Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The behaviour of sunflower oleosomes at the interfaces
Dimitris
Karefyllakis
a,
Atze
Jan van der Goot
a and
Constantinos V.
Nikiforidis
 *b
*b
aFood Process Engineering, Wageningen University, The Netherlands
bBiobased Chemistry and Technology, Wageningen University, The Netherlands. E-mail: costas.nikiforidis@wur.nl
First published on 15th May 2019
Abstract
Oleosomes are particles equipped with a sophisticated membrane, comprising a continuous monolayer of phospholipids and hydrophobic proteins, which covers the triglyceride core and grants them extreme physical and chemical stability. The noteworthy qualities of oleosomes have attracted strong interest for their incorporation in emulsion formulations; however, little is known about their emulsifying properties and their behaviour on interfaces. For these reasons, oleosomes were isolated from sunflower seeds (96.2 wt% oil, 3.1 wt% protein) and used as an emulsifier for the stabilization of O/W and W/O interfaces. In both cases, oleosomes showed high interfacial and emulsifying activity. Individual oleosome particles had a broad size distribution from 0.4 to 10.0 μm and it was observed that the membrane of the larger oleosomes (>1–5 μm) was disrupted and its fractions participated in the newly formed interface. Oleosomes with a smaller diameter (<1 μm) seemed to have survived the applied mild emulsification step as a great number of them could be observed both in the bulk of the emulsions and on the interface of the emulsion droplets. This phenomenon was more pronounced for the W/O interface where oleosomes were absorbed intact in a manner similar to a Pickering mechanism. However, when the triglycerides were removed from the core of oleosomes in order to focus more on the effect of the membrane, the remaining material formed sub-micron spherical particles, which clearly acted as Pickering stabilisers. These findings showcase the intriguing behaviour of oleosomes upon emulsification, especially the crucial role of their membrane. The study demonstrates relevance for applications where immiscible liquid phases are present.
Introduction
Nature has been always a great source of inspiration regarding innovations in science and technology. Emulating life's unique developments leads to the fabrication of bioinspired and bioderived materials with high functionality. These biobased materials can offer evolved-through-the-millennia solutions to practical challenges humanity faces. A characteristic example of such a resourceful engineering can be found in plant seeds where the protection of oil (mostly triacylglycerides) against oxidation and its arduous mixing with hydrophilic systems has been resolved by nature with organelles called oleosomes.1The primary function of oleosomes is to store the seed energy source, during seed dormancy and protect them against environmental stresses.2 To achieve this, oleosomes are equipped with a sophisticated membrane that covers the triacylglyceride core and grants them extreme physical and chemical stability.1,3 The membrane consists of a continuous monolayer of phospholipids to which a number of uniquely hydrophobic proteins (oleosins, caleosins and steroleosins) are embedded. Oleosins are the dominant ones and all three groups of proteins have long hydrophobic domains that are anchored into the oil phase, while their amphiphilic termini rest on the hydrophilic oleosome surface.4
When applying pressing or organic solvent extraction the oleosome membrane is disrupted and the oil core is extracted. However, in order to extract intact oleosomes, an environmentally friendly aqueous extraction process has to be followed. When intact oleosomes are extracted, the aforementioned noteworthy qualities of physical and chemical stability are conveyed to the resulting “natural” oil-in-water emulsions.5,6 Besides stability, these biobased emulsions bear additional conveniences as a consequence of their pre-existence considering that neither emulsifiers nor the high energy consuming step of homogenization is required.7 Therefore, scientists from different disciplines are currently investigating their in situ properties and the potential applications of these pre-emulsified systems in several fields like foods and pharmaceuticals.8–10
Nonetheless, to deeply understand the molecular interactions in the oleosome membrane and also explore new potential applications, it is important to investigate the properties of oleosomes when an extrinsic oil/water interface is present. Until today it remains unknown what happens to the structure of the oleosomes and how their membrane behaves once new interfaces are created. It is not certain yet whether their hydrophobic core merges with the lipid phase and their membrane participates in the formation of the newly available interface or if oleosomes remain intact acting as dispersed spherical particles. To study this was the aim of this work. Oleosomes were extracted from sunflower seeds and interfacial tensiometry combined with electron and confocal microscopy techniques was used to track the behaviour of the oleosomes on oil/water interfaces. Oleosomes from sunflower seeds were chosen due to their big size7 that could be more easily observed with the applied microscopy techniques.
Experimental section
Materials
Dehulled and not additionally treated sunflower seeds were ordered from Notenstore (Bergschenhoek, The Netherlands). Sunflower oil (SF oil) was purchased from the local market and was filtered with silica (MP Alumina N-Super I, MP Biomedicals, Germany)11 to remove any polar compounds. Sunflower protein isolate (SFPI) of high purity (96 wt%) was isolated during a previous work.12 Petroleum ether, ethanol, potassium monobasic dihydrate, potassium phosphate dibasic, sodium hydroxide and hydrochloric acid were all purchased from Sigma Aldrich (Sigma, USA) and were of analytical grade. For all analyses, ultrapure water was used.Methods
Extraction of sunflower oleosomes (SFOs)
SFOs were extracted from sunflower seeds using an aqueous method developed by Nikiforidis et al.13 with slight modifications. First, seeds were soaked overnight in an aqueous solution of pH 8, adjusted with 4 M NaOH, and stored at 4 °C. After overnight soaking (16 hours), the excess aqueous solution was drained off using a strainer. The seeds were then ground with demi water in the ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (w/v) for 90 seconds in a laboratory blender at maximum speed. The slurry was then passed through a commercial juicer in order to remove the solid leftovers. The crude extract obtained as filtrate was centrifuged at 10
7 (w/v) for 90 seconds in a laboratory blender at maximum speed. The slurry was then passed through a commercial juicer in order to remove the solid leftovers. The crude extract obtained as filtrate was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g at 4 °C for 30 min. The cream layer was then carefully removed and subsequently re-suspended in aqueous solution pH 8 at 1
000g at 4 °C for 30 min. The cream layer was then carefully removed and subsequently re-suspended in aqueous solution pH 8 at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio (w/v) and centrifuged under the same conditions. This washing cycle was repeated once more.
4 ratio (w/v) and centrifuged under the same conditions. This washing cycle was repeated once more.
Protein and oil content
The protein content was determined by Dumas analysis (Nitrogen analyzer FlashEA 1112 series, Thermo Scientific, The Netherlands). The protein factor used to calculate the percentage of protein was 5.8.14 The oil content was obtained using an automated Soxhlet device (Buchi B-811, Buchi Labortechnik AG, Flawil, Switzerland) for 12 h using petroleum ether. All the analyses were performed in triplicates.Defatting the SFO cream
Part of the SFO cream was left to dry at 35 °C over the course of 2 days. The dried cream was then subjected to oil extraction using an automated Soxhlet device using petroleum ether (Buchi B-811, Buchi Labortechnik AG, Flawil, Switzerland) for 12 h. The resulting powder was left in the open air until all the solvent was evaporated and was called defatted SFO.Emulsion preparation
Emulsions were prepared by dispersing 0.5 wt% of the SFO, SFPI and the defatted SFO in aquatic 0.1 M phosphate buffer pH 8 and stirred for 30 min. Sunflower oil was then slowly added and a coarse emulsion (10 wt% and 90 wt% in sunflower oil for O/W and W/O emulsions respectively) was made using a rotor-stator homogenizer (9500 rpm, 3 min) (UltraTurrax, IKA, California, United states).Dynamic interfacial properties
Interfacial tension measurements at the O/W and W/O interfaces were recorded using an automated drop tensiometer (ADT) (Tracker, Teclis-IT Concept, France). The protein concentration was equalised in both cases (0.001% and 0.01% w/v for O/W and W/O, respectively). Stripped sunflower oil was used and the emulsifiers were left to diffuse from the inside of the droplet towards the interface and from the cuvette towards the interface, respectively. The initial droplet volume was set to 16 μL, with corresponding droplet area of 30 mm2. The interfacial tension was monitored for 3 h while keeping the droplet volume constant. The Stokes–Einstein equation was used to roughly estimate the scale of the diffusion rate during the beginning of the absorption. The Stokes–Einstein equation is: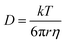 | (1) |
Confocal laser scanning microscopy (CLSM)
CLSM images were obtained at room temperature using a LEICA TCS SP5 Confocal Laser Scanning Microscope (Leica Microsystems GmbH., Mannheim, Germany) equipped with an inverted microscope (model Leica DMI6000), containing a set of four visible light lasers. The used objectives were HC PL APO 10×/0.40 CS and HC PL APO 20×/0.70 IMM/CORR CS. Digital image files were acquired in 1024 × 1024 pixel resolution and were analysed with the program Zeiss SLM image examiner (Zeiss group, Oberkochen, Germany). Samples were carefully placed on a microscope slide and stained with a droplet of aquatic Nile Blue 1.0 wt%.Cryo-scanning electron microscope imaging (cryo-SEM)
Dry SFO cream was glued onto a brass sample holder by carbon glue (Leit-C, Neubauer Chemicalien, Germany) and subsequently frozen by the use of liquid nitrogen. The sample holder was fitted into the transfer cryogenic Leica holder. All manipulations were carried out under liquid nitrogen. The Leica sample holder was transferred in a non-dedicated cryo-preparation system (MED 020/VCT 100, Leica, Vienna, Austria) onto a sample stage at −93 °C. In this cryo-preparation chamber, the samples were immediately fractured and freeze-dried for 23 min at −93 °C at 1.3 × 10−6 mbar to remove contaminated water vapour. The samples were sputter-coated with a layer of 4 nm tungsten at the same temperature and transferred cryo-shielded into the field emission scanning microscope (Magellan 400, FEI, Eindhoven, the Netherlands), onto a sample stage equilibrated at −122 °C at 4 × 10−7 mbar. The analysis was performed with SE at 2 kV, 13 pA. All images were recorded digitally.Results and discussion
The membrane structure of oleosomes
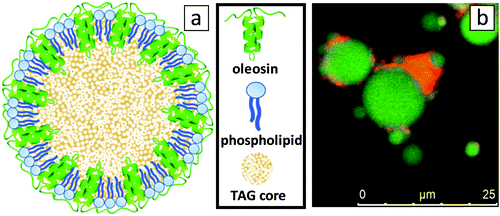
The detailed configuration of the oleosome membrane has long been investigated; however, even until today a general consensus has not been reached entirely.4 An illustration of the generally accepted oleosome model can be seen in Fig. 1a and is valid for the majority of oilseeds, including sunflower. To investigate the structure of the extracted sunflower oleosomes, confocal microscopy was employed. As shown in Fig. 1b, sunflower oleosomes were spherical droplets that appeared in various diameters from sub-micron sizes until a few μm. The initial size of sunflower oleosomes is expected to be lower (∼1 μm),7 therefore the presence of large oleosomes (∼10 μm) could be attributed to partial coalescence during the extraction procedure. Despite that the tight packing and absence of bulk water inside the cells7 allow the survival of oleosomes, agitation and extensive hydrophobic forces between neighbouring oleosomes is probably forcing them to aggregate and to a certain extent coalesce. In addition, confocal microscopy revealed that besides the interfacial proteins, there is still a minor amount of extrinsic proteins present, which is shown in the CLSM image in red colour (Fig. 1b). It has been reported that storage proteins are co-extracted with oleosomes from sunflower seeds and despite the applied washing steps they are not completely removed.7 They interact through hydrophobic forces with the oleosome interfacial proteins and can act as bridges between neighbouring oleosomes. Nevertheless, the protein content of the SFO on a dry basis was 3.1 wt%, while the oil content was 96.2 wt%. The oil/protein ratio in isolated oleosomes from different seeds is in this range,15 therefore we presume that the amount of extrinsic proteins is minor.Oleosomes at the O/W interface
The behaviour of oleosomes on interfaces is still largely unknown as it is uncertain what happens to their membrane upon mixing with bulk oil, homogenization and absorption. It has been proven though that isolated oleosomes, and their membrane components, are highly interfacially active.16–18 However, when mixed with high amounts of extraneous proteins (protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oleosome 3
oleosome 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
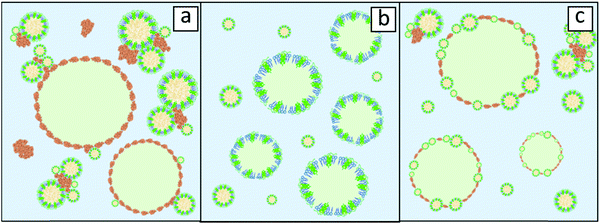
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), oleosomes appeared to have little to no impact on the emulsification efficiency.19 This indicates that the presence of storage proteins should be taken into account when the interfacial behaviour of the oleosomes is examined as extraneous proteins might influence it severely when present in high concentration. This scenario is schematically illustrated in Fig. 2a, where it is illustrated that storage proteins are hindering the absorption of oleosomes at the droplet interface by bridging them and by dominating the oil/water interface.
1), oleosomes appeared to have little to no impact on the emulsification efficiency.19 This indicates that the presence of storage proteins should be taken into account when the interfacial behaviour of the oleosomes is examined as extraneous proteins might influence it severely when present in high concentration. This scenario is schematically illustrated in Fig. 2a, where it is illustrated that storage proteins are hindering the absorption of oleosomes at the droplet interface by bridging them and by dominating the oil/water interface.
The removal of storage proteins simplifies the picture and allows safer conclusions on oleosome behaviour. When the absorption of purified soybean oleosomes at the air–water interface was examined, it was showcased that rupture of these organelles presumably occurred and that fragments of their membrane (oleosin/phospholipid mixtures) participated in the stabilization of the interface.20 Similar findings were reported for emulsions stabilised by purified soybean oleosomes where analysis of cryo-SEM images revealed an oil droplet interface free from intact oleosomes pointing also towards the potential rupture of these organelles upon absorption.16 A schematic representation of such an emulsion, stabilised by fragmented oleosomes, is shown in Fig. 2b. One last scenario could be that oleosomes do not disintegrate upon absorption but they remain intact at the O/W interface. Detailed surface pressure studies for soybean oleosomes and reconstituted oleosomes revealed that intact oleosomes can indeed stabilise the O/W interface; however, after long time intervals, oleosomes seem to collapse followed by rearrangements of their components at the interface.20,21 This could signify that oleosomes do not rupture but act as Pickering stabilizers on the O/W interface. Pickering stabilisation is a mechanism of stabilisation of a membrane interface by solid particles that do not deform upon absorption like proteins do.22 An illustration of such a scenario is represented in Fig. 2c.
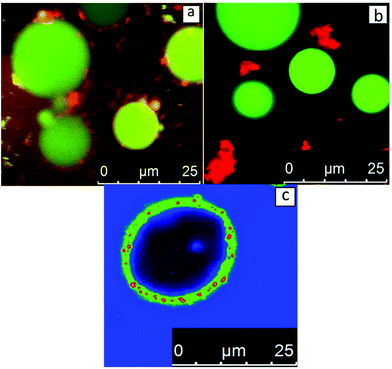
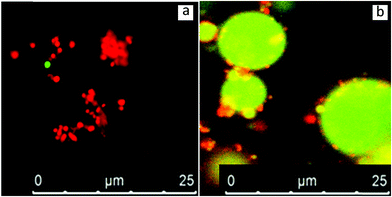
In order to clarify which scenario describes the behaviour of sunflower oleosomes more accurately, the isolated SFO was used as an emulsifier for the stabilization of oil-in-water emulsions. CLSM analysis of an emulsion stabilized by SFO is presented in Fig. 3a. For comparison, CLSM images of an emulsion stabilised with sunflower protein isolate are shown in Fig. 3b. As it is shown in Fig. 3b, there were a few protein aggregates in the continuous phase (red colour), while absorbed proteins on the interface formed a very thin homogeneous film since no red colour could be observed. On the other hand, when SFO was used a different type of oil droplet interface was observed. In this case, dense red coloured particles are observed on the interface. This indicates that a material that contains protein is absorbed, which should be the SFO. The diameter of the formed oil droplets during homogenization had been deliberately chosen to be big (>10 μm) in order to easily distinguish them from the SFO (≤10 μm). However, no droplets in the range of 1–10 μm could be observed, an indication that the oleosomes that had this size (Fig. 1a), probably merge with the newly formed oil droplets. At the same time, the smaller oleosomes (<5 μm) seem to remain stable or absorb or participate on the oil/water interface.
The nature of the spherical particles on the interface is not a trivial subject to elaborate on and the amount of certainty one can have on the topic can be limited. A certain amount of disintegration of oleosomes is probably occurring (at least for the larger ones). It is not expected however, that when oleosomes eventually rupture that their membrane breaks down completely until individual molecules are formed. This means that neither oleosins nor phospholipids are expected to form pure micelles. Oleosome membranes are known for their strength, hence the strong association of its elements such that it is more logical that the result of oleosome rupture will be membrane fragments rather than phospholipid and oleosin micelles/aggregates. Certainly a big number of phospholipids can escape and stabilise the interface before intact oleosomes do. But even in this scenario a certain participation of oleosins can be expected as it has been reported that phospholipid/oleosin mixed systems stabilise emulsions with greater efficiency than oleosins or phospholipids separately.18 All in all, it can be confidently stated that oleosin and/or phospholipid micelles cannot completely explain the spherical particles observed in Fig. 3a and that fragment aggregates or intact oleosomes should be employed as explanations. Intact oleosomes seem to be able to act as Pickering stabilisers but certainly they are not the only stabilizing agent in our system. Membrane fragments should be considered as well.
Signal enhancement of the produced SFO stabilised emulsions, shown in Fig. 3c, leads to better visualisation of the elements present at the interface. It is important to state though that due to the signal enhancement the interface is probably not to scale. It became apparent that a material that contains protein (now with green) has been absorbed at the interface giving a strong signal. However, the most interesting observation is the red spherical particles that are uniformly arranged on the droplet interface. One possible explanation is that these particles could be insoluble oleosin micelles. If upon emulsification the oleosome membrane disintegrates to its molecules, the formation of oleosin micelles could be a possibility. Oleosins are known to aggregate rapidly in aqueous dispersions and form micelles due to their strong hydrophobic nature.23 In the same study, the size of these micelles/aggregates was reported to be around 20 nm. The scale of this size would be too small for the sensitivity of the CLSM technique which means that these particles could not be oleosin micelles. An additional reason pointing towards this direction is that the displacement of oleosins from the membrane has been reported to be really difficult to occur24 suggesting that oleosin will not be present in pure micelles but mixed with phospholipids in membrane fragments.
The colour difference in Fig. 3c indicates that a non-protein material is indeed absorbed onto the interface. However, only very recently the shell thickness of oleosomes was calculated employing small angle neutron scattering and it was shown that the size of the membrane only amounts to around 9 nm.25 Taking these into account, it seems that due to their size, membrane fragments composed of oleosins and phospholipids by themselves would not be visible on the interface due to the limitations of the technique. At the emulsification conditions (pH 8) about 10 wt% of sunflower proteins are insoluble.12 These insoluble proteins do not absorb at the interface and can be seen as red aggregates in Fig. 3b. It cannot be excluded that these aggregated proteins, together with the membrane fragments of the oleosomes could indeed constitute a possible explanation regarding the presence of particles at the interfaces in Fig. 3a and c. However, it is not certain that these aggregates could assume such a spherical shape as the spheres of red colour observed in Fig. 3c. If the spherical shape is taken into consideration, one can assume that these small spheres are oleosomes that have been absorbed intact on the interface of the new oil droplet. This could signify that the smaller oleosomes are apparently more stable than the bigger ones and do not rupture but act as Pickering stabilizers on the O/W interface. In addition, membrane material of the larger oleosomes that merged with the newly formed oil droplets could also participate in the interface. In detailed interfacial studies of purified soybean oleosomes on the air–water interface, it was suggested that rupture of all the oleosomes occurs and that their membrane fragments absorb on the interface.26 In another research where the behaviour of pure soybean oleosomes was studied on the O/W interface, the same explanation was suggested for the stabilization of the interface.16 In both studies, it is supported that the oleosomes initially absorb intact on the interface and subsequently disintegrate into interfacially active membrane fragments. From our results, sunflower oleosomes seemed to either rupture (larger oleosomes) or act as Pickering stabilisers (smaller oleosomes) depending on their size.
Oleosomes at the W/O interface
While some theories have been reported for the behaviour of oleosomes at the O/W interface, nothing is known about how oleosomes behave at W/O interfaces. In this situation where oleosomes are exposed to a non-polar continuous phase, it is possible the impact would be different and even amplified. For these reasons W/O emulsions were prepared with the SFO and the analysis results using CLSM are shown in Fig. 4. Due to a mild homogenization step, large water droplets with diameters ranging from 5 to 20 μm were formed. No dispersed oleosomes were observed in the continuous oil phase, showing that the bigger oleosomes ruptured and merged with the oil phase. It can also be seen from Fig. 4 that the water droplets were stabilized by small spherical particles with a diameter of up to 3 μm. It can be concluded that water droplets are stabilized through a Pickering mechanism by the smaller oleosomes that initially occurred in SFO and remain intact.27–30 Similar to the case of O/W emulsions (Fig. 3), the water/oil interface can at the same time be stabilized by membrane material from the bigger ruptured oleosomes. Through this investigation, it can be hypothesized that sunflower oleosomes with a diameter below 5 μm remain stable and possess the capacity to stabilize O/W or W/O interfaces through the Pickering mechanism, while larger oleosomes (>5 μm) rupture and merge with the oil phase. One possible reason behind the rupture of larger oleosomes could be the influx of lipids through holes on their membrane. It could be possible that the membrane of larger oleosomes does not uniformly cover the triglyceride core but in some areas the penetration of lipids might be easier. This in turn might cause additional swelling of the oleosome until the point of stretchability of the membrane is maximised and the oleosome collapses. On the other hand, for smaller oleosomes this might not be occurring due to a more compact and less penetrable membrane.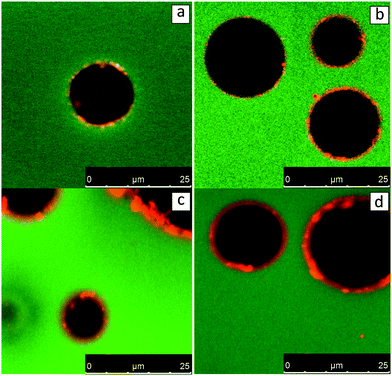 | ||
| Fig. 4 CLSM-imaging of W/O emulsions stabilised by sunflower oleosome. Samples are stained by Nile Blue showing lipids in green and proteins in red. | ||
Interfacial activity of oleosomes at the O/W and W/O interfaces
In order to investigate the interfacial activity of the sunflower oleosomes pendant droplet tensiometry analysis was performed. As it can be seen in Fig. 5, the behaviour of SFO on interfaces was directly compared with SFPI dispersions at both O/W and W/O interfaces. The protein concentration was equalised in both cases (0.001% and 0.01% w/v for O/W and W/O interfaces, respectively).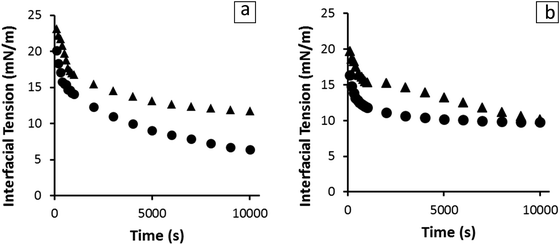 | ||
| Fig. 5 Interfacial tension at the O/W (a) and W/O (b) interfaces as a function of absorption time in the presence of SFO (●) and SFPI (▲) at constant protein concentration (0.001% and 0.01% w/v). | ||
In both cases, the dispersions showed significant interfacial activity. However, the SFO seemed to absorb faster at the initial stages of both measurements and decreased the interfacial activity to a lower value at equilibrium state, (∼6 mN m−1) compared to that of SFPI (∼12 mN m−1). The differences between the absorption profiles of the two dispersions indicate that besides storage proteins that are present in minor amounts, oleosomes and also material from their membrane are absorbed at the interface. Therefore the scenario depicted in Fig. 3a does not hold true when the concentration of storage proteins is that small but maybe only when proteins clearly dominate in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 protein
1 protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oleosome ratio, as it has been previously published.19 The interfacial activity of sunflower oleosomes was significantly lower than those of both crude and purified soybean oleosomes, which resulted in an O/W interfacial tension of 12–15 mN m−1.16,20,31 The lowest value of interfacial tension achieved by SFO could be explained from the partial coalescence of SFO during extraction that resulted in bigger and less stable oleosomes. The membrane of those oleosomes can rupture in the presence of a non-polar phase, while its fragments, together with intact oleosomes, can absorb on the interface. The oleosome membrane fragments, which comprised of oleosome proteins and phospholipids are proven to be highly interfacially active.32 The scale of the diffusion rate can be roughly estimated from eqn (1) where the diffusion rate and the size of the particle are inversely related. This equation assumes a spherical shape of the particles which is true in the case of both the sunflower oleosomes and sunflower globular proteins. The fact that the absorption rate of SFO is faster than the SFPI is surprising as the storage proteins are smaller molecules (average diameter ∼10 nm
oleosome ratio, as it has been previously published.19 The interfacial activity of sunflower oleosomes was significantly lower than those of both crude and purified soybean oleosomes, which resulted in an O/W interfacial tension of 12–15 mN m−1.16,20,31 The lowest value of interfacial tension achieved by SFO could be explained from the partial coalescence of SFO during extraction that resulted in bigger and less stable oleosomes. The membrane of those oleosomes can rupture in the presence of a non-polar phase, while its fragments, together with intact oleosomes, can absorb on the interface. The oleosome membrane fragments, which comprised of oleosome proteins and phospholipids are proven to be highly interfacially active.32 The scale of the diffusion rate can be roughly estimated from eqn (1) where the diffusion rate and the size of the particle are inversely related. This equation assumes a spherical shape of the particles which is true in the case of both the sunflower oleosomes and sunflower globular proteins. The fact that the absorption rate of SFO is faster than the SFPI is surprising as the storage proteins are smaller molecules (average diameter ∼10 nm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) and are expected to diffuse faster, due to the inverse relation of diffusion rate and molecular size, towards the interface compared to the sunflower oleosomes (average diameter ∼1 μm
12) and are expected to diffuse faster, due to the inverse relation of diffusion rate and molecular size, towards the interface compared to the sunflower oleosomes (average diameter ∼1 μm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19). However, the concentration of oleosomes was significantly higher than the one of proteins which means that directly after the formation of the droplet a number of them could absorb readily on it, especially the smaller ones. After diffusion is completed, and oleosomes are absorbed on the interface, oleosome membrane fragments can further decrease the interfacial tension.
19). However, the concentration of oleosomes was significantly higher than the one of proteins which means that directly after the formation of the droplet a number of them could absorb readily on it, especially the smaller ones. After diffusion is completed, and oleosomes are absorbed on the interface, oleosome membrane fragments can further decrease the interfacial tension.
Defatting oleosomes and properties of their membrane
In an effort to showcase the role of the oleosome membrane to stabilise emulsions, it was decided to isolate the membrane without degrading it and subsequently study it with microscopy imaging. For this reason, SFO cream was defatted. To visualise the morphology of the particles in greater detail, the SFO was analysed with cryo-SEM before and after the defatting step. The obtained images are presented in Fig. 6. Prior to lipid removal (Fig. 6a), spherical particles with an average diameter of around 1 μm were observed with a relatively smooth surface that could be potentially identified as oleosomes. After the solvent extraction, particles of similar size were detected but with slightly distorted shape and severely punctured surface (Fig. 6b). The observed holes are most likely caused during the penetration of the solvent inside the oleosome and also during the exit of the triglycerides from the core. After the extraction is completed the remaining material seems to be an empty spherical particle composed with the remaining membrane.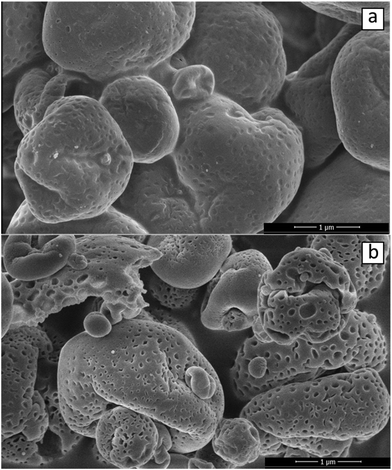 | ||
| Fig. 6 Cryo-SEM imaging of (a) SFO material (b) and of the same sample after removing the triglycerides with an organic solvent. | ||
As it can be observed in Fig. 7, the defatted SFO (Fig. 7a) was then used to stabilize O/W emulsions (Fig. 7b). From Fig. 7a, and complementary to Fig. 6b, it becomes obvious that oil was completely removed from oleosomes and only the surviving membrane fractions are depicted with red, probably due to the present oleosome proteins. Only particles of a few micron or submicron sizes could be observed in Fig. 7a. What is interesting is that most of these particles are clearly spherical. One could argue that all the observed particles are tiny oleosomes which could not be accessed by the solvent hinting towards an incomplete defatting. This is somewhat true, as a small number of green spheres (fat droplets) could be also observed in Fig. 7a meaning that a few tiny oleosomes might have survived. However, the great majority of them were red stained (protein molecules). This kind of sphere was not observed during CLSM-imaging of an SFO dispersion (Fig. 1b). Proteins clearly dominate their structure and if they were not of spherical shape it could be argued that they are just storage protein aggregates. However, storage protein aggregates are usually amorphous, as it can be seen in Fig. 2b. Similarities can be found with the comparable system of lipophilic protein particles extracted from defatted soy flour and composed of oleosin and phospholipids.33 After sonication of the lipophilic protein fraction, the particles in this study were suggested to possess a core–shell structure, with oleosins in the centre and phospholipids surrounding them, and an average size of 140 nm. Therefore, even after oil extraction the components of oleosomes appear to form spherical particles in aqueous dispersions. Alternatively, it could be hypothesised that when the solvent molecules extract the triglycerides out from the core of the oleosomes, the latter do not disintegrate into amorphous aggregates but shrink instead. This would be in agreement with the punctured particles of slightly distorted shape presented in Fig. 6b. The probability that these particles are oleosin micelles should not be considered. The size of these particles is too large for them to be oleosin micelles (reported size ∼20 nm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23) which is too small to be observed with this technique. It could be true that the spherical particles can be multiple oleosin micelles aggregated together. However, a quick observation in Fig. 7a assures us that the spherical particles do not aggregate beyond the size of 1 μm and when they do, their individual shapes can still be obvious.
23) which is too small to be observed with this technique. It could be true that the spherical particles can be multiple oleosin micelles aggregated together. However, a quick observation in Fig. 7a assures us that the spherical particles do not aggregate beyond the size of 1 μm and when they do, their individual shapes can still be obvious.
 | ||
| Fig. 7 CLSM-imaging of (a) defatted SFO cream dispersion and of (b) an O/W emulsion stabilised by it. Samples are stained by Nile Blue showing lipids in green and proteins in red. | ||
When these particles were used as emulsifiers to stabilize an O/W emulsion, a great number of them could be observed on the surface of the emulsion droplets (Fig. 7b). Independently from the nature of these small spheres, they seem to widely absorb at the O/W interface without rupturing. These particles seem to be acting in a similar way as in Fig. 3a, b and 4 and as in those cases, the Pickering mechanism can also be employed here for the explanation of the observed phenomena. These particles do not seem to rupture upon absorption but maintain their spherical shape and act as Pickering stabilisers. Excellent emulsion stabilization has been reported for soy protein/phospholipid nanoparticles although it was suggested that they disintegrate upon absorption.33
An illustration of the possible oleosome configurations after defatting is presented in Fig. 8. Fig. 8a shows an intact oleosome membrane with an empty core, which as observed in Fig. 6b, might be one of the configurations. Another configuration can be seen in Fig. 8b, where phospholipids form a type of micelle and through hydrophobic interactions carry the oleosome hydrophobic proteins. A similar model has been proposed for defatted soybean oleosin/phospholipid mixtures.33
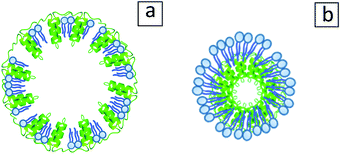 | ||
| Fig. 8 Illustration of possible configurations that may be adopted by the oleosomes after the removal of lipids away from their core. (a) Empty punctured oleosome shell model. (b) Core–shell model with oleosin molecules in the core and phospholipids on the outside layer, inspired by Gao et al.33 | ||
All in all, the obtained spherical particles after the defatting of oleosomes are either composite protein/phospholipid particles or non-disintegrated oleosome membrane shells. Both systems seem to survive the applied conditions and act as Pickering particles showcasing the important role of the oleosome membrane when stabilizing an interface.
Conclusions
The noteworthy qualities of oleosomes have attracted strong interest for their incorporation in colloidal formulations where two immiscible phases are present. Especially, when external oil is added to an aqueous oleosome dispersion, it is not certain what happens to the structure of the oleosomes and how their membrane behaves once they reach the interface. Through this work, it became clear that SFOs are capable of significantly decreasing the interfacial activity of both O/W and W/O interfaces. Additionally, a clear difference in the behaviour of oleosomes depending on their size was observed upon emulsification. SFOs larger than 5 μm were not detected when extrinsic oil was present and after applying a mild emulsification step. These results indicate that oleosomes with big diameter are prone to rupture upon mixing with bulk oil, while the material from their membrane can absorb on the newly formed interface. Smaller oleosomes proved to be more resilient as a great number of them seemed to be able to act as Pickering stabilisers and absorb on the interface of the emulsion droplets. After the removal of the triglycerides from the core, intact but empty oleosome shells or oleosome membrane fractions were formed that could act as interfacial stabilisers. Therefore, it was showcased that the role of the membrane and its elements was of paramount importance. These findings showcase the behaviour of sunflower oleosomes and the oleosome membrane fractions upon emulsification and are of great relevance for future applications where bulk oil or immiscible phases are present.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work took place within the framework of the Institute for Sustainable Process Technology (ISPT).References
- J. Tzen and A. Huang, J. Cell Biol., 1992, 117, 327–335 CrossRef CAS PubMed.
- J. D. Bewley and M. Black, Seeds, Springer, 1994, pp. 1–33 Search PubMed.
- O. A. Karkani, N. Nenadis, C. V. Nikiforidis and V. Kiosseoglou, Food Chem., 2013, 139, 640–648 CrossRef CAS PubMed.
- A. H. Huang, Plant Physiol., 2018, 176, 1894–1918 CrossRef.
- D. Iwanaga, D. A. Gray, I. D. Fisk, E. A. Decker, J. Weiss and D. J. McClements, J. Agric. Food Chem., 2007, 55, 8711–8716 CrossRef CAS PubMed.
- I. D. Fisk, D. A. White, M. Lad and D. A. Gray, Eur. J. Lipid Sci. Technol., 2008, 110, 962–968 CrossRef CAS.
- C. Nikiforidis, V. Kiosseoglou and E. Scholten, Food Res. Int., 2013, 52, 136–141 CrossRef CAS.
- B. Chen, D. J. McClements, D. A. Gray and E. A. Decker, Food Chem., 2012, 132, 1514–1520 CrossRef CAS PubMed.
- C.-J. Chiang, C.-C. Lin, T.-L. Lu and H.-F. Wang, Nanotechnology, 2011, 22, 415102 CrossRef PubMed.
- C. V. Nikiforidis, C. G. Biliaderis and V. Kiosseoglou, Food Chem., 2012, 134, 64–73 CrossRef CAS.
- C. Berton, C. Genot and M.-H. Ropers, J. Colloid Interface Sci., 2011, 354, 739–748 CrossRef CAS PubMed.
- D. Karefyllakis, S. Altunkaya, C. C. Berton-Carabin, A. J. Van Der Goot and C. V. Nikiforidis, Food Hydrocolloids, 2017, 326–334 CrossRef CAS.
- C. V. Nikiforidis and V. Kiosseoglou, J. Agric. Food Chem., 2009, 57, 5591–5596 CrossRef CAS PubMed.
- E. Martínez-Force, N. T. Dunford and J. J. Salas, Sunflower: chemistry, production, processing, and utilization, Elsevier, 2015 Search PubMed.
- J. Tzen, G. Lie and A. Huang, J. Biol. Chem., 1992, 267, 15626–15634 CAS.
- T. Ishii, K. Matsumiya, Y. Nambu, M. Samoto, M. Yanagisawa and Y. Matsumura, Food Struct., 2017, 12, 64–72 CrossRef.
- C. V. Nikiforidis, C. Ampatzidis, S. Lalou, E. Scholten, T. D. Karapantsios and V. Kiosseoglou, Soft Matter, 2013, 9, 1354–1363 RSC.
- M. Deleu, G. Vaca-Medina, J.-F. Fabre, J. Roïz, R. Valentin and Z. Mouloungui, Colloids Surf., B, 2010, 80, 125–132 CrossRef CAS PubMed.
- D. Karefyllakis, H. Octaviana, A. J. van der Goot and C. V. Nikiforidis, Food Hydrocolloids, 2019, 88, 75–85 CrossRef CAS.
- G. Waschatko, B. Schiedt, T. A. Vilgis and A. Junghans, J. Phys. Chem. B, 2012, 116, 10832–10841 CrossRef CAS PubMed.
- S. Bettini, A. Santino, G. Giancane and L. Valli, Colloids Surf., B, 2014, 122, 12–18 CrossRef CAS PubMed.
- S. U. Pickering, J. Chem. Soc., Trans., 1907, 91, 2001–2021 RSC.
- K. B. Vargo, N. Sood, T. D. Moeller, P. A. Heiney and D. A. Hammer, Langmuir, 2014, 30, 11292–11300 CrossRef CAS PubMed.
- C. V. Nikiforidis and V. Kiosseoglou, Food Hydrocolloids, 2011, 25, 1063–1068 CrossRef CAS.
- B. I. Zielbauer, A. J. Jackson, S. Maurer, G. Waschatko, M. Ghebremedhin, S. E. Rogers, R. K. Heenan, L. Porcar and T. A. Vilgis, J. Colloid Interface Sci., 2018, 197–204 CrossRef CAS PubMed.
- G. Waschatko, A. Junghans and T. A. Vilgis, Faraday Discuss., 2012, 158, 157–169 RSC.
- Y. Feng and Y. Lee, Food Hydrocolloids, 2016, 56, 292–302 CrossRef CAS.
- A. Ye, X. Zhu and H. Singh, Langmuir, 2013, 29, 14403–14410 CrossRef CAS PubMed.
- Y. Tan, K. Xu, C. Liu, Y. Li, C. Lu and P. Wang, Carbohydr. Polym., 2012, 88, 1358–1363 CrossRef CAS.
- C. C. Berton-Carabin and K. Schroën, Annu. Rev. Food Sci. Technol., 2015, 6, 263–297 CrossRef CAS PubMed.
- S. Maurer, G. Waschatko, D. Schach, B. I. Zielbauer, J. Dahl, T. Weidner, M. Bonn and T. A. Vilgis, J. Phys. Chem. B, 2013, 117, 13872–13883 CrossRef CAS PubMed.
- É. Roux, S. Baumberger, M. A. Axelos and T. Chardot, J. Agric. Food Chem., 2004, 52, 5245–5249 CrossRef PubMed.
- Z.-M. Gao, J.-M. Wang, N.-N. Wu, Z.-L. Wan, J. Guo, X.-Q. Yang and S.-W. Yin, J. Agric. Food Chem., 2013, 61, 7838–7847 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |