 7553–7560 CrossRef CAS.
- A. Mantion, A. G. Guex, A. Foelske, L. Mirolo, K. M. Fromm, M. Painsi and A. Taubert, Soft Matter, 2008, 4, 606–617 RSC.
- H. Basit, A. Pal, S. Sen and S. Bhattacharya, Chem. – Eur. J., 2008, 14, 6534–6545 CrossRef CAS.
- A. Pal, H. Basit, S. Sen, V. K. Aswal and S. Bhattacharya, J. Mater. Chem., 2009, 19, 4325–4334 RSC.
- J. Zhang, X. Wang, B. Zhao and C. Li, Chem. Lett., 2006, 40–41 CrossRef.
- S. Chatterjee and A. K. Nandi, Chem. Commun., 2011, 47, 11510–11512 RSC.
- M. O. M. Piepenbrock, N. Clarke and J. W. Steed, Soft Matter, 2011, 7, 2412–2418 RSC.
- T. J. Trivedi, K. S. Rao and A. Kumar, Green Chem., 2014, 16, 320–330 RSC.
- C. García-Astrain, C. Chen, M. Burón, T. Palomares, A. Eceiza, L. Fruk, M. Á. Corcuera and N. Gabilondo, Biomacromolecules, 2015, 16, 1301–1310 CrossRef.
- S. K. Mandal, S. Brahmachari and P. K. Das, ChemPlusChem, 2014, 79, 1733–1746 CAS.
- S. Brahmachari, S. K. Mandal and P. K. Das, PLoS One, 2014, 9, e106775 CrossRef.
- B. Simmons, S. Li, V. T. John, G. L. McPherson, C. Taylor, D. K. Schwartz and K. Maskos, Nano Lett., 2002, 2, 1037–1042 CrossRef CAS.
- G. Tan, M. Singh, J. He, V. T. John and G. L. McPherson, Langmuir, 2005, 21, 9322–9326 CrossRef CAS.
- P. D. Wadhavane, R. E. Galian, M. A. Izquierdo, J. Aguilera-Sigalat, F. Galindo, L. Schmidt, M. I. Burguete, J. Pérez-Prieto and S. V. Luis, J. Am. Chem. Soc., 2012, 134, 20554–20563 CrossRef CAS.
- X. Yan, Y. Cui, Q. He, K. Wang and J. Li, Chem. Mater., 2008, 20, 1522–1526 CrossRef CAS.
- L. Korala, L. Li and S. L. Brock, Chem. Commun., 2012, 48, 8523–8525 RSC.
- L. F. F. F. Gonçalves, F. K. Kanodarwala, J. A. Stride, C. J. R. Silva, M. R. Pereira and M. J. M. Gomes, J. Photochem. Photobiol., A, 2014, 285, 21–29 CrossRef.
- E. D. Sone, E. R. Zubarev and S. I. Stupp, Angew. Chem., Int. Ed., 2002, 41, 1706–1709 CrossRef.
- P. Xue, R. Lu, Y. Huang, M. Jin, C. Tan, C. Bao, Z. Wang and Y. Zhao, Langmuir, 2004, 20, 6470–6475 CrossRef CAS.
- N. Lopez, F. Illas and G. Pacchioni, J. Am. Chem. Soc., 1999, 121, 813–821 CrossRef CAS.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710–712 CrossRef CAS.
- X. Feng, G. E. Fryxell, L. Q. Wang, A. Y. Kim, J. Liu and K. M. Kemner, Science, 1997, 276, 923–926 CrossRef CAS.
- Z. R. Tian, W. Tong, J. Y. Wang, N. G. Duan, V. V. Krishnan and S. L. Suib, Science, 1997, 276, 926–930 CrossRef CAS.
- R. S. Singh, V. K. Rangari, S. Sanagapalli, V. Jayaraman, S. Mahendra and V. P. Singh, Sol. Energy Mater. Sol. Cells, 2004, 82, 315–330 CrossRef CAS.
- M. Dhayal, S. D. Sharma, C. Kant, K. K. Saini and S. C. Jain, Surf. Sci., 2008, 602, 1149–1154 CrossRef CAS.
- S. S. Mali, S. K. Desai, D. S. Dalavi, C. A. Betty, P. N. Bhosale and P. S. Patil, Photochem. Photobiol. Sci., 2011, 10, 1652–1658 RSC.
- L. Xu and H. K. Lee, Anal. Chem., 2007, 79, 5241–5248 CrossRef CAS.
- R. A. Caruso, J. H. Schattka and A. Greiner, Adv. Mater., 2001, 13, 1577–1579 CrossRef CAS.
- M. Suzuki, Y. Nakajima, T. Sato, H. Shirai and K. Hanabusa, Chem. Commun., 2006, 377–379 RSC.
- S. Dutta, D. Das, A. Dasgupta and P. K. Das, Chem. – Eur. J., 2010, 16, 1493–1505 CrossRef CAS PubMed.
- R. K. Das, S. Bhat, S. Banerjee, C. Aymonier, A. Loppinet-Serani, P. Terech, U. Maitra, G. Raffy, J. P. Desvergne and A. Guerzo, J. Mater. Chem., 2011, 21, 2740–2750 RSC.
- A. Kotal, T. K. Paira, S. Banerjee and T. K. Mandal, Langmuir, 2010, 26, 6576–6582 CrossRef CAS.
- E. Taboada, L. N. Feldborg, A. P. del Pino, A. Roig, D. B. Amabilino and J. Puigmartí-Luis, Soft Matter, 2011, 7, 2755–2761 RSC.
- Z. Yang, H. Gu, J. Du, J. Gao, B. Zhang, X. Zhang and B. Xu, Tetrahedron, 2007, 63, 7349–7357 CrossRef CAS.
- J. H. Lee, R. Ivkov and R. Blumenthal, J. Nanomed. Biother. Discovery, 2014, 4, 3 Search PubMed.
- Y. Ono, K. Nakashima, M. Sano, Y. Kanekiyo, K. Inoue, J. Hojob and S. Shinkai, Chem. Commun., 1998, 1477–1478 RSC.
- Y. Wei, Y. Wang, C. Wei, Q. Zhao, Y. Yan, J. Yang and J. Huang, RSC Adv., 2015, 5, 106005–106011 RSC.
- I. Maity, M. K. Manna, D. B. Rasale and A. K. Das, ChemPlusChem, 2014, 79, 413–420 CrossRef CAS.
- B. Yan, J. C. Boyer, D. Habault, N. R. Branda and Y. Zhao, J. Am. Chem. Soc., 2012, 134, 16558–16561 CrossRef CAS.
- N. Choudhary, S. Hwang and W. Choi, in Handbook of Nanomaterials Properties, ed. B. Bhushan, D. Luo, S. R. Schricker, W. Sigmund and S. Zauscher, Springer, Berlin, 2014 Search PubMed.
- T. Ishi-i, J. H. Jung and S. Shinkai, J. Mater. Chem., 2000, 10, 2238–2240 RSC.
- T. Ishi-i, R. Iguchi, E. Snip, M. Ikeda and S. Shinkai, Langmuir, 2001, 17, 5825–5833 CrossRef CAS.
- M. Shirakawa, N. Fujita and S. Shinkai, J. Am. Chem. Soc., 2003, 125, 9902–9903 CrossRef CAS PubMed.
- X. Yang, G. Zhang, D. Zhang and D. Zhu, Langmuir, 2010, 26, 11720–11725 CrossRef CAS.
- C. Zhang, J. Wang, J. J. Wang, M. Li, X. L. Yang and H. B. Xu, Chem. – Eur. J., 2012, 18, 14954–14956 CrossRef CAS.
- K. Oishi, T. Ishi-i, M. Sano and S. Shinkai, Chem. Lett., 1999, 1089–1090 CrossRef CAS.
- T. Ishi-i, Y. Ono and S. Shinkai, Chem. Lett., 2000, 808–809 CrossRef CAS.
- X. Yang, G. Zhang, D. Zhang, J. Xiang, G. Yang and D. Zhu, Soft Matter, 2011, 7, 3592–3598 RSC.
- P. Xue, Q. Xu, P. Gong, C. Qian, Z. Zhang, J. Jia, X. Zhao, R. Lu, A. Renb and T. Zhang, RSC Adv., 2013, 3, 26403–26411 RSC.
- I. D. Tevis, W.-W. Tsai, L. C. Palmer, T. Aytun and S. I. Stupp, ACS Nano, 2012, 6, 2032–2040 CrossRef CAS.
- C. Lee, X. Wei, J. W. Kysar and J. Hone, Science, 2008, 321, 385–388 CrossRef CAS.
- A. A. Balandin, S. Ghosh, W. Bao, I. Calizo, D. Teweldebrhan, F. Miao and C. N. Lau, Nano Lett., 2008, 8, 902–907 CrossRef CAS.
- X. Du, I. Skachko, A. Barker and E. Y. Andrei, Nat. Nanotechnol., 2008, 3, 491–495 CrossRef CAS.
- S. K. Samanta, K. S. Subrahmanyam, S. Bhattacharya and C. N. R. Rao, Chem. – Eur. J., 2012, 18, 2890–2901 CrossRef CAS.
- Q. Y. Cheng, D. Zhou, Y. Gao, Q. Chen, Z. Zhang and B. H. Han, Langmuir, 2012, 28, 3005–3010 CrossRef CAS.
- Y. Xu, K. Sheng, C. Li and G. Shi, ACS Nano, 2010, 4, 4324–4330 CrossRef CAS.
- S. Y. Qin, X. J. Liu, R. X. Zhuo and X. Z. Zhang, Macromol. Chem. Phys., 2012, 213, 2044–2051 CrossRef CAS.
- H. Bai, C. Li, X. Wangb and G. Shi, Chem. Commun., 2010, 46, 2376–2378 RSC.
- P. Xing, X. Chu, G. Du, M. Ma, S. Li and A. Hao, Colloid Polym. Sci., 2014, 292, 3223–3231 CrossRef CAS.
- S. K. Mandal, D. Mandal and P. K. Das, ChemPlusChem, 2016, 81, 213–221 CrossRef CAS.
- P. Xing, X. Chu, S. Li, M. Ma and A. Hao, ChemPhysChem, 2014, 15, 2377–2385 CrossRef CAS.
- K. Das, S. Maiti, M. Ghosh, D. Mandal and P. K. Das, J. Colloid Interface Sci., 2013, 395, 111–118 CrossRef CAS PubMed.
- S. Iijima, Nature, 1991, 354, 56–58 CrossRef CAS.
- E. Miyako, T. Itho, Y. Nara and T. Hirotsu, Chem. – Eur. J., 2009, 15, 7520–7525 CrossRef CAS.
- A. Pal, B. S. Chhikara, A. Govindaraj, S. Bhattacharya and C. N. R. Rao, J. Mater. Chem., 2008, 18, 2593–2600 RSC.
- S. Bhattacharyya, S. Guillot, H. Dabboue, J. F. Tranchant and J. P. Salvetat, Biomacromolecules, 2008, 9, 505–509 CrossRef CAS.
- Z. Wang and Y. Chen, Macromolecules, 2007, 40, 3402–3407 CrossRef CAS.
- J. Zou, J. Liu, A. S. Karakoti, A. Kumar, D. Joung, Q. Li, S. I. Khondaker, S. Seal and L. Zhai, ACS Nano, 2010, 4, 7293–7302 CrossRef CAS.
- S. K. Mandal, T. Kar and P. K. Das, Chem. – Eur. J., 2013, 19, 12486–12496 CrossRef CAS.
- V. C. Moore, M. S. Strano, E. H. Haroz, R. H. Hauge and R. E. Smalley, Nano Lett., 2003, 3, 1379–1382 CrossRef CAS.
- R. Pignatello, S. Guccione, S. Forte, C. Di Giacomo, V. Sorrenti, L. Vicari, G. Uccello Barretta, F. Balzano and G. Puglisi, Bioorg. Med. Chem., 2004, 12, 2951–2964 CrossRef CAS.
- B. G. Cousins, A. K. Das, R. Sharma, Y. Li, J. P. McNamara, I. H. Hillier, I. A. Kinloch and R. V. Ulijn, Small, 2009, 5, 587–590 CrossRef CAS.
- S. Brahmachari, D. Das and P. K. Das, Chem. Commun., 2010, 46, 8386–8388 RSC.
- S. Brahmachari, M. Ghosh, S. Dutta and P. K. Das, J. Mater. Chem. B, 2014, 2, 1160–1173 RSC.
- M. Ghosh, S. Brahmachari and P. K. Das, Macromol. Biosci., 2014, 14, 1795–1806 CrossRef CAS.
- M. Ghosh and P. K. Das, Colloids Surf., B, 2016, 142, 367–376 CrossRef CAS.
- K. Das, S. Nimushakavi, A. Chaudhuri and P. K. Das, ChemMedChem, 2017, 12, 738–750 CrossRef CAS.
- S. Zhu, Q. Meng, L. Wang, J. Zhang, Y. Song, H. Jin, K. Zhang, H. Sun, H. Wang and B. Yang, Angew. Chem., Int. Ed., 2013, 52, 3953–3957 CrossRef CAS.
- H. Huang, C. Li, S. Zhu, H. Wang, C. Chen, Z. Wang, T. Bai, Z. Shi and S. Feng, Langmuir, 2014, 30, 13542–13548 CrossRef CAS.
- G. H. G. Ahmed, R. B. Laino, J. A. G. Calzon and M. E. D. Garcia, Talanta, 2015, 132, 252–257 CrossRef CAS.
- S. Nandi, S. Kolusheva, R. Malishev, A. Trachtenberg, T. P. Vinod and R. Jelinek, Chem. – Eur. J., 2015, 21, 7755–7759 CrossRef CAS.
- A. Ghosh, B. Parasar, T. Bhattacharyya and J. Dash, Chem. Commun., 2016, 52, 11159–11162 RSC.
- S. Sarkar, K. Das and P. K. Das, Langmuir, 2016, 32, 3890–3900 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |