Interface engineering for light-driven water oxidation: unravelling the passivating and catalytic mechanism in BiVO4 overlayers†
Guiji
Liu
ab,
Johanna
Eichhorn
ab,
Chang-Ming
Jiang
 ab,
Mary C.
Scott
ef,
Lucas H.
Hess
ab,
John M.
Gregoire
ab,
Mary C.
Scott
ef,
Lucas H.
Hess
ab,
John M.
Gregoire
 c,
Joel A.
Haber
c,
Joel A.
Haber
 c,
Ian D.
Sharp
c,
Ian D.
Sharp
 abd and
Francesca M.
Toma
abd and
Francesca M.
Toma
 *ab
*ab
aChemical Sciences Division, Lawrence Berkeley National Laboratory, 1 Cyclotron Road, Berkeley, California 94720, USA
bJoint Center for Artificial Photosynthesis, Lawrence Berkeley National Laboratory, 1 Cyclotron Road, Berkeley, California 94720, USA. E-mail: fmtoma@lbl.gov
cJoint Center for Artificial Photosynthesis, California Institute of Technology, Pasadena, CA 91125, USA
dWalter Schottky Institut and Physik Department, Technische Universität München, Am Coulombwall 4, 85748 Garching, Germany
eDepartment of Materials Science and Engineering, University of California Berkeley, Berkeley, USA
fNational Center for Electron Microscopy, Molecular Foundry, Lawrence Berkeley National Laboratory, Berkeley, USA
First published on 12th October 2018
Abstract
Artificial photosynthetic approaches require the combination of light absorbers interfaced with overlayers that enhance charge transport and collection to perform catalytic reactions. Despite numerous efforts that have coupled various catalysts to light absorbing semiconductors, the optimization of semiconductor/catalyst as well as catalyst/electrolyte interfaces and the identification of the role of the catalyst still remain a key challenge. Herein, we assemble (NiFeCoCe)Ox multi-component overlayers, interfaced with bismuth vanadate photoanodes, and determine the roles of different elements on promoting interfacial charge transfer and catalytic reaction over competitive photocarrier recombination loss processes. Through this understanding, and aided by complementary macroscopic photoelectrochemical measurements and nanoscale atomic force microscopy techniques, a bifunctional (CoFeCe/NiFe)Ox overlayer was rationally engineered. The resulting multi-functional coating yields BiVO4 photoanodes with almost 100% efficient surface collection of holes under oxygen evolving reaction conditions. The (CoFeCe)Ox component excels at efficient capture and transport of photogenerated holes in BiVO4 through the availability of redox active states, whereas (NiFe)Ox plays a vital role in reducing charge recombination at the BiVO4/electrolyte interface. In addition, this study supports the hypothesis that catalytic sites act as electronically active trap states on uncoated BiVO4 photoanodes.
1. Introduction
The adverse effect of intensive fossil fuel usage on our environment has motivated the search for clean and renewable energy technologies. However, for many of these technologies, energy storage still remains a limiting factor.1 By converting the large but intermittent solar energy flux into chemical fuels, artificial photosynthesis holds promise to overcome this challenge and provide a sustained energy supply for our societal needs.2,3 Photoelectrochemical (PEC) water splitting and CO2 reduction represent intriguing routes to produce clean fuels using sunlight.4–7 As the electrons and protons needed for solar fuel production hinge on the oxidation of water, an efficient photoanode is a prerequisite for a viable solar fuel technology.Among promising semiconductor photoanode materials, bismuth vanadate (BiVO4) has emerged as one of the most investigated systems in unassisted water-splitting devices, as it captures a substantial portion of the visible spectrum and possesses band energetics that are well-suited for the oxygen evolution reaction (OER).8 However, the bare surface of this material suffers from poor catalytic activity for the OER and possesses high concentrations of electronically active surface states, leading to significant charge recombination losses9,10 and corrosion.11 Numerous studies have demonstrated that BiVO4 needs to be paired with catalysts to achieve suitable photocurrents for the OER.12–19 However, different working mechanisms have been reported for these catalysts, depending on the physicochemical nature of the BiVO4/catalyst interface. For example, Durrant, et al. concluded that the origin of the enhanced photocurrent resulting from a cobalt phosphate (CoPi) catalyst treatment is primarily due to improved charge transfer across the BiVO4/electrolyte interface rather than the catalytic function of CoPi.20 This argument was strengthened by a recent report of van de Krol, et al., which highlights that CoPi passivates the surface states of BiVO4 without significantly influencing the charge transfer kinetics.21 On the other hand, Gamelin and co-workers came to a different conclusion that CoPi promoted charge transfer for the OER, comparing with H2O2 as a hole scavenger.13 In addition, Li, et al., argued that a cobalt borate (CoBi) catalyst accelerates interfacial charge transfer at the BiVO4/electrolyte interface.14 Choi, et al., demonstrated that a FeOOH overlayer creates a favorable interface with BiVO4 as well as acts as an OER catalyst.15
The different interpretations of the improvement mechanisms for the proposed overlayers highlight the variety of demands placed on the catalyst coating, as well as the challenge in discerning the prominent function(s) due to the confluence of these phenomena during photoelectrochemical reactions. Specifically, a good catalyst layer deposited on a light absorber needs to out-compete the surface states in capturing holes in order to be efficient for OER catalysis. The rate of these competing kinetic pathways determines whether the passivation mechanism will dominate over the catalytic one or vice versa. Disentangling the respective passivation and catalytic roles of light absorber overlayers is currently needed to provide the necessary insight for further development of highly efficient and stable integrated photoanodes. In order to address this issue, multi-component overlayers with complimentary hole transport and catalytic functions are practically required. While a few examples of multi-component catalytic layers have been reported to improve the efficiency of BiVO4,16,17 the charge transfer and collection mechanism in these overlayers still remains unclear.
Here, via an individual component analysis, we rationally design (NiFeCoCe)Ox multi-component coatings on BiVO4 photoanodes to investigate how the specific properties of the light absorber/catalyst interface define the interfacial charge transfer and collection mechanism, as well as to simultaneously optimize the passivating and catalytic functions. Based on our high-throughput direct discovery of BiVO4/multi-component functional interfaces,18,19 we deposit (NiFeCoCe)Ox multi-component overlayers by photoelectrochemical deposition (PED). PED is a straightforward method that is well suited for producing diverse compositions of coatings and creating interfacial structures with BiVO4.22 In addition, PED inherently places catalysts at specific locations on the BiVO4/electrolyte interface where the photogenerated holes are most readily available without coating the underlying FTO.15 However, to the best of our knowledge, attempts to deposit controlled multi-element compositions by this versatile technique are rare.22
To clarify the role of the different components and enable the rational design of a targeted multi-component overlayer, we studied the individual (Ni, Fe, Co, Ce) oxides in multi-component (NiFe, CoFeCe, and CoFeCe/NiFe) oxides. Thus, we demonstrate that an optimized light absorber/overlayer interface necessitates a dual hole transport/catalyst coating. We combined a suite of photoelectrochemical (PEC) and microscopy techniques to characterize the nanoscale distribution and functionality of the catalyst on the photoanode surface. Thus, we correlate this information with macroscopic PEC measurements to provide a deeper understanding of charge transfer at the light absorber/overlayer interface. Analysis of the role of the individual overlayer components shows that while CeOx is excellent at passivating active surface states, it reduces the BiVO4 catalytic activity under OER conditions. Specifically, the CeOx overlayer produces discontinuous films that together with the photoelectrochemical findings support that surface trap states in BiVO4 may be responsible for OER catalysis. In addition, using PEC characterization we identify (NiFe)Ox as the most active surface catalyst, and (CoFeCe)Ox as an interfacial charge transfer and collection layer in (NiFeCoCe)Ox multi-component coatings. Furthermore, we investigate the interface energetics and dynamics of multi-component BiVO4/(CoFeCe)Ox using open circuit potential (OCP) and transient photocurrent (TPC) measurements. These measurements demonstrate that (CoFeCe)Ox is able to extract and collect holes from BiVO4 by introducing surface redox states for the OER. Based on the understanding of (NiFeCoCe)Ox, we optimize the BiVO4/overlayer interface by sequentially integrating (CoFeCe)Ox as a hole transport and collection layer and (NiFe)Ox as a surface catalyst with BiVO4. The resulting photoanode achieved near-complete suppression of interface losses between 0.8 and 1.23 V vs. reverse hydrogen electrode (RHE) for solar water oxidation. In addition, we utilize atomic force microscopy (AFM) to discern and map the overlayer morphology on top of the BiVO4 photoanode and provide subsequent insights with photoconductive AFM (pc-AFM) measurements to reveal that the holes collected by (CoFeCe)Ox can be efficiently used by (NiFe)Ox for water oxidation. These findings shed light on interface engineering and optimization with BiVO4/catalyst assemblies, thereby offering guidelines to accelerate the discovery of functional components for PEC water splitting devices.
2. Results and discussion
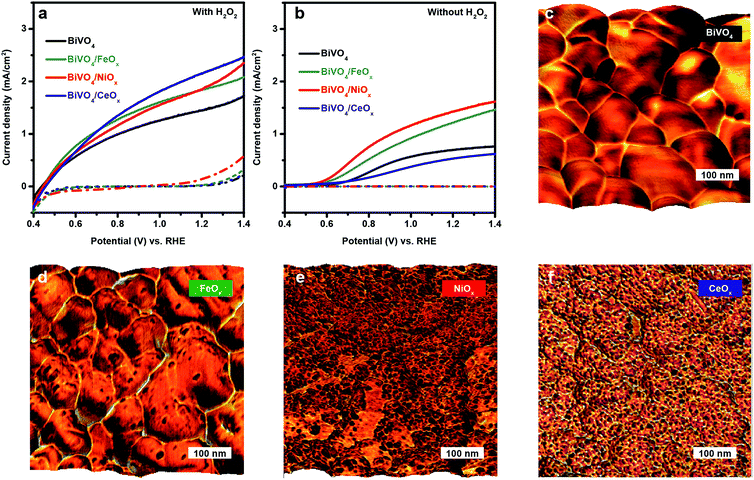
In the present work, spin-coated, undoped BiVO4 thin films on fluorine doped tin oxide (FTO)/glass substrates were prepared as previously reported.11 As a starting point for understanding the properties of BiVO4/(NiFeCoCe)Ox multi-component overlayers, we investigate the specific role of Ni, Fe, Co and Ce by individually depositing each overlayer component (NiOx, FeOx, CoOx and CeOx) on BiVO4 films with PED under constant current density. J–E measurements in 0.1 M NaOH solution with and without H2O2 as a hole scavenger were conducted (Fig. 1a and b). Measurements with H2O2 as a hole scavenger are performed to minimize recombination losses associated with slow catalytic processes and assess the quality of the semiconductor/overlayer assembly for capturing light and separating/transporting charge. In the absence of H2O2, the measured photocurrent is lower due to the significant competition between the sluggish kinetics of the OER and surface recombination.The J–E curves in the presence of H2O2 (Fig. 1a) show onset potentials at 0.4 V vs. RHE for BiVO4/NiOx, BiVO4/FeOx, BiVO4/CeOx and pristine BiVO4 photoanodes. FeOx shows slightly more favourable onset characteristics at 0.4–0.8 V vs. RHE. As the applied potential increases, the corresponding photocurrent densities of all the samples increase rapidly, as expected since charge extraction is a function of the applied potential. The pristine BiVO4 photoanode exhibits a photocurrent density of 1.5 mA cm−2 at 1.23 V vs. RHE, whereas BiVO4/NiOx, BiVO4/FeOx, and BiVO4/CeOx show about 1.9, 1.9, and 2.2 mA cm−2, respectively, at the same potential. Unexpectedly, while CeOx is not catalytically active for the OER,23 BiVO4/CeOx exhibits the highest photocurrent density in the presence of H2O2 at 0.8–1.4 V vs. RHE. The enhanced performance of BiVO4 on different overlayers may be attributed to improved light absorption or charge separation/transport. According to UV-Vis measurements, the addition of overlayers with PED barely alters light absorption of BiVO4 (Fig. S1†). Moreover, all the samples exhibit similar onset potentials (∼0.4 V vs. RHE) as shown in Fig. 1a, thus implying similar interfacial energetics for driving charge separation.
Therefore, the enhanced performances of BiVO4/NiOx, BiVO4/FeOx, and BiVO4/CeOx with H2O2 may be ascribed to facilitated charge transport by passivating intrinsic surface states of BiVO4. Specifically, CeOx is the most effective at passivating surface states at the high potential region, and FeOx is excellent at passivating surface states at the onset region. These observations agree well with previous reports on the excellent interface properties of BiVO4/FeOx (ref. 16) and BiVO4/CeOx.24
By comparing the photocurrents for water and H2O2 oxidation, we can examine the surface hole injection efficiencies in BiVO4/catalyst assemblies as Phole injection = JH2O/JH2O2. The corresponding J–E curves of NiOx, FeOx, and CeOx loaded BiVO4 photoanodes without H2O2 are shown in Fig. 1b. BiVO4/NiOx and BiVO4/FeOx exhibit an enhanced photocurrent density at 1.23 V vs. RHE (1.3 and 1.5 mA cm−2) with respect to bare BiVO4 (0.7 mA cm−2). However, relative to the pristine BiVO4, the BiVO4/CeOx assembly shows a significantly reduced photocurrent density for water oxidation over the entire operating potential range. This result contrasts sharply with the superior performance of BiVO4/CeOx in the presence of H2O2 (Fig. 1a), thus confirming that the catalytic activity of CeOx for the OER is very poor. Thus, a large portion of the holes that reach the surface tend to undergo recombination instead of interfacial charge transfer, despite the favourable properties of CeOx for passivating electronically active surface defects.
In order to estimate the probability that the holes reaching the BiVO4 surface can effectively contribute to the OER, the hole injection efficiency at 1.23 V vs. RHE was calculated for these BiVO4/catalyst assemblies (Table S1†). For pristine BiVO4, the hole injection efficiency is only 48% at 1.23 V vs. RHE. This finding reveals significant kinetic competition between surface recombination and water oxidation, which is in line with previous reports.14,15 Once coupled with NiOx or FeOx, this value goes up to 79% or 67%, respectively. This result is in good agreement with the enhanced photocurrent with respect to pristine BiVO4 in Fig. 1b, thereby implying that both NiOx and FeOx retain BiVO4 catalytic activity and introduce catalytic sites for the OER onto BiVO4 surfaces. However, CeOx results in a hole injection efficiency of 27% at 1.23 V vs. RHE. Thus, CeOx appears to dramatically reduce the overall catalytic activity of the photoelectrode, presumably due to coating the moderately active semiconductor surface with a more chemically inert material, CeOx.
In order to better understand the role of each individual component, we analysed the morphology and nanomechanical adhesion properties of the overlayer with atomic force microscopy (AFM). This technique allows for direct visualization of the distribution of different oxide overlayer nanoparticles on the BiVO4 semiconductor. Specifically, we use the topography images overlayed by the corresponding adhesion maps to provide nanoscale information on the surface structure of the BiVO4/overlayer assembly. Fig. 1c–f show the overlay images for bare BiVO4, BiVO4/FeOx, BiVO4/NiOx, and BiVO4/CeOx, respectively. The pristine BiVO4 film exhibits individual BiVO4 grains ranging between 50 and 200 nm in size with an overall smooth surface. After depositing FeOx, NiOx and CeOx, it is possible to identify 5–10 nm nanoparticles dispersed on the surfaces of the BiVO4 grains with different degrees of coverage. Specifically, it is interesting to notice that CeOx particles are homogenously yet discontinuously dispersed on top of the BiVO4 light absorber (Fig. 1f). This morphology together with the PEC measurements support that while CeOx passivates electronically active trap states in BiVO4, it also reduces the catalytically active states. Consequently, this finding leads us to conclude that the surface trap states may be the catalytic states of bare BiVO4, thus explaining why the BiVO4/CeOx shows reduced catalytic activity towards the OER.
In contrast to NiOx, FeOx, and CeOx, CoOx did not show a significant photoresponse under either front or back-side illumination but greatly enhanced the dark current for the OER (Fig. S2a†). It should also be noted that CoOx was only deposited for 5 min, as the potential required for deposition increased sharply (Fig. S2b†), whereas the standard deposition time for all other samples was 60 min. This modified deposition approach avoided massive growth of CoOx on BiVO4, and prevented CoOx from affecting light absorption of BiVO4 (Fig. S2c†). CoOx deposition eliminates the built-in potential and, thus, the driving force for photocarrier separation. A similar phenomenon was observed for the case of IrOx on WO3, which yields an ohmic solid/solid contact.25 This conclusion explains the observation that the BiVO4/CoOx anode behaves like a conducting electrocatalyst for the OER. Thus, the BiVO4/CoOx junction energetics are poorly suited for creating efficient photoelectrodes.
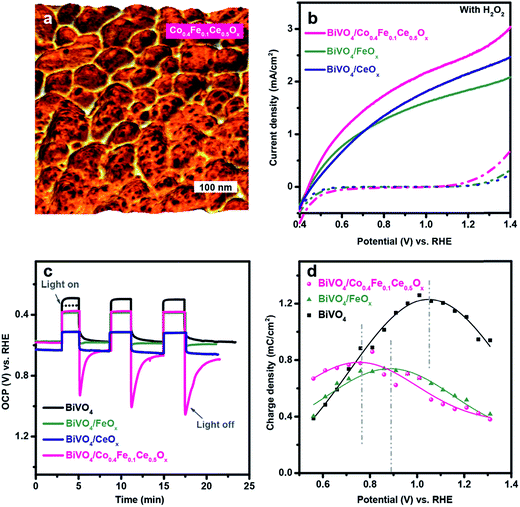
Based on the above PEC characterization and the charge injection efficiency values of the individual overlayer components, we summarized the individual roles of the metal oxide overlayers in Fig. S3.† It is clear that NiOx is the main catalytic component in the (NiFeCoCe)Ox multi-component coating. On the other hand, CeOx and CoOx modify the interfacial properties of BiVO4, by passivating electronically active trap states and decreasing the built in potential, respectively. In addition, FeOx has a moderate impact on both catalytic and passivating functions. Based on these insights, we rationally designed, synthesized, and tested a multi-layer coating with the aim of optimizing both the semiconductor/overlayer interface and the catalytic activity. To this end, we modified NiOx with FeOx to form a Ni0.8Fe0.2Ox catalytic layer at the outer surface by PED using mixed 0.08 M nickel(II) nitrate hexahydrate, and 0.02 M iron nitrate(III) nonahydrate as the plating solution, which provides a superior catalytic activity relative to the pure NiOx or FeOx (Fig. S4†). Given the beneficial interface of BiVO4/FeOx and BiVO4/CeOx, we attempted to combine FeOx and CeOx into an interface layer for efficiently collecting holes over the entire operating potential range. However, the combination of the Fe and Ce species results in poor coating of BiVO4 with PED, which greatly limits the performance of BiVO4/(FeCe)Ox (Fig S5a and c†). The different morphologies of CeOx and (FeCe)Ox indicate that the interaction between the Fe and Ce species leads to a different growth process of the ternary oxide, as supported by the enlarged potential for depositing (FeCe)Ox (Fig. S5b†). Interestingly, we found that incorporating 80% of Co into the (FeCe) precursor solution not only leads to the uniform coating of (CoFeCe)Ox on BiVO4 with PED but also offers additional redox active sites that are available for hole collection and transport, as demonstrated below. The X-ray photoelectron spectroscopy (XPS) measurements revealed that the deposited (CoFeCe)Ox consists of Co(II/III), Fe(III) and Ce(III/IV) in the form of oxyhydroxides (Fig. S6†). Since XPS is a surface sensitive technique, inductively coupled plasma mass spectrometry (ICP-MS) was used for complementary analysis of the overall composition of (CoFeCe)Ox. The ICP-MS results reveal that (CoFeCe)Ox is composed of Co: 40%, Fe: 10% and Ce: 50% (Co0.4Fe0.1Ce0.5Ox), thus indicating a preferential deposition of CeOx on BiVO4 during PED. In addition, energy-dispersive X-ray spectroscopy (EDX) maps, obtained on the scanning transmission electron microscope (STEM), highlight the presence of the overlayer atop the BiVO4 (Fig. S7†), which shows uniform coating on BiVO4 (Fig. 2a). As a result, BiVO4/Co0.4Fe0.1Ce0.5Ox shows superior onset characteristics as well as an optimal photocurrent density of 2.4 mA cm−2 at 1.23 V vs. RHE with H2O2, which is higher than BiVO4/CeOx (2.2 mA cm−2), BiVO4/FeOx (1.9 mA cm−2) or BiVO4/(FeCe)Ox(1.2 mA cm−2) (Fig. S5a†) demonstrating that the BiVO4/Co0.4Fe0.1Ce0.5Ox interface is excellent for transporting holes.
To investigate the interface energetics of the Co0.4Fe0.1Ce0.5Ox layer, we monitored the open circuit potential (OCP) profiles and the charge carrier dynamics using transient photocurrent (TCP) measurements (Fig. 2c and d). For a photoelectrode, the changes of OCP reflect charge migration driven by the internal energetics at the electrode/electrolyte interface.17 As shown in Fig. 2c, the OCP of the pristine BiVO4 photoanode in darkness is at 0.6 V vs. RHE. Under illumination, the OCP of BiVO4 immediately reached an initial value (black dashed line), which further increased over time. This effect indicates that holes are accumulating on the surface under illumination, which can be ascribed to the saturation of the surface states by photo-induced processes, analogous to the recently reported photocharging effect on BiVO4.26 Upon addition of CeOx, the OCP of BiVO4 shifts positively, which indicates the physical passivation effect of CeOx on partially removing surface states of BiVO4, in line with previous findings.24 In contrast, the addition of FeOx, or Co0.4Fe0.1Ce0.5Ox marginally alters the resting potential of BiVO4 under dark conditions, suggesting that these overlayers provide marginal physical passivation of intrinsic surface states on BiVO4. However, the photocharging processes of BiVO4 disappear upon overlayer loading, which indicates those overlayers may offer alternative paths for hole transfer other than through the intrinsic surface states, therefore functionally passivating surface states of BiVO4. This finding can be further supported by the different transient OCPs upon switching off the light. When the light is switched off, the OCP of BiVO4 slowly decays to the same position as in the dark. This slow process reveals that the photogenerated holes originally accumulated on the surface of BiVO4 under illumination and charge recombination with the majority of electrons is required to re-establish equilibrium when illumination is stopped. In contrast, after depositing CeOx on the top, the OCP of BiVO4/CeOx goes back to the same position as in the dark without an apparent decay process within the time scales detectable with our measurements. The absence of a decay process further supports the passivation effect of CeOx, as mentioned earlier. When the light is turned off, we observe a different behaviour with FeOx and Co0.4Fe0.1Ce0.5Ox. Specifically, the OCP of BiVO4/FeOx immediately jumps to a new value, which is 10 mV higher than the initial dark equilibrium value and shows an “opposite” charge recombination decay, compared to the pristine BiVO4. This phenomenon is even more pronounced with Co0.4Fe0.1Ce0.5Ox. The OCP of BiVO4/Co0.4Fe0.1Ce0.5Ox jumps to a position which is 300 mV higher than the initial dark equilibrium value and the “opposite” charge recombination decay for BiVO4/Co0.4Fe0.1Ce0.5Ox is much more significant than that for BiVO4/FeOx. The presence of an offset with respect to the initial dark equilibrium suggests that there might be a change in the oxidation state of the FeOx and Co0.4Fe0.1Ce0.5Ox layers, compatible with the presence of photogenerated holes of BiVO4. In other words, this finding proves that FeOx and Co0.4Fe0.1Ce0.5Ox can extract and collect photogenerated holes from BiVO4 under illumination, building up positively charged species, thereby establishing a new interfacial energetic alignment. The behavior of the Co0.4Fe0.1Ce0.5Ox overlayer implies that the hole extraction ability mainly results from the Co species. Indeed, in the absence of Co, the OCP profile of (FeCe)Ox shows no apparent charge recombination decay upon switching off the light (Fig. S8†), which further demonstrates the important role of Co redox active species in extracting holes from BiVO4. Notably, BiVO4/Co0.4Fe0.1Ce0.5Ox is exposed to light for only 3 min and requires about 60 min to recover the original resting level of OCP in the dark (Fig. S9†). These results indicate that hole extraction and collection from BiVO4 into Co0.4Fe0.1Ce0.5Ox is energetically favourable.
To gain more insight into the charge carrier dynamics between (CoFeCe)Ox and BiVO4 under operating conditions, TPC measurements were employed using a pulsed 340 nm LED and a continuous white light source. The photocurrent transients upon light perturbation are indicative of capacitive charge accumulation, which offers the possibility for electrons to recombine. The number of charges accumulated at the electrode/electrolyte interface can be qualitatively determined by integrating the photocurrent transients during the monitored surface recombination process. The potential peaks of the accumulation maximum most probably represent the energy levels of the surface redox states, which shed light on the interfacial charge capture/collection on BiVO4 surfaces.27–29 To depict this clearly, a plot of the accumulated charge versus potential is shown in Fig. 2d, which is obtained by integrating the photocurrent transients measured in Fig. S10.† Pristine BiVO4 shows the most pronounced charge accumulation at the electrode/electrolyte interface. When covering the BiVO4 photoanode surface with overlayers, charge accumulation is attenuated and occurs at more cathodic potentials. The cathodic shift provides strong evidence that the overlayers out-compete the intrinsic surface states for capturing holes, thus providing alternative paths for hole transfer. In particular, the addition of FeOx cathodically shifts the maximum charge accumulation, indicating that the Fe redox states have more driving force to capture and accumulate holes over the intrinsic surface states on BiVO4. In contrast, almost no charge accumulation can be calculated from the TPC results of BiVO4/CeOx (Fig. S10c†), once again, confirming the passivation effect that prevents hole accumulation via surface states. Interestingly, adding Ce into FeOx leads to a great reduction in the magnitude of charge accumulation, further indicating that Ce contributes to removing surface states (Fig. S11†). These measurements, together with the OCP results, revealed the role of CeOx in passivating surface states of BiVO4 and creating a favourable interface for hole transport, consistent with our previous study on the BiVO4/CeOx interface.24 Moreover, a further shift in the energy levels of redox states to 0.7 V vs. RHE is observed due to the presence of Co0.4Fe0.1Ce0.5Ox. This finding suggests that Co offers additional redox states for accepting surface holes. Interestingly, CV measurements of BiVO4/Co0.4Fe0.1Ce0.5Ox in the dark also show redox features at 0.7 V vs. RHE, produced by Co and Fe species (Fig. S12†). Given the findings above, it is very likely that Co0.4Fe0.1Ce0.5Ox acts as an interface component that extracts/captures photogenerated holes from BiVO4, out-competing intrinsic surface states by providing surface redox states (Co and Fe species), which lie at more favorable energy levels for hole capture from BiVO4, while CeOx mainly provides excellent interface quality with BiVO4 for hole transport by passivating the intrinsic surface states of the semiconductor.
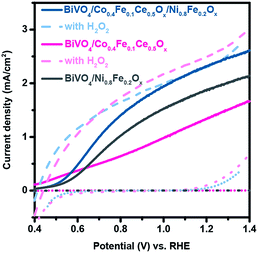
Although the Co0.4Fe0.1Ce0.5Ox coatings excel at efficient capture and collection of photogenerated holes in BiVO4, the significant differences of the BiVO4/Co0.4Fe0.1Ce0.5Ox photoanode for H2O2 and H2O oxidation suggests that the holes collected by Co0.4Fe0.1Ce0.5Ox cannot be efficiently used for the OER, leading to significant surface recombination losses, as shown in Fig. 3. Accordingly, we further assembled a Ni0.8Fe0.2Ox catalytic layer on top of the BiVO4/Co0.4Fe0.1Ce0.5Ox photoanode, as presented in Fig. S13.† As a result, BiVO4/Co0.4Fe0.1Ce0.5Ox/Ni0.8Fe0.2Ox photoanode shows superior performance to BiVO4/Co0.4Fe0.1Ce0.5Ox and BiVO4/Ni0.8Fe0.2Ox photoanode for OER. In addition, the J–E curve of the BiVO4/Co0.4Fe0.1Ce0.5Ox/Ni0.8Fe0.2Ox photoanode for the OER approaches that one for H2O2 oxidation. Strikingly, 85–100% of the surface collected holes are used by Ni0.8Fe0.2Ox for the OER at 0.8–1.23 V vs. RHE, as determined by comparing the J–E curves of BiVO4/Co0.4Fe0.1Ce0.5Ox/Ni0.8Fe0.2Ox with (dashed line) and without H2O2 hole scavenger (solid line). This finding demonstrates the almost complete elimination of interfacial charge recombination losses with interface optimization. Moreover, the PEC performances of the BiVO4/Co0.4Fe0.1Ce0.5Ox/Ni0.8Fe0.2Ox and BiVO4/Co0.4Fe0.1Ce0.5Ox photoanodes show similar results with H2O2. These results demonstrate that the addition of Ni0.8Fe0.2Ox greatly improves the interfacial hole transfer of the BiVO4/Co0.4Fe0.1Ce0.5Ox photoanode for the OER without altering its charge separation/transport abilities, whereas Co0.4Fe0.1Ce0.5Ox remedies losses at the BiVO4/Ni0.8Fe0.2Ox interface. To demonstrate the generality of our findings, we further performed the PEC characterization of BiVO4, BiVO4/Co0.4Fe0.1Ce0.5Ox and BiVO4/Co0.4Fe0.1Ce0.5Ox/Ni0.8Fe0.2Ox at pH 7 (0.1 M KPi), and at pH 9 (0.1 M NaBi).30 As a result, the addition of Co0.4Fe0.1Ce0.5Ox and Co0.4Fe0.1Ce0.5Ox/Ni0.8Fe0.2Ox overlayers exhibit similar effect on BiVO4 as in the pH 13 electrolyte (Fig. S14†)
In order to provide further insights into the charge-transport mechanism through the BiVO4/Co0.4Fe0.1Ce0.5Ox/Ni0.8Fe0.2Ox interfaces, we investigated the current distributions in BiVO4/Ni0.8Fe0.2Ox, BiVO4/Co0.4Fe0.1Ce0.5Ox, and BiVO4/Co0.4Fe0.1Ce0.5Ox/Ni0.8Fe0.2Ox by pc-AFM in the dark and under illumination. As shown in Fig. S15† and 4, pc-AFM measurements in the dark at an applied sample bias of 1.75 V reveal bright spots in the current map. We previously observed similar bright spots on pristine BiVO4 and assigned these to shunts between the metal-coated probe and the underling FTO substrate.31 The photoelectrodeposited catalysts are sparsely distributed on the BiVO4 surface, and preferentially deposited on top of the grains, not on the grain boundaries or pinholes, as demonstrated with the overlay maps of BiVO4/Co0.4Fe0.1Ce0.5Ox in Fig. 2a and BiVO4/Ni0.8Fe0.2Ox, BiVO4/Co0.4Fe0.1Ce0.5Ox/Ni0.8Fe0.2Ox in Fig. S16.† Therefore, only a minor role is assigned to a shunt pathway compared to charge transport across the FTO/BiVO4/overlayer.32
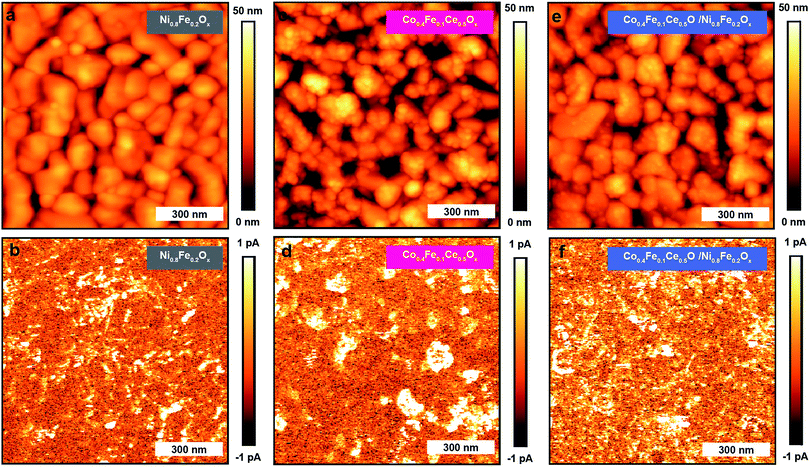 | ||
| Fig. 4 The pc-AFM measurements. Topographic and current maps collected for BiVO4/Ni0.8Fe0.2Ox (a and b), BiVO4/Co0.4Fe0.1Ce0.5Ox (c and d), and BiVO4/Co0.4Fe0.1Ce0.5Ox/Ni0.8Fe0.2Ox (e and f). | ||
Furthermore, pc-AFM measurements were also conducted using above band gap illumination (Eph = 3.06 eV, 100 mW cm−2). Maps of the current under illumination (Fig. 4b, d and f), for the BiVO4/Ni0.8Fe0.2Ox and the BiVO4/Co0.4Fe0.1Ce0.5Ox anodes exhibit spots of increased conductivity, whereas an almost homogenous current distribution is observed for the BiVO4/Co0.4Fe0.1Ce0.5Ox/Ni0.8Fe0.2Ox anode. In all three samples, under illumination the current mainly increases on top of the grain and not at the grain boundaries. The corresponding median photocurrents are 0.08 pA for BiVO4/Ni0.8Fe0.2Ox, 0.28 pA for BiVO4/Co0.4Fe0.1Ce0.5Ox, and 0.38 pA for BiVO4/Co0.4Fe0.1Ce0.5Ox/Ni0.8Fe0.2Ox. These findings are in agreement with the PEC measurements presented above, which showed improved performance for BiVO4/Co0.4Fe0.1Ce0.5Ox/Ni0.8Fe0.2Ox. The higher photocurrent value of BiVO4/Co0.4Fe0.1Ce0.5Ox compared to BiVO4/Ni0.8Fe0.2Ox might indicate that hole extraction/collection from BiVO4 is more feasible via the Co0.4Fe0.1Ce0.5Ox overlayer. Accordingly, the photo-conductivity of BiVO4/Ni0.8Fe0.2Ox is greatly enhanced once Co0.4Fe0.1Ce0.5Ox is inserted between Ni0.8Fe0.2Ox and BiVO4. These results not only confirm the role of Co0.4Fe0.1Ce0.5Ox as a hole mediator/collector, but also verify that the holes collected by Co0.4Fe0.1Ce0.5Ox can be efficiently used by Ni0.8Fe0.2Ox to drive the OER.
3. Conclusions
In summary, we have investigated the critical interfacial roles of individual component metal oxides in the (NiFeCoCe)Ox multi-component overlayers by interfacing each of them with BiVO4. We have identified (NiFe)Ox as the surface catalytic component and (CoFeCe)Ox as the charge capture/collection component via available redox active states, by using PEC characterization with and without a H2O2 hole scavenger. The charge capture/collection mechanism of (CoFeCe)Ox from BiVO4 was also studied in detail with OCP and TPC measurements. Accordingly, we further optimized BiVO4/(NiFeCoCe)Ox by serially interfacing the (CoFeCe)Ox hole collector and (NiFe)Ox catalyst with BiVO4. In addition, our findings corroborate that BiVO4 surface trap states may be catalytically active for OER catalysis. The resulting integrated photoanode almost eliminates interfacial losses across the BiVO4/electrolyte interface over the range of 0.8–1.23 V vs. RHE for the OER. The effective charge transfer through the (CoFeCe)Ox hole collector and (NiFe)Ox catalyst interfaces was further verified by pc-AFM measurements. This study highlights the roles of (NiFeCoCe)Ox multi-component overlayers in preferentially capturing holes over the surface states of BiVO4 as well as catalyzing the OER. This work demonstrates the significance of interface optimization in the multi-component overlayer-integrated BiVO4 photoanodes, establishing foundational knowledge to design the next generation of integrated photo-active materials for solar fuel production.4. Experimental
4.1 Fabrication of BiVO4 films
Spin-coated bismuth vanadate (BiVO4) thin films were deposited on the fluorine-doped tin oxide (FTO)-coated side of a 10 cm × 10 cm glass plate (TEC-15 Sigma Aldrich), following a procedure from our previous report.11 The FTO/glass substrates were thoroughly washed with isopropanol (Sigma Aldrich, ≥98%), detergent (Alconex) in deionized water, and pure deionized water, dried with a nitrogen gun and treated with an ozone cleaner (Jelight Model 42) for 10 min.For spin coating, typically, 15 mL of a 0.2 M solution of bismuth(III) nitrate pentahydrate (Sigma Aldrich, ≥98%) in acetylacetone (Sigma Aldrich, ≥99%) and 100 mL of a 0.03 M solution of vanadium(IV)-oxyacetylacetonate in acetylacetone were prepared separately and sonicated for 10 min. Then, the two solutions were mixed together and further sonicated for 5 min. The resulting solution was filtered with 0.45 μm nylon filters (Thermo Fisher Scientific), and dispensed onto the 10 cm × 10 cm FTO/glass plate. The substrate was then spun two times at 1000 rpm for 6 s on a spin coater (Laurell Technologies) with an acceleration rate of 150 rpm s−1 and annealed in air for 10 min at 500 °C in a muffle furnace (Cole-Parmer). This spin coating-annealing procedure was repeated nine times. After the last spin coating cycle, the substrate was annealed for 2 h at 500 °C to yield a final thickness of ∼50 nm.
4.2 Photoelectrochemical deposition (PED) of catalysts
Catalysts were produced via PED on the BiVO4 electrodes. A 0.1 M solution of various metal nitrates (nickel(II) nitrate hexahydrate, Sigma Aldrich, ≥97%; iron nitrate(III) nonahydrate Sigma Aldrich, ≥98%; cobalt(II) nitrate hexahydrate, Strem chemicals, ≥99%; and cerium nitrate(III) hexahydrate, Sigma Aldrich, ≥99%) was used as an electrolyte. PED was conducted in an undivided cell at a constant current density of 50 μA cm−2 over a period of 60 min under AM 1.5G-simulated sunlight. The BiVO4 electrode was used as the working electrode, Ag/AgCl as the reference electrode and a Pt wire as the counter electrode. The light irradiation came from the back side of electrodes. The 0.1 M metal nitrate precursor solution of (CoFeCe)Ox was prepared by mixing 0.08 M cobalt(II) nitrate hexahydrate, 0.01 M iron nitrate(III) nonahydrate and 0.01 M cerium nitrate(III) hexahydrate. Likewise, the 0.1 M metal nitrate precursor solution of (NiFe)Ox was prepared by mixing 0.08 M nickel(II) nitrate hexahydrate, and 0.02 M iron nitrate(III) nonahydrate. The reported ratios in the chemical formulae were provided by ICP-MS results. The BiVO4/Co0.4Fe0.1Ce0.5Ox/Ni0.8Fe0.2Ox sample was assembled by depositing Co0.4Fe0.1Ce0.5Ox for 15 min and then Ni0.8Fe0.2Ox for the rest 45 min.4.3 UV-Vis transmission and reflectance measurements
Transmission and spectral reflectance measurements were performed on a Shimadzu SolidSpec-3700 UV/Vis/NIR spectrometer using an integrating sphere.4.4 Photoelectrochemical (PEC) and open circuit potential (OCP) measurements
The PEC test was conducted in a single-compartment, three-electrode electrochemical cell with a potentiostat (Bio-Logic, SP-300) under simulated AM 1.5G solar light irradiation (100 mW cm−2, 16S-300-002, Solar Light). The fabricated electrode, a platinum wire, and Ag/AgCl electrode were used as the working, counter, and reference electrodes, respectively. Unless expressly mentioned, 0.1 M NaOH aqueous solution (pH = 13) was used as an electrolyte after saturation with N2 gas for 20 min. The measured potentials were converted to V vs. RHE (ERHE = EAg/AgCl + 0.193 V + 0.059 V × pH). The photocurrent was measured by linear sweep voltammetery with a scan rate of 10 mV s−1. The light irradiation came from the front side of the electrodes for all cases. OCP measurements were performed under open circuit condition in the same electrolyte. The OCP reading in the dark or under illumination was obtained after a stabilization process in the dark (10 min).4.5 Transient photocurrent (TPC) measurements
TPC measurements were conducted in a similar setup to PEC measurements. The main difference is the working electrode was exposed to a continuous ∼100 mW cm−2 light bias from a home-built white light-emitting diode (LED) array. A 340 nm LED (M340L4, ThorLabs) with ∼10 nm spectral full width at half maximum (FWHM) and ∼10 mW cm−2 power density was used as the modulating light source, alternating between 150 ms on-time and 203 ms off-time. The applied potential was increased from 0.56 to 1.36 V vs. RHE at 0.05 V intervals. After passing through a current pre-amplifier (SR570, Stanford Research Systems), the photocurrent transients induced by the 340 nm LED were collected at a frequency of 105 Hz and averaged over 100 light pulses.4.6 X-ray photoelectron spectroscopy (XPS) and inductively coupled plasma mass spectrometry (ICP-MS)
The near-surface chemical composition was analyzed by XPS using a monochromatized Al Kα source (hν = 1486.6 eV) on a Kratos Axis Ultra DLD system with a pass energy of 20 eV. Spectral fitting was conducted using the Casa XPS analysis software. Spectral energy positions were corrected by shifting the C 1s core level position to 284.8 eV and curves were fit with quasi-Voigt lines following Shirley background subtraction. ICP-MS was performed using an Agilent 7900 system run with the He mode. The internal standard was Ge for Ni, Fe, Co and Tb for Ce, selected based on their first ionization potentials and M/Z. The samples were digested with 1 mL of 70% HNO3 (>99.999% trace metals basis, 225711, Aldrich) and diluted to 5 mL in total with 1% HNO3 solution as the original sample solutions.4.7 Transmission electron microscopy (TEM) measurements
TEM measurements were conducted on a FEI TitanX 60-300 microscope at NCEM using a double tilt holder and an accelerating voltage of 200 kV. The images were acquired with a HAADF detector for STEM. EDS was collected using a Bruker windowless EDS detector.4.8 Atomic force microscopy (AFM) measurements
All the AFM measurements were performed with a commercial AFM system (Bruker Dimension Icon). PeakForce Quantitative Nanoscale Mechanical (PF-QNM) mode was used for recording the topography and adhesion maps simultaneously. For all the PF-QNM measurements, uncoated Si-probes (RFESP-75) with spring constants of 3.0 N m−1 were used.For pc-AFM mapping, PeakForce TUNA mode was used, whereby a positive bias of 1.75 V was applied to the FTO back contact. For all the pc-AFM measurements, the sample was illuminated from the FTO-side using a specially designed illumination setup.31 As the light source, a CW laser diode with a wavelength of 405 nm and an illumination intensity of about 100 mW cm−1 was used. The pc-AFM measurements were performed with conductive PtIr-coated probes (Bruker SCM-PIT) exhibiting a nominal spring constant of 2.8 N m−1.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study is based on work performed at the Joint Center for Artificial Photosynthesis, a DOE Energy Innovation Hub, supported through the Office of Science of the U.S. Department of Energy under Award Number DE-SC0004993. Work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231.Notes and references
- J. Su and L. Vayssieres, ACS Energy Lett., 2016, 1, 121–135 CrossRef CAS.
- N. S. Lewis, Science, 2007, 315, 798–801 CrossRef CAS PubMed.
- S. Dahl and I. Chorkendorff, Nat. Mater., 2012, 11, 100–101 CrossRef CAS PubMed.
- M. Gratzel, Nature, 2001, 414, 338–344 CrossRef CAS PubMed.
- M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori and N. S. Lewis, Chem. Rev., 2010, 110, 6446–6473 CrossRef CAS PubMed.
- X. Chen, C. Li, M. Gratzel, R. Kostecki and S. S. Mao, Chem. Soc. Rev., 2012, 41, 7909–7937 RSC.
- B. A. Pinaud, J. D. Benck, L. C. Seitz, A. J. Forman, Z. Chen, T. G. Deutsch, B. D. James, K. N. Baum, G. N. Baum, S. Ardo, H. Wang, E. Miller and T. F. Jaramillo, Energy Environ. Sci., 2013, 6, 1983–2002 RSC.
- I. D. Sharp, J. K. Cooper, F. M. Toma and R. Buonsanti, ACS Energy Lett., 2017, 2, 139–150 CrossRef CAS.
- F. F. Abdi, T. J. Savenije, M. M. May, B. Dam and R. van de Krol, J. Phys. Chem. Lett., 2013, 4, 2752–2757 CrossRef CAS.
- B. Pattengale and J. Huang, Phys. Chem. Chem. Phys., 2017, 19, 6831–6837 RSC.
- F. M. Toma, J. K. Cooper, V. Kunzelmann, M. T. McDowell, J. Yu, D. M. Larson, N. J. Borys, C. Abelyan, J. W. Beeman and K. M. Yu, Nat. Commun., 2016, 7, 12012 CrossRef PubMed.
- H. Ye, H. S. Park and A. J. Bard, J. Phys. Chem. C, 2011, 115, 12464–12470 CrossRef CAS.
- D. K. Zhong, S. Choi and D. R. Gamelin, J. Am. Chem. Soc., 2011, 133, 18370–18377 CrossRef CAS PubMed.
- C. Ding, J. Shi, D. Wang, Z. Wang, N. Wang, G. Liu, F. Xiong and C. Li, Phys. Chem. Chem. Phys., 2013, 15, 4589–4595 RSC.
- J. A. Seabold and K.-S. Choi, J. Am. Chem. Soc., 2012, 134, 2186–2192 CrossRef CAS PubMed.
- T. W. Kim and K. S. Choi, Science, 2014, 343, 990–994 CrossRef CAS PubMed.
- M. Zhong, T. Hisatomi, Y. Kuang, J. Zhao, M. Liu, A. Iwase, Q. Jia, H. Nishiyama, T. Minegishi, M. Nakabayashi, N. Shibata, R. Niishiro, C. Katayama, H. Shibano, M. Katayama, A. Kudo, T. Yamada and K. Domen, J. Am. Chem. Soc., 2015, 137, 5053–5060 CrossRef CAS PubMed.
- D. Guevarra, A. Shinde, S. K. Suram, I. D. Sharp, F. M. Toma, J. A. Haber and J. M. Gregoire, Energy Environ. Sci., 2016, 9, 565–580 RSC.
- A. Shinde, D. Guevarra, G. Liu, I. D. Sharp, F. M. Toma, J. M. Gregoire and J. A. Haber, ACS Appl. Mater. Interfaces, 2016, 8, 23696–23705 CrossRef CAS PubMed.
- Y. Ma, A. Kafizas, S. R. Pendlebury, F. L. Formal and J. R. Durrant, Adv. Funct. Mater., 2016, 26, 4951–4960 CrossRef CAS.
- C. Zachaus, F. F. Abdi, L. M. Peter and R. van de Krol, Chem. Sci., 2017, 8, 3712–3719 RSC.
- G. V. Govindaraju, G. P. Wheeler, D. Lee and K.-S. Choi, Chem. Mater., 2017, 29, 355–370 CrossRef CAS.
- J. A. Haber, E. Anzenburg, J. Yano, C. Kisielowski and J. M. Gregoire, Adv. Energy Mater., 2015, 5, 1402307 CrossRef.
- A. Shinde, G. Li, L. Zhou, D. Guevarra, S. K. Suram, F. M. Toma, Q. Yan, J. A. Haber, J. B. Neaton and J. M. Gregoire, J. Mater. Chem. A, 2016, 4, 14356–14363 RSC.
- J. M. Spurgeon, J. M. Velazquez and M. T. McDowell, Phys. Chem. Chem. Phys., 2014, 16, 3623–3631 RSC.
- B. J. Trzesniewski and W. A. Smith, J. Mater. Chem. A, 2016, 4, 2919–2926 RSC.
- F. Le Formal, K. Sivula and M. Grätzel, J. Phys. Chem. C, 2012, 116, 26707–26720 CrossRef CAS.
- F. A. L. Laskowski, J. Qiu, M. R. Nellist, S. Z. Oener, A. M. Gordon and S. W. Boettcher, Sustainable Energy Fuels, 2018 Search PubMed.
- G. Liu, J. Shi, F. Zhang, Z. Chen, J. Han, C. Ding, S. Chen, Z. Wang, H. Han and C. Li, Angew. Chem., Int. Ed., 2014, 53, 7295–7299 CrossRef CAS PubMed.
- L. Zhou, A. Shinde, D. Guevarra, F. M. Toma, H. S. Stein, J. M. Gregoire and J. A. Haber, ACS Appl. Energy Mater., 2018, 1, 5766–5771 CAS.
- J. Eichhorn, C. Kastl, J. K. Cooper, D. Ziegler, A. M. Schwartzberg, I. D. Sharp and F. M. Toma, Nat. Commun., 2018, 9, 2597 CrossRef PubMed.
- J. Qiu, H. Hajibabaei, M. R. Nellist, F. A. L. Laskowski, S. Z. Oener, T. W. Hamann and S. W. Boettcher, ACS Energy Lett., 2018, 3, 961–969 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8se00473k |
| This journal is © The Royal Society of Chemistry 2019 |