Sustainable production of ethylene from bioethanol over hierarchical ZSM-5 nanosheets†
Sirawit
Shetsiri‡
a,
Anawat
Thivasasith‡
a,
Kachaporn
Saenluang
a,
Wannaruedee
Wannapakdee
a,
Saros
Salakhum
a,
Piraya
Wetchasat
a,
Somkiat
Nokbin
b,
Jumras
Limtrakul
c and
Chularat
Wattanakit
 *a
*a
aDepartment of Chemical and Biomolecular Engineering, School of Energy Science and Engineering and Nanocatalysts and Nanomaterials for Sustainable Energy and Environment Research Network of NANOTEC, Vidyasirimedhi Institute of Science and Technology, Rayong 21210, Thailand. E-mail: chularat.w@vistec.ac.th
bDepartment of Chemistry, Faculty of Science, Kasetsart University, Bangkok 10900, Thailand
cDepartment of Materials Science and Engineering, School of Molecular Science and Engineering, Vidyasirimedhi Institute of Science and Technology, Rayong 21210, Thailand
First published on 1st October 2018
Abstract
Hierarchical aluminosilicate nanosheets composed of an MFI structure with various Si/Al ratios have been successfully prepared via a one-pot hydrothermal process with the aid of tetrabutylphosphonium hydroxide (TBPOH) as the bifunctional structure-directing agent (SDA). The MFI zeolite nanosheets exhibit outstanding properties, such as an extremely high external surface area and appropriate acidic properties. To illustrate the beneficial effect of the hierarchical structure of the zeolite nanosheets towards green and sustainable catalytic conversion of renewable resources to high value-added chemicals, direct bioethanol dehydration to ethylene over Brønsted-acid MFI nanosheets has been studied from both experimental and theoretical points of view. Interestingly, the hierarchical structure strongly effects an increase in surface acid density at the external surfaces, eventually resulting in an improvement in ethylene selectivity. The results obtained from density functional theory (DFT) calculations also reveal that ethylene is possibly produced over the Brønsted acid sites located at the external surfaces, whereas diethyl ether (DEE) formation is the predominant pathway over the internal acid sites of MFI. From these findings, hierarchical ZSM-5 nanosheets consisting of a high fraction of active sites at the external silanol surfaces can significantly enhance the selectivity of ethylene from ethanol/bioethanol dehydration.
1 Introduction
Ethylene is one of the most crucial light olefinic compounds in the petrochemical industry because it is widely used as a building block for various chemicals ranging from plastics and solvents to antifreeze agents.1,2 Although there are many vital processes that can be used to produce ethylene, for example, conventional naphtha cracking using steam or thermal techniques,1,2 there are serious problems with fossil fuel depletion, which has been increasing over the past few decades, and environmental and global warming issues. In order to circumvent these problems, alternative resources are considered as very important feedstocks for chemical production.3,4 Bioethanol is a well-known renewable organic substance obtained via the fermentation process of biomass.5–7 Because of a large increase in its production volume compared to its limited demand in a few applications, it is anticipated that excess bioethanol will allow the development of novel technology for the production of value-added compounds from bioethanol in the very near future.8,9 Therefore, the catalytic conversion of bioethanol via the dehydration route is one of the most fascinating processes for the production of high value-added chemicals such as ethylene.The dehydration of ethanol is typically performed using various solid-acid catalysts, in particular, metal oxides,10–15 heteropoly acids,16–20 and zeolites.5,21–34 There have been several reports on the catalytic performance of ethanol conversion on acidic zeolites obtained under different reaction conditions.5,21–34 Compared to Al2O3 and amorphous SiO2, zeolites with various topologies exhibit significantly higher yields of ethylene.35,36 However, at low temperatures (<573 K) the main product is diethyl ether (DEE).35,36 Because the dehydration of ethanol to ethylene is an endothermic reaction,35,36 a high reaction temperature is required (>723 K), and therefore a large proportion of higher hydrocarbons or/and aromatic products is also observed (>35% of the yield of hydrocarbons at 625 K using H-ZSM-5).37 This behavior eventually leads to fast catalyst deactivation due to coke formation inside their microporous structures.
An alternative way to improve the efficiency of zeolite-based catalysts for the selective dehydration of ethanol to ethylene is the use of hierarchical zeolites due to their outstanding properties, such as high external surface area and porosity, resistance to coke formation, and improved catalyst lifetime.38 Typically, such materials have been successfully prepared with one of the following two main approaches: (i) top-down or post treatment methods via desilication (alkaline treatment) or dealumination (acid treatment); (ii) bottom-up or direct synthesis methods via using hard templates (carbon allotropes) or soft templates (e.g., surfactants, cationic polymers, amphiphilic organosilane surfactants).39–47 Recently, various hierarchical zeolites with nanosheet assembled structures have been thoroughly developed using designed amphiphilic organosilane surfactants or quaternary ammonium/phosphonium cations.48–54 Such materials exhibit fascinating properties, in particular, the presence of interstitial pore space between nanosheet layers.48,49,51,54
Although there are some reports on modified HZSM-5 zeolite catalysts for ethanol dehydration at low temperatures (220–350 °C), exhibiting acceptable values for the ethanol conversion (70–100%) and ethylene selectivity (86–100%) (see the ESI Table S1†), the feasibility of using hierarchical zeolite nanosheet assemblies for selective ethanol dehydration to ethylene, and in particular, that of applying them in the dehydration of bioethanol feedstock, has not yet been demonstrated even though they would have many benefits, such as the two-fold advantage of external surface areas and unique acidic surface properties. In addition, an interpretation of the structural and acidic properties of zeolite nanosheets relating to their catalytic activity and product selectivity has not yet been studied.
In the present study, we have attempted to modify the ZSM-5 zeolite with nanosheet structures in a wide range of Si/Al ratios to improve the selective production of ethylene from ethanol and bioethanol resources. The modified nanosheet catalysts have been successfully synthesized via a one-pot hydrothermal synthesis in the presence of a quaternary phosphonium cation (tetrabutylphosphonium hydroxide, TBPOH) as a bifunctional structure-directing agent (SDA). Their structures were characterized by means of X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM), NH3 temperature programmed desorption (NH3-TPD), pyridine adsorption experiments and 27Al and 29Si MAS NMR spectroscopy. In addition, to maximize the efficiency of the catalysts in terms of activity, the ethylene selectivity and catalyst lifetime for the dehydration of ethanol to ethylene, several experimental parameters, including the Si/Al ratio of the catalyst, the reaction temperature, and the weight hourly space velocity (WHSV), have been systematically studied. Finally, mechanistic perspectives of ethanol dehydration to ethylene and DEE on nanosheet zeolite surfaces were also investigated using a density functional theory (DFT) approach to gain insights into the effect of hierarchical surfaces on the promotion of the catalytic selectivity of ethylene.
2 Experimental section
2.1 Preparation of the catalysts
Hierarchical MFI nanosheets with various Si/Al ratios were synthesized using a modified literature method.51,54 In a typical procedure, the first aqueous solution consisted of 8.7 g of tetraethyl orthosilicate (Sigma-Aldrich, >99.0%), 8.6 g of tetrabutylphosphonium hydroxide (TBPOH) solution (Sigma-Aldrich, 40 wt% in H2O) as the SDA, and aluminium isopropoxide (Sigma-Aldrich, >98.0%) in varying amounts to vary the Si/Al ratio of the samples. To prepare the second synthesis solution, 0.0208 g of sodium hydroxide (Sigma-Aldrich, >98.0%) was dissolved in 2.3175 g of deionized water. Subsequently, the first solution was added dropwise into the second solution under vigorous stirring at room temperature for 12 h to obtain a homogeneous mixture. The gel solution was then transferred to a Teflon-lined stainless steel autoclave and crystallized at 130 °C for 48 h. The solid powder was precipitated using a centrifugal technique and washed with deionized water until neutral pH of the supernatant fraction was obtained. Finally, the sample was dried overnight at 110 °C and then calcined at 650 °C for 8 h to remove the SDA and the other contaminants. The synthesized solid was converted to H+ form with three consecutive ion-exchange steps with 0.1 M NH4Cl solution at 80 °C for 2 h. The obtained powder was dried and finally calcined at 650 °C for 6 h. The catalyst was defined ZSM5-HieNS(x), where x is the actual Si/Al ratio.To prepare the conventional ZSM-5 catalyst, a synthesized precursor containing 3.50 g of tetraethyl orthosilicate (Sigma-Aldrich, >99.0%), 2.02 g of tetrapropylammonium hydroxide solution (Sigma-Aldrich, 1 M in H2O), 0.0276 g of sodium aluminate (Sigma-Aldrich, >99.5%, anhydrous) and 0.0693 g of sodium hydroxide (Sigma-Aldrich, >98.0%) in 10.465 g of deionized water was stirred at room temperature for 2 h. Then, this was transferred to a stainless-steel autoclave for hydrothermal synthesis at 180 °C for 72 h.51,55 Finally, a solid sample was calcined at 650 °C for 8 h. The acid catalyst was obtained by applying the above-mentioned ion-exchange procedure. The obtained conventional ZSM-5 was denoted ZSM5-CON(x), where x is the actual Si/Al ratio.
2.2 Characterization of the catalysts
Powder X-ray diffraction (XRD) patterns were obtained using a Bruker D8 ADVANCE diffractometer and CuKα radiation (30 kV, 40 mA) with a step size of 0.02° and a scan rate of 1° min−1 in the two theta (2θ) range of 5 to 50°. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were used to reveal the catalyst morphologies and were performed using JEOL JSM-7600F (at 2 kV) and TECNAI 20 TWIN (at 200 kV), respectively.Wavelength-dispersive X-ray fluorescence spectrometry (WDXRF) was used to analyze the elemental composition of the samples and was performed using a Bruker S8 Tiger instrument. The textural properties were determined through an N2 adsorption/desorption technique at 77 K performed on BELSORP-max apparatus. The BET specific surface area (SBET), and the external surface area (Sext), together with the micropore volume (Vmicro), and the mesopore size distribution were calculated using the Brunauer–Emmett–Teller (BET) method, the t-plot method, and the Barrett–Joyner–Halenda (BJH) model, respectively.
To investigate the acidic properties, NH3 temperature programmed desorption (NH3-TPD) profiles were recorded using a BELL-CATII analyzer. Typically, a catalyst (0.05 g) was pretreated under the flow of He at 500 °C with a heating rate of 10 °C min−1. Subsequently, a diluted ammonia feed (7.5 v/v% of NH3 in He) was introduced for 0.5 h. The NH3-TPD profiles were recorded by increasing the temperature from 100 to 700 °C with a heating rate of 10 °C min−1 in the flow of He. To investigate the coke content, O2 temperature programmed oxidation (O2-TPO) was performed on a BELL-CATII analyzer with the same pretreatment conditions as those in the NH3-TPD experiments. After pretreatment, the feed of a diluted oxygen (5 v/v% of O2 in He) with a total mass flow rate of 50 mL min−1 was introduced, and the temperature was linearly increased from 50 to 700 °C using a heating rate of 5 °C min−1. FTIR spectra were recorded in the range 4000–800 cm−1 using a Bruker Vertex V70v instrument. The spectra were gained at a 2 cm−1 resolution and averaged over 64 scans. Firstly, the zeolite sample was heated to 550 °C at a rate of 2 °C min−1 under 20 v/v% of O2 in He. To determine the density of acid sites in the zeolites, pyridine was introduced for about 10 min into the chamber at its vapor pressure at room temperature. Then, the pyridine molecules were desorbed by evacuation for 1 h at 150 °C. Afterwards, a spectrum was recorded at 150 °C. To calculate the total amount of Brønsted and Lewis acid sites, the molar extinction coefficient values 0.73 and 1.11 cm μmol−1 were applied, respectively.
The nature of the aluminium sites and the environment of the silicon species in the zeolite framework were investigated using 27Al and 29Si MAS NMR spectroscopy operating at the magnetic fields 17.6 T and 9.4 T, respectively, performed on an AVANCE III HD (400 MHz) Digital NMR spectrometer (Bruker).
2.3 Catalytic studies of ethanol and bioethanol dehydration
To carry out the catalytic study of ethanol/bioethanol dehydration, the reaction was performed using a continuous fixed-bed tubular reactor with a tube with an inner diameter of 3/8 inches. The catalyst was sieved into particle sizes in the range 450–850 μm to control the particle size distribution and packed in the middle of the reactor tube between thin layers of glass wool. Prior to the catalytic test, the catalyst was pretreated at 500 °C under the flow of N2 (50 mL min−1) for 2 h. Subsequently, the denatured ethanol (99.99%, QRec) or bioethanol (Sapthip company) in N2 carrier gas (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 molar ratio) was introduced into the reactor at the reaction temperature range 200 to 400 °C with weight hourly space velocities (WHSV) in the range 10.0 to 30.0 h−1. To prevent the condensation of liquid products, the inlet and outlet zones were heated to 185 °C. The products were analyzed using an online gas chromatograph (GC) (Agilent 7820A) equipped with an FID detector and a PoraBOND Q capillary column (10 m × 3 μm × 0.25 mm) at an interval time of 1 h. The chromatogram of the reaction mixture is shown in Fig. S1.† The mass balance of all the experiments was calculated from the calibration curve of each component and their values were in the range 99.03 ± 0.07% (Fig. S2†). The conversion of ethanol (XEtOH) and the product selectivity (Si) were estimated using eqn (1) and (2), respectively:
2.5 molar ratio) was introduced into the reactor at the reaction temperature range 200 to 400 °C with weight hourly space velocities (WHSV) in the range 10.0 to 30.0 h−1. To prevent the condensation of liquid products, the inlet and outlet zones were heated to 185 °C. The products were analyzed using an online gas chromatograph (GC) (Agilent 7820A) equipped with an FID detector and a PoraBOND Q capillary column (10 m × 3 μm × 0.25 mm) at an interval time of 1 h. The chromatogram of the reaction mixture is shown in Fig. S1.† The mass balance of all the experiments was calculated from the calibration curve of each component and their values were in the range 99.03 ± 0.07% (Fig. S2†). The conversion of ethanol (XEtOH) and the product selectivity (Si) were estimated using eqn (1) and (2), respectively: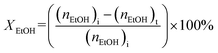 | (1) |
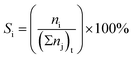 | (2) |
2.4 Computational studies
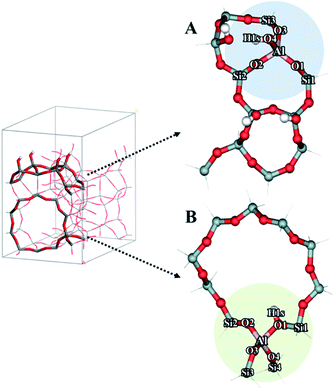
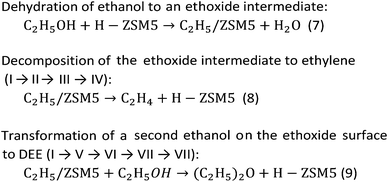
Density functional theory (DFT) calculations were performed to investigate the mechanism of the dehydration of ethanol to ethylene over the Brønsted acid sites on different surfaces of the MFI framework. The 12T quantum cluster models taken from the MFI zeolite lattice structure56 were used to represent the Brønsted acid sites at the external surfaces of hierarchical ZSM-5 and the internal surfaces of the bulk MFI framework as shown in Fig. 9A and B, respectively. To keep the crystalline structure of the MFI framework, the dangling bonds of the surface oxygen atoms were terminated by H atoms at the distance 1.47 Å![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57–59 and the Si–H bonds were aligned in the directions of the Si–O bonds of the zeolite structure. To represent the active site at the external MFI surfaces, the 12T model was generated from the outer surface of the zeolite60 (Fig. 9A). An aluminium atom was substituted at the T12 position and a Brønsted acid site was generated by adding a hydroxyl group on this aluminum site.61 In addition, three hydroxyl groups were added to the silicon atoms at the T7, T9, and T10 positions to represent surface silanol groups, as can be seen in Fig. 9A.60,61 For the model to represent the active site at the internal surface of the MFI framework corresponding to the bulk MFI catalyst, it was constructed using the 12T quantum cluster generated which was composed from the intersecting straight and zigzag channels of MFI with the replacement of an aluminium atom at the T12 position to generate the Brønsted acid site58 (Fig. 9B).
57–59 and the Si–H bonds were aligned in the directions of the Si–O bonds of the zeolite structure. To represent the active site at the external MFI surfaces, the 12T model was generated from the outer surface of the zeolite60 (Fig. 9A). An aluminium atom was substituted at the T12 position and a Brønsted acid site was generated by adding a hydroxyl group on this aluminum site.61 In addition, three hydroxyl groups were added to the silicon atoms at the T7, T9, and T10 positions to represent surface silanol groups, as can be seen in Fig. 9A.60,61 For the model to represent the active site at the internal surface of the MFI framework corresponding to the bulk MFI catalyst, it was constructed using the 12T quantum cluster generated which was composed from the intersecting straight and zigzag channels of MFI with the replacement of an aluminium atom at the T12 position to generate the Brønsted acid site58 (Fig. 9B).
The M06-2X density functional was used to perform all calculations. This method can describe the van der Waals interactions and is suitable for studying various catalytic reactions over zeolite surfaces.57,62,63 During the geometry optimizations, the 12T active region and adsorbates were allowed to relax, while the remaining hydrogen atoms were fixed at crystallographic coordinates. All calculations were performed with the GAUSSIAN 09 code using the basis set 6-31G(d,p). The interaction energies (ΔE) of the intermediates and products were calculated with eqn (3):
| ΔE = Ecomplex − (Ezeolite + Eadsorbate) | (3) |
3 Results
3.1 Physicochemical properties of the hierarchical ZSM-5 catalysts (ZSM5-HieNS)
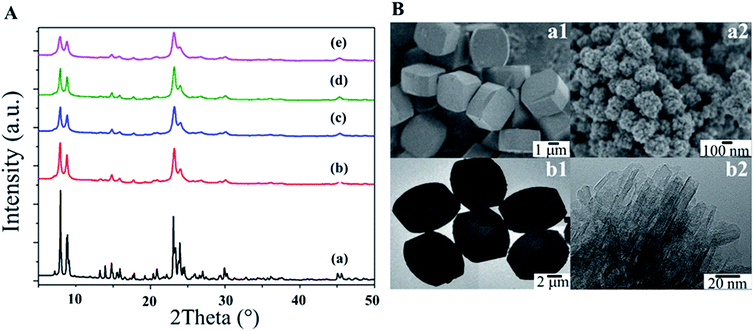
The XRD patterns of all the synthesized samples indicate that the synthesized samples contain the MFI framework, for which the main characteristic peaks appeared at 2θ of 7.8, 8.7, 23.1, 23.8 and 24.3°, corresponding to (101), (111), (501), (303) and (313),64,65 respectively, as shown in Fig. 1A. The relative crystallinity calculated with respect to a conventional MFI (MFI-CON(50)) as a reference, and the average crystallite size calculated with the Scherrer equation from the peak area in the range of 2θ 22 to 25°![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55,66 are summarized in Table S2.† Clearly the relative crystallinity values of the hierarchical nanosheets are above 90% compared to those of the bulk zeolite, implying that the crystalline structures are well-preserved even in the presence of hierarchical structures. Interestingly, an increase in the relative crystallinity relating to a slightly larger average crystallite size is observed as a function of increasing Si/Al ratio.
55,66 are summarized in Table S2.† Clearly the relative crystallinity values of the hierarchical nanosheets are above 90% compared to those of the bulk zeolite, implying that the crystalline structures are well-preserved even in the presence of hierarchical structures. Interestingly, an increase in the relative crystallinity relating to a slightly larger average crystallite size is observed as a function of increasing Si/Al ratio.
The morphologies of the as-synthesized samples were observed using SEM and TEM microscopic techniques (Fig. 1B). As expected, MFI-CON(38) exhibits a bulk structure with cubic-shaped crystals (Fig. 1B(a1 and b1)). In contrast, the MFI-HieNS samples exhibit a different structure due to spherical assemblies of nanolayers (Fig. 1B (a2 and b2), and Fig. S3† for the hierarchical samples with different Si/Al ratios). In addition, the particle size distribution and nanosheet thickness of the nanolayers are demonstrated in Fig. S4 and S5,† respectively. Compared to MFI-CON(38), MFI-HieNS(37) has a smaller particle size which is in the range 224.09 ± 41.25 nm. However, when increasing the Si/Al ratio, the particle size is slightly increased to 255.87 ± 31.48 nm for the MFI-HieNS(159) sample. The thickness of the as-synthesized nanosheets is approximately 8–9 nm.
The textural properties of the as-synthesized samples were investigated with N2 physisorption measurements as shown in Fig. 2 and Table 1. The adsorption–desorption isotherm of MFI-CON(38) corresponds to a type I isotherm due to micropore filling in the microporous network at a low relative pressure (P/P0) (Fig. 2A(a)). However, the nanosheet series of MFI catalysts exhibit a combined isotherm which is between type I and IV isotherms, in which a hysteresis loop at high P/P0, due to a capillary condensation within the additional secondary pores, is observed (Fig. 2A). This makes it clear that the zeolite nanosheets are composed of microporous features due to their zeolite characteristics together with mesopores/macropores obtained from the assemblies of nanolayers and the inter-particle voids. This behavior eventually leads to an increase in external surface area and pore volume (Table 1).
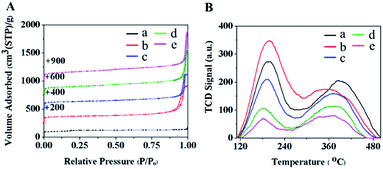 | ||
| Fig. 2 (A) N2-sorption isotherms and (B) NH3-TPD profiles of (a) MFI-CON(38), (b) MFI-HieNS(37), (c) MFI-HieNS(75), (d) MFI-HieNS(103), and (e) MFI-HieNS(159). | ||
| Sample | Si/Ala | Surface area (m2 g−1) | Pore volume (cm3 g−1) | S ext/SBETh | V ext/Vtotali | ||||
|---|---|---|---|---|---|---|---|---|---|
| S BET | S Micro | S ext | V total | V micro | V ext | ||||
| a Si/Al ratio was determined using an XRF technique. b S BET: BET specific surface area. c S Micro: micropore surface area. d S ext: external surface area. e V total: total pore volume obtained at P/P0 = 0.99. f V micro: micopore volume. g V ext: external pore volume calculated from the difference between the total pore volume and micropore volume. h The fraction of external surface area. i The proportional external volume. | |||||||||
| MFI-CON(38) | 38 | 405 | 390 | 15 | 0.22 | 0.18 | 0.03 | 0.04 | 0.16 |
| MFI-HieNS(37) | 37 | 451 | 286 | 165 | 1.02 | 0.11 | 0.90 | 0.37 | 0.89 |
| MFI-HieNS(75) | 75 | 562 | 412 | 150 | 1.10 | 0.22 | 0.88 | 0.27 | 0.80 |
| MFI-HieNS(103) | 103 | 505 | 352 | 153 | 1.07 | 0.17 | 0.89 | 0.30 | 0.84 |
| MFI-HieNS(159) | 159 | 589 | 433 | 156 | 1.05 | 0.25 | 0.81 | 0.26 | 0.77 |
The chemisorption profiles and acidic properties of all the samples were investigated using the NH3 temperature programmed desorption (TPD) technique as shown in Fig. 2B and Table 2. All the synthesized samples exhibit two predominant NH3 desorption bands at the low-temperature desorption peak (190 to 205 °C) and the high-temperature desorption peak (375 to 400 °C) corresponding to the characteristics of weak acid sites and strong acid sites, respectively.67 Typically, the weak acid sites relate to the non-framework Al species or the Si–OH in the structural defects, which can interact with base molecules.68 As can be seen in Table 2, MFI-HieNS(37) and MFI-CON(38) have an equivalent total acid density, however the ratio of the weak to the strong acid density of the nanosheet samples obviously increases. This might be explained by the change in the microporous structure and the addition of secondary mesoporous networks leading to an increase in the external surface properties. Obviously, the ratio of the weak to the strong acid densities continuously decreases as a function of the Si/Al ratio, relating to a decrease in the number of strong acid sites when increasing the Si/Al ratio.
| Sample | Desorption temperature (°C) | Acidity amount (μmol g−1) | W/Sf | Q2/Q4g | Q3/Q4h | |||
|---|---|---|---|---|---|---|---|---|
| Low DESa | High DESb | Weakc (W) | Strongd (S) | Totale | ||||
| a Low temperature desorption peak (DES). b High temperature desorption peak (DES). c Weak acid density. d Strong acid density. e Total acid density. f The ratio of the weak and strong acid density; the weak acid density, strong acid density and total acid density were calculated using a deconvoluted Gaussian distribution. g The ratio of Q2 to Q4. h The ratio of Q3 to Q4 obtained using the 29Si NMR technique. | ||||||||
| MFI-CON(38) | 201 | 396 | 90 | 130 | 220 | 0.69 | 0.50 | 4.83 |
| MFI-HieNS(37) | 203 | 376 | 100 | 110 | 221 | 0.91 | 0.74 | 7.09 |
| MFI-HieNS(75) | 202 | 385 | 70 | 100 | 170 | 0.70 | 0.70 | 6.81 |
| MFI-HieNS(103) | 196 | 379 | 30 | 60 | 90 | 0.50 | 0.63 | 4.91 |
| MFI-HieNS(159) | 193 | 374 | 20 | 60 | 80 | 0.33 | 0.59 | 3.89 |
To investigate the nature of the aluminium and silicon species in the zeolite framework, the 27Al and 29Si MAS-NMR techniques were used, respectively. The 27Al-NMR spectra of all the synthesized samples are illustrated in Fig. S6.† The tetrahedral aluminium in the frameworks corresponds to the peak at the chemical shift 55 ppm, while the extra-aluminum framework (EFAl), an oligomeric alumina with a low degree of hydration/hydroxylation, relates to the signal at the chemical shift 0 ppm.69 Although hierarchical ZSM-5 exhibits the presence of EFAl species, a large proportion of the aluminum sites are due to the tetrahedral aluminium framework. This is also consistent with the pyridine adsorption results, confirming the presence of both Brønsted acid sites and Lewis acid sites (in Fig. S7 and Table S3†). Interestingly, the content ratio of the Brønsted acid sites to the Lewis acid sites of MFI-HieNS is obviously smaller than that of MFI-CON. The presence of EFAl species might be related to the removal of aluminium during the calcination at high temperature. The presence of EFAl species together with that of the Brønsted acid sites (BAS) of tetrahedral aluminium in the framework also provides a synergistic effect between cationic EFAl and vicinal BAS and can promote some catalytic reactions, such as catalytic cracking and dehydration.70–72
To reveal the environment of the Si and Al frameworks, 29Si-NMR spectra were obtained and are illustrated in Fig. 3. They indicate the presence of the following three characteristic peaks: (i) Si surrounded by two Si or Al atoms and two hydroxyl groups (Q2); (ii) Si surrounded by three Si or Al atoms and one hydroxyl group (Q3); (iii) Si surrounded by four Si or Al atoms (Q4). They appear at the chemical shifts −93, −103, and −113 ppm for Q2, Q3, and Q4, respectively.73 Compared to the conventional HZSM-5 (MFI-CON(38)), the hierarchical nanosheet with a similar Si/Al ratio (MFI-HieNS(37)) exhibits a significantly higher ratio of Q2/Q4 and Q3/Q4 (Table 2), which corresponds to a large number of Si or Al atoms sitting on the external surfaces or zeolite defects to form silanol surfaces. It is therefore reasonable to assume that the modified zeolites are composed of a large number of silanol defect sites or disturbed tetrahedral coordinated aluminium sites due to increased external surface area obtained by introducing a secondary mesoporous network. In addition, the Q2/Q4 and Q3/Q4 ratios obviously increase with decreasing Si/Al ratio. The reason for this increase in the ratio of silanol defects to tetrahedral frameworks relates to the fact that a zeolite with a low Si/Al ratio demonstrates a high possibility of the formation of a disturbed aluminum tetrahedral coordinated state of aluminum,74 which is also exhibited by the broad signal in the 27Al NMR spectra in the range 25 to 50 ppm (Fig. S6B†). In addition, the high ratio of silanol surfaces to bridging hydroxyls (Brønsted sites) of the hierarchical zeolite nanosheet can be confirmed by the high proportion of O–H stretching from terminal Si–OH groups at 3740 cm−1 with respect to bridging hydroxyls (Brønsted sites), which appeared at 3600 cm−1, whereas this ratio is obviously lower in the case of the conventional zeolite (Fig. S8†).
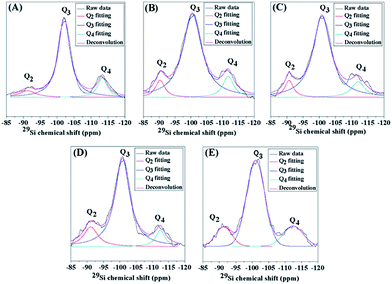 | ||
| Fig. 3 29Si-NMR spectra of (A) MFI-CON(38), and (B–E) MFI-HieNS with the Si/Al ratios 37, 75, 103, and 159, respectively. | ||
3.2 Catalytic conversion of ethanol through dehydration to ethylene
| 2C2H5OH → C2H5OC2H5 + H2O, ΔH0298 = −25.1 kJ mol−1 | (4) |
| C2H5OH → C2H4 + H2O, ΔH0298 = +44.9 kJ mol−1 | (5) |
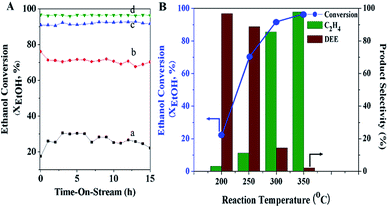
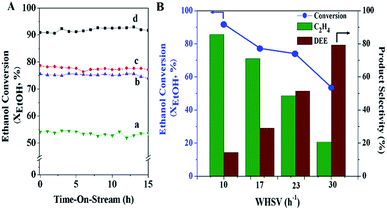
The effect of space velocity on the catalytic performance was studied by variation in terms of the weight hourly space velocity, WHSV (10, 17, 23, and 30 h−1), under atmospheric pressure at the reaction temperature 300 °C over the MFI-HieNS(37) catalyst. As expected, the activity of ethanol conversion significantly decreases from 91.9% to 53.6% when increasing the WHSV from 10 to 30 h−1 (Fig. 5A). The selectivity of ethylene decreases from 85.7% to 20.7%, whereas the selectivity of DEE increases from 14.3% to 79.2% at WHSV values of 10 and 30 h−1, respectively, as shown in Fig. 5B. The reason for the change in activity and product distribution as a function of WHSV relates to the fact that a low space velocity allows a long time for the reaction mixture to be in contact with the catalyst, and therefore a high conversion of the reactant can be observed.76,77 In addition, the higher ethylene selectivity at a low WHSV might be explained by the direct decomposition of diethyl ether (DEE) to ethylene. This reaction pathway is the competing reaction with direct dehydration of ethanol to ethylene, and may occur depending on the relative partial pressures of ethanol and DEE, which can be controlled with specific reaction conditions.35,78,79 Therefore, it is reasonable to propose that the reaction pathway when using a low space velocity of reactant proceeds via a parallel series reaction, including the following three reactions: (i) direct conversion of ethanol into ethylene; (ii) ethanol transformation to DEE; (iii) decomposition of DEE to ethylene79 (eqn (6)).
| C2H5OC2H5 → 2C2H4 + H2O, ΔH0298 = +115.7 kJ mol−1 | (6) |
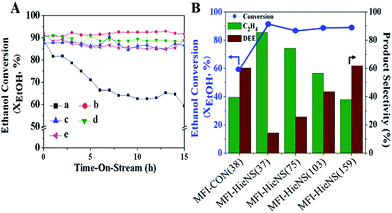
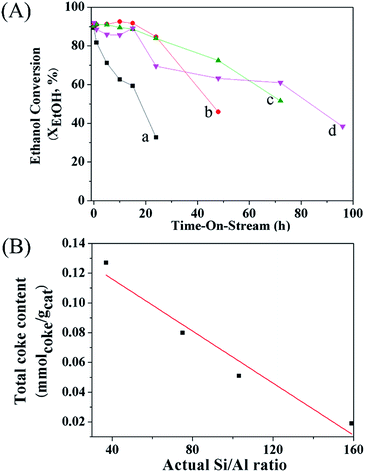
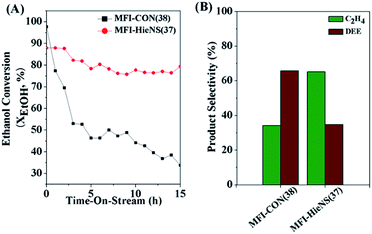
In addition, the catalytic performance of MFI-HieNS with different Si/Al ratios was also investigated (Fig. 6B). This clearly shows that a high ethanol conversion (∼86.9% to 91.9%) is still observed irrespective of the Si/Al ratio. However, the product selectivity significantly changes. For example, the ethylene selectivity gradually decreases when increasing the Si/Al ratio. The highest selectivity of ethylene ∼85.5% is found when using the MFI-HieNS(37) catalyst. The reason for this increase in the ethylene selectivity as a function of decreasing Si/Al ratio relates to the fact that a lower Si/Al ratio corresponds to a high acid density and this may increase the possibility of primary dehydration of the alcohol and decomposition of the ether intermediates to light olefins.38,80,81 It is well-known that the major disadvantage of a bulk zeolite for ethanol conversion is the short catalyst lifetime. To further confirm the benefits of hierarchical structures for catalytic stability, a stability test was carried out over the synthesized catalysts under the same reaction conditions, as shown in Fig. 7A. Clearly, MFI-CON(38) shows fast deactivation of the catalyst even after 5 h of TOS and the time taken to reach 50% ethanol conversion is less than 20 h. Compared to conventional HZSM-5 with a similar Si/Al ratio (MFI-CON(38)), MFI-HieNS(37) has greatly improved catalytic stability. This makes it clear that the hierarchical structures can enhance the stability of the catalyst. In addition, the stability of the catalyst also depends on the Si/Al ratio. It was found that a high Si/Al ratio (MFI-HieNS(103)) can increase the catalyst stability. However, the activity of a sample in which the Si/Al ratio is too high (MFI-HieNS(159)) becomes lower. The reason for this decrease in activity relates to the fact that there are insufficient acid sites, resulting in suppression of the catalytic reaction.
To verify the accumulated coke deposit in the zeolite structures, the coke contents derived from O2-TPO profiles (Fig. S9†) to represent the coke species over MFI-HieNS(37) and MFI-CON(38) are shown in Fig. 7B. The results indicate that MFI-HieNS(37) can reduce the amount of the coke species (0.127 mmolcoke gcat−1), whereas a significantly higher amount of coke can be observed over MFI-CON(38) (0.151 mmolcoke gcat−1). In addition, when increasing the Si/Al ratio, the total amount of coke deposits is obviously reduced as can be seen in Fig. 7 because a low acid density eventually leads to suppression of the hydrocarbon pool pathway.82,83
To investigate the regeneration of the catalysts, the catalytic performance of the hierarchical MFI catalysts was investigated after catalyst regeneration by the burning of coke species. It was found that hierarchical ZSM-5 exhibits high ethanol conversion and high selectivity of ethylene (∼90–95%) even after regeneration of the catalysts (Fig. S10†). In addition the morphologies of the catalysts can be well preserved (Fig. S11–S12, and Table S4†).
3.3 Mechanistic studies
As stated above, the hierarchical ZSM-5 nanosheets dramatically increase the ethylene yield of the ethanol conversion compared to that of the bulk ZSM-5. To understand the reaction mechanism of ethanol dehydration to ethylene, we also carried out a series of calculations using computational approaches. In this study, we focused on the above-mentioned mechanisms, including direct conversion of ethanol to ethylene and ethanol transformation to DEE.79To compare the reaction mechanisms over different structures of catalysts, two different models, composed of the Brønsted acid sites on the external silanol surfaces (containing Q3 species) and the internal surfaces of the bulk MFI framework (containing Q4 species), have been used to represent the structures of hierarchical ZSM-5 (Fig. 9A) and a conventional one (Fig. 9B), respectively.
To represent the hierarchical ZSM-5 surfaces, in which the external silanol surface (Fig. 9A) is predominant, a hydroxyl group was added to the aluminium atom (T12 site) of the MFI framework to create a Brønsted acid site and other three hydroxyl groups were introduced at the silicon atom of the external surface of MFI.60,61 As can be seen in Fig. 9A, the bond distances of O4⋯H1s and O4⋯Al are 0.97 and 1.72 Å, respectively. Furthermore, the average bond distance between the Al atom and its three neighboring oxygens (O1, O2, and O3) atoms is 1.77 Å. As for the structure representing the acid site at the internal surfaces of a conventional MFI framework (Fig. 9B), the 12T cluster covers the intersecting straight and zigzag channels, in which an aluminum atom was substituted at the T12 position to generate a Brønsted acid site.58 The bond distances of O1⋯Al and O2⋯Al are 1.83 and 1.68 Å, respectively. The bond distance of the Brønsted acid site (O1–H1s) is 0.97 Å.
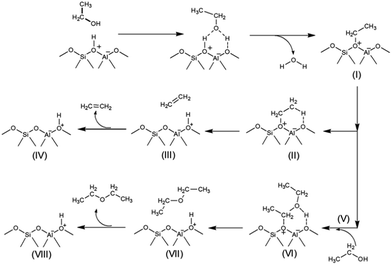
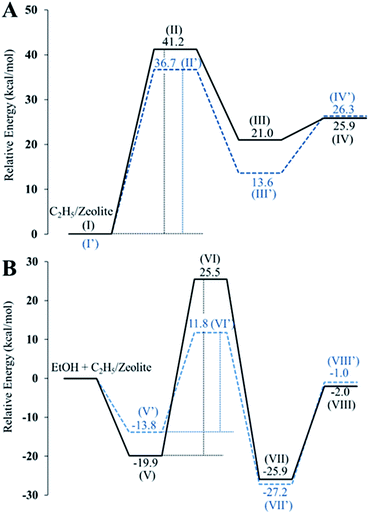
In strong contrast to this, ethanol dehydration to DEE starts from the co-adsorption of a second ethanol over the ethoxide surface (Fig. 11B(V) and (V′)). The oxygen atom of the co-adsorbed ethanol moves closer to the carbon atom of the ethoxide species, and the hydrogen atom of the hydroxy group of the second ethanol is simultaneously transferred to the oxygen atom of the zeolite framework. As for the external surface model of MFI, the bond distance between the O5 of ethanol and the C1 of the ethoxy species is shortened from 3.10 to 2.47 Å. Meanwhile the bond distance between H1e and O5 of the hydroxy group of ethanol is elongated from 0.97 to 0.98 Å and the H1e of the hydroxy group interacts with the O2 of the zeolite framework with a bond length of 1.76 Å. This reaction step requires an activation energy of 45.5 kcal mol−1. Compared to that in the external surface model, the transition state over the internal surfaces of MFI can occur by a similar pathway, however it requires a significantly lower activation energy of 25.6 kcal mol−1. Finally, diethyl ether is formed over the external and internal surfaces of MFI (Fig. 11B(VII) and (VII′)). As expected, ethoxide decomposition to ethylene and DEE requires lower activation energies over the internal surfaces than over the external surfaces of the MFI zeolite. This behavior relates to the higher acid strength of the Brønsted acid sites at the internal surfaces compared to that of the acid sites at the external surfaces.87
From the calculation observations, the ethoxide species can be converted to either ethylene or DEE as products over the external surfaces of hierarchical ZSM-5 and the internal surfaces of the bulk MFI framework. However, ethylene formation possibly occurs over the Brønsted acid site at the external surfaces because the surface silanol groups can induce a charge between the intermediate molecules and the zeolite surfaces. In contrast, diethyl ether formation is preferably carried out over the internal acid sites, confirmed by a large difference in activation energies between ethylene formation and the DEE pathway.
These observations are also consistent with the experimental point of view that ethylene is possibly produced over the hierarchical nanosheets, which have the characteristic of the external silanol surfaces (Q2 and Q3 species), whereas diethyl ether (DEE) formation is the predominant pathway over the internal Brønsted acid sites of the bulk MFI. It is therefore reasonable to assume that the hierarchical ZSM-5 nanosheets consisting of a high fraction of external surfaces compared to bulk ZSM-5 can significantly enhance the selectivity of ethylene from ethanol dehydration. These observations also support the details obtained from previous works which reveal that ethylene is possibly generated from direct ethanol conversion or indirect generation from ether, or that both routes contribute to ethene formation.88,89 In particular, the surface acid sites of the hierarchical zeolite can promote direct ethanol conversion to ethylene.
4 Conclusions
Hierarchical MFI zeolite nanosheet catalysts have been successfully synthesized via a one-pot hydrothermal method with the aid of TBPOH as a bifunctional SDA. These zeolites had improved physicochemical properties with outstanding external surface area and appropriate acid properties due to a large proportion of external surfaces in the hierarchical structure. To illustrate the beneficial effect of zeolite nanosheets towards green and sustainable catalytic conversion of renewable resources to high value-added chemicals, direct bioethanol dehydration to ethylene over Brønsted acid MFI nanosheets has been studied from both experimental and theoretical points of view. With their superior properties, the hierarchical ZSM-5 nanosheets can greatly enhance ethanol conversion (>90%), with a high selectivity of ethylene (>85%) and high catalyst stability in the ethanol dehydration process at moderate temperatures (300–350 °C). The computational point of view reveals that ethylene is possibly produced over the Brønsted acid sites at the external surfaces whereas diethyl ether formation is the predominant pathway over the internal Brønsted acid sites of MFI. This information provides insight into the fact that the Brønsted acid sites at the external surfaces of the hierarchical ZSM-5 nanosheets can significantly enhance the selectivity of ethylene from ethanol dehydration. This opens up new perspectives on the hierarchical structures not only improving the catalytic performance due to a reduction in coke formation but also the weak acid sites at the external surfaces enhancing the selectivity of ethylene.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants from the Vidyasirimedhi Institute of Science and Technology (VISTEC), the Thailand Research Fund (TRF) (MRG6180099), the Office of Higher Education Commission (OHEC), and the Junior Research Fellowship Program of the French Embassy in Thailand. This work has also been partially supported by the National Nanotechnology Center (NANOTEC), NSTDA, Ministry of Science and Technology, Thailand, through its program of Research Network NANOTEC (RNN). Furthermore, the authors would like to acknowledge the Frontier Research Center (FRC) at VISTEC for the technical support.References
- Y. Gucbilmez, T. Dogu and S. Balci, Ind. Eng. Chem. Res., 2006, 45, 3496–3502 CrossRef CAS.
- C. J. Pereira, Science, 1999, 285, 670–671 CrossRef CAS.
- B. Liu, L. France, C. Wu, Z. Jiang, V. L. Kuznetsov, H. A. Al-Megren, M. Al-Kinany, S. A. Aldrees, T. Xiao and P. P. Edwards, Chem. Sci., 2015, 6, 5152–5163 RSC.
- Y. Li, L. Li and J. Yu, Chem, 2017, 3, 928–949 CrossRef CAS.
- J. Bi, X. Guo, M. Liu and X. Wang, Catal. Today, 2010, 149, 143–147 CrossRef CAS.
- Q. Sheng, K. Ling, Z. Li and L. Zhao, Fuel Process. Technol., 2013, 110, 73–78 CrossRef CAS.
- L. Wang, R. Templer and R. J. Murphy, Energy Environ. Sci., 2012, 5, 8281–8293 RSC.
- J. Sun and Y. Wang, ACS Catal., 2014, 4, 1078–1090 CrossRef CAS.
- J. Zhang, Q. Qian, M. Cui, C. Chen, S. Liu and B. Han, Green Chem., 2017, 19, 4396–4401 RSC.
- G. Chen, S. Li, F. Jiao and Q. Yuan, Catal. Today, 2007, 125, 111–119 CrossRef CAS.
- M. M. Doheim, S. A. Hanafy and G. A. El-Shobaky, Mater. Lett., 2002, 55, 304–311 CrossRef CAS.
- H. Knözinger, H. Bühl and K. Kochloefl, J. Catal., 1972, 24, 57–68 CrossRef.
- B. C. Shi and B. H. Davis, J. Catal., 1995, 157, 359–367 CrossRef CAS.
- N. Takezawa, C. Hanamaki and H. Kobayashi, J. Catal., 1975, 38, 101–109 CrossRef.
- X. Zhang, R. Wang, X. Yang and F. Zhang, Microporous Mesoporous Mater., 2008, 116, 210–215 CrossRef CAS.
- V. V. Bokade and G. D. Yadav, Appl. Clay Sci., 2011, 53, 263–271 CrossRef CAS.
- A. Ciftci, D. Varisli, K. Cem Tokay, N. Aslı Sezgi and T. Dogu, Chem. Eng. J., 2012, 207–208, 85–93 CrossRef CAS.
- J. Gurgul, M. Zimowska, D. Mucha, R. P. Socha and L. Matachowski, J. Mol. Catal. A: Chem., 2011, 351, 1–10 CrossRef CAS.
- T. Okuhara, T. Arai, T. Ichiki, K. Y. Lee and M. Misono, J. Mol. Catal., 1989, 55, 293–301 CrossRef CAS.
- Y. Saito and H. Niiyama, J. Catal., 1987, 106, 329–336 CrossRef CAS.
- A. T. Aguayo, A. G. Gayubo, A. Atutxa, M. Olazar and J. Bilbao, Ind. Eng. Chem. Res., 2002, 41, 4216–4224 CrossRef CAS.
- D. E. Bryant and W. L. Kranich, J. Catal., 1967, 8, 8–13 CrossRef CAS.
- R. Le Van Mao, P. Levesque, G. McLaughlin and L. H. Dao, Appl. Catal., 1987, 34, 163–179 CrossRef CAS.
- R. Le Van Mao, T. M. Nguyen and G. P. McLaughlin, Appl. Catal., 1989, 48, 265–277 CrossRef CAS.
- F. F. Madeira, N. S. Gnep, P. Magnoux, S. Maury and N. Cadran, Appl. Catal., A, 2009, 367, 39–46 CrossRef CAS.
- W. R. Moser, C.-C. Chiang and R. W. Thompson, J. Catal., 1989, 115, 532–541 CrossRef CAS.
- J. Ouyang, F. Kong, G. Su, Y. Hu and Q. Song, Catal. Lett., 2009, 132, 64–74 CrossRef CAS.
- C. B. Phillips and R. Datta, Ind. Eng. Chem. Res., 1997, 36, 4466–4475 CrossRef CAS.
- K. Ramesh, Y. L. E. Goh, C. G. Gwie, C. Jie, T. J. White and A. Borgna, J. Porous Mater., 2012, 19, 423–431 CrossRef CAS.
- K. Ramesh, L. M. Hui, Y.-F. Han and A. Borgna, Catal. Commun., 2009, 10, 567–571 CrossRef CAS.
- K. Ramesh, C. Jie, Y.-F. Han and A. Borgna, Ind. Eng. Chem. Res., 2010, 49, 4080–4090 CrossRef CAS.
- I. Takahara, M. Saito, M. Inaba and K. Murata, Catal. Lett., 2005, 105, 249–252 CrossRef CAS.
- N. Zhan, Y. Hu, H. Li, D. Yu, Y. Han and H. Huang, Catal. Commun., 2010, 11, 633–637 CrossRef CAS.
- D. Zhang, R. Wang and X. Yang, Catal. Lett., 2008, 124, 384–391 CrossRef CAS.
- T. K. Phung and G. Busca, Chem. Eng. J., 2015, 272, 92–101 CrossRef CAS.
- T. K. Phung, L. Proietti Hernández, A. Lagazzo and G. Busca, Appl. Catal., A, 2015, 493, 77–89 CrossRef CAS.
- M. Inaba, K. Murata, M. Saito and I. Takahara, React. Kinet. Catal. Lett., 2006, 88, 135–141 CrossRef CAS.
- K. A. Tarach, J. Tekla, W. Makowski, U. Filek, K. Mlekodaj, V. Girman, M. Choi and K. Gora-Marek, Catal. Sci. Technol., 2016, 6, 3568–3584 RSC.
- M. Choi, H. S. Cho, R. Srivastava, C. Venkatesan, D.-H. Choi and R. Ryoo, Nat. Mater., 2006, 5, 718–723 CrossRef CAS PubMed.
- W. Fan, M. A. Snyder, S. Kumar, P.-S. Lee, W. C. Yoo, A. V. McCormick, R. Lee Penn, A. Stein and M. Tsapatsis, Nat. Mater., 2008, 7, 984–991 CrossRef CAS PubMed.
- B. T. Holland, L. Abrams and A. Stein, J. Am. Chem. Soc., 1999, 121, 4308–4309 CrossRef CAS.
- C. J. H. Jacobsen, C. Madsen, J. Houzvicka, I. Schmidt and A. Carlsson, J. Am. Chem. Soc., 2000, 122, 7116–7117 CrossRef CAS.
- Y. Tao, H. Kanoh and K. Kaneko, J. Am. Chem. Soc., 2003, 125, 6044–6045 CrossRef CAS PubMed.
- H. Wang and T. J. Pinnavaia, Angew. Chem., Int. Ed., 2006, 45, 7603–7606 CrossRef CAS PubMed.
- D. P. Serrano, J. Aguado, J. M. Escola, J. M. Rodríguez and Á. Peral, Chem. Mater., 2006, 18, 2462–2464 CrossRef CAS.
- M. Opanasenko, M. Shamzhy, F. Yu, W. Zhou, R. E. Morris and J. Cejka, Chem. Sci., 2016, 7, 3589–3601 RSC.
- T. C. Keller, S. Isabettini, D. Verboekend, E. G. Rodrigues and J. Perez-Ramirez, Chem. Sci., 2014, 5, 677–684 RSC.
- M. Choi, K. Na, J. Kim, Y. Sakamoto, O. Terasaki and R. Ryoo, Nature, 2009, 461, 246–249 CrossRef CAS PubMed.
- D. Xu, G. R. Swindlehurst, H. Wu, D. H. Olson, X. Zhang and M. Tsapatsis, Adv. Funct. Mater., 2014, 24, 201–208 CrossRef CAS.
- K. Rodponthukwaji, C. Wattanakit, T. Yutthalekha, S. Assavapanumat, C. Warakulwit, W. Wannapakdee and J. Limtrakul, RSC Adv., 2017, 7, 35581–35589 RSC.
- W. Wannapakdee, C. Wattanakit, V. Paluka, T. Yutthalekha and J. Limtrakul, RSC Adv., 2016, 6, 2875–2881 RSC.
- P. Wuamprakhon, C. Wattanakit, C. Warakulwit, T. Yutthalekha, W. Wannapakdee, S. Ittisanronnachai and J. Limtrakul, Microporous Mesoporous Mater., 2016, 219, 1–9 CrossRef CAS.
- T. Yutthalekha, D. Suttipat, S. Salakhum, A. Thivasasith, S. Nokbin, J. Limtrakul and C. Wattanakit, Chem. Commun., 2017, 53, 12185–12188 RSC.
- X. Zhang, D. Liu, D. Xu, S. Asahina, K. A. Cychosz, K. V. Agrawal, Y. Al Wahedi, A. Bhan, S. Al Hashimi, O. Terasaki, M. Thommes and M. Tsapatsis, Science, 2012, 336, 1684–1687 CrossRef CAS PubMed.
- A. J. J. Koekkoek, C. H. L. Tempelman, V. Degirmenci, M. Guo, Z. Feng, C. Li and E. J. M. Hensen, Catal. Today, 2011, 168, 96–111 CrossRef CAS.
- E. M. Flanigen, J. M. Bennett, R. W. Grose, J. P. Cohen, R. L. Patton, R. M. Kirchner and J. V. Smith, Nature, 1978, 271, 512–516 CrossRef CAS.
- B. Boekfa, P. Pantu, M. Probst and J. Limtrakul, J. Phys. Chem. C, 2010, 114, 15061–15067 CrossRef CAS.
- T. Maihom, S. Wannakao, B. Boekfa and J. Limtrakul, Chem. Phys. Lett., 2013, 556, 217–224 CrossRef CAS.
- W. Panjan, J. Sirijaraensre, C. Warakulwit, P. Pantu and J. Limtrakul, Phys. Chem. Chem. Phys., 2012, 14, 16588–16594 RSC.
- C. E. Hernandez-Tamargo, A. Roldan and N. H. de Leeuw, J. Solid State Chem., 2016, 237, 192–203 CrossRef CAS.
- D. Zhai, L. Zhao, Y. Liu, J. Xu, B. Shen and J. Gao, Chem. Mater., 2015, 27, 67–74 CrossRef CAS.
- B. Boekfa, S. Choomwattana, P. Khongpracha and J. Limtrakul, Langmuir, 2009, 25, 12990–12999 CrossRef CAS PubMed.
- T. Maihom, B. Boekfa, J. Sirijaraensre, T. Nanok, M. Probst and J. Limtrakul, J. Phys. Chem. C, 2009, 113, 6654–6662 CrossRef CAS.
- S. K. Jesudoss, J. J. Vijaya, K. Kaviyarasu, L. J. Kennedy, R. Jothi Ramalingam and H. A. Al-Lohedan, RSC Adv., 2018, 8, 481–490 RSC.
- M. Liu, J. Li, W. Jia, M. Qin, Y. Wang, K. Tong, H. Chen and Z. Zhu, RSC Adv., 2015, 5, 9237–9240 RSC.
- O. Ben Mya, M. Bita, I. Louafi and A. Djouadi, MethodsX, 2018, 5, 277–282 CrossRef PubMed.
- N. Katada, H. Igi and J.-H. Kim, J. Phys. Chem. B, 1997, 101, 5969–5977 CrossRef CAS.
- S. Kouva, J. Kanervo, F. Schüβler, R. Olindo, J. Lercher and A. Krause, Chem. Eng. Sci., 2013, 89, 40–48 CrossRef CAS.
- D. Ma, Y. Lu, L. Su, Z. Xu, Z. Tian, Y. Xu, L. Lin and X. Bao, J. Phys. Chem. B, 2002, 106, 8524–8530 CrossRef CAS.
- S. Li, A. Zheng, Y. Su, H. Zhang, L. Chen, J. Yang, C. Ye and F. Deng, J. Am. Chem. Soc., 2007, 129, 11161–11171 CrossRef CAS PubMed.
- S. Li, S.-J. Huang, W. Shen, H. Zhang, H. Fang, A. Zheng, S.-B. Liu and F. Deng, J. Phys. Chem. C, 2008, 112, 14486–14494 CrossRef CAS.
- C. Liu, G. Li, E. J. M. Hensen and E. A. Pidko, ACS Catal., 2015, 5, 7024–7033 CrossRef CAS.
- R. Ravishankar, C. E. A. Kirschhock, P.-P. Knops-Gerrits, E. J. P. Feijen, P. J. Grobet, P. Vanoppen, F. C. De Schryver, G. Miehe, H. Fuess, B. J. Schoeman, P. A. Jacobs and J. A. Martens, J. Phys. Chem. B, 1999, 103, 4960–4964 CrossRef CAS.
- G. Engelhardt, U. Lohse, A. Samoson, M. Mägi, M. Tarmak and E. Lippmaa, Zeolites, 1982, 2, 59–62 CrossRef CAS.
- D. Varisli, T. Dogu and G. Dogu, Chem. Eng. Sci., 2007, 62, 5349–5352 CrossRef CAS.
- K. K. Ramasamy and Y. Wang, Catal. Today, 2014, 237, 89–99 CrossRef CAS.
- Z. S. B. Sousa, C. O. Veloso, C. A. Henriques and V. Teixeira da Silva, J. Mol. Catal. A: Chem., 2016, 422, 266–274 CrossRef CAS.
- M. A. Christiansen, G. Mpourmpakis and D. G. Vlachos, ACS Catal., 2013, 3, 1965–1975 CrossRef CAS.
- M. Zhang and Y. Yu, Ind. Eng. Chem. Res., 2013, 52, 9505–9514 CrossRef CAS.
- W. Choopun and S. Jitkarnka, J. Cleaner Prod., 2016, 135, 368–378 CrossRef CAS.
- C.-Y. Wu and H.-S. Wu, ACS Omega, 2017, 2, 4287–4296 CrossRef CAS.
- M. Hartmann, A. G. Machoke and W. Schwieger, Chem. Soc. Rev., 2016, 45, 3313–3330 RSC.
- H. Zhang, Z. Hu, L. Huang, H. Zhang, K. Song, L. Wang, Z. Shi, J. Ma, Y. Zhuang, W. Shen, Y. Zhang, H. Xu and Y. Tang, ACS Catal., 2015, 5, 2548–2558 CrossRef CAS.
- R. Jérôme, R. Pascal and C. Céline, ChemCatChem, 2017, 9, 2176–2185 CrossRef.
- H. Chiang and A. Bhan, J. Catal., 2010, 271, 251–261 CrossRef CAS.
- P. M. Mortensen, J. D. Grunwaldt, P. A. Jensen, K. G. Knudsen and A. D. Jensen, Appl. Catal., A, 2011, 407, 1–19 CrossRef CAS.
- F. Sannino, M. Pansini, A. Marocco, B. Bonelli, E. Garrone and S. Esposito, Phys. Chem. Chem. Phys., 2015, 17, 28950–28957 RSC.
- K. Alexopoulos, M. John, K. Van der Borght, V. Galvita, M.-F. Reyniers and G. B. Marin, J. Catal., 2016, 339, 173–185 CrossRef CAS.
- S. A. Kadam and M. V. Shamzhy, Catal. Today, 2018, 304, 51–57 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8se00392k |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2019 |