Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Mechanism of hydrogen peroxide formation by lytic polysaccharide monooxygenase
Octav
Caldararu
 a,
Esko
Oksanen
a,
Esko
Oksanen
 bc,
Ulf
Ryde
bc,
Ulf
Ryde
 a and
Erik D.
Hedegård
*a
a and
Erik D.
Hedegård
*a
aDivision of Theoretical Chemistry, Lund University, Chemical Centre, P. O. Box 124, SE-221 00 Lund, Sweden. E-mail: octav.caldararu@teokem.lu.se; erik.hedegard@teokem.lu.se
bEuropean Spallation Source ESS ERIC, P. O. Box 176, SE-221 00 Lund, Sweden
cDepartment of Biochemistry and Structural Biology, Lund University, Chemical Centre, P. O. Box 124, SE-221 00 Lund, Sweden
First published on 29th August 2019
Abstract
Correction for ‘Mechanism of hydrogen peroxide formation by lytic polysaccharide monooxygenase’ by Octav Caldararu et al., Chem. Sci., 2019, 10, 576–586.
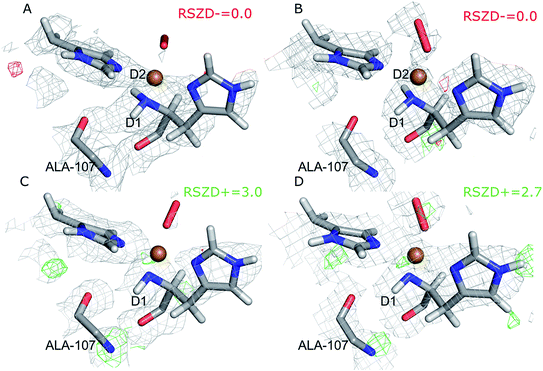
In the original article, two technical errors were made during the calculation of the nuclear scattering-length density maps for Fig. 2 and 3. As noted in the original article, joint refinement of the AA10 LPMO was conducted, even though the unit cells of the X-ray and the neutron crystals were slightly different (PDB IDs 5VG0 and 5VG1).1 Both the traditional joint refinement and the quantum refinement were performed in the X-ray unit cell, but the nuclear density maps in Fig. 2 and 3 were calculated in the neutron unit cell. Furthermore, atom D1 was incorrectly considered as H when making Fig. 3B and D (all of the refinements correctly considered D1 as deuterium).
Corrected Fig. 2 and 3 are now reported as shown, with the nuclear density maps calculated in the X-ray unit cell and with the correct assignment of all of the deuterium atoms. The RSZD values for the N-terminus group have also been re-calculated.
The conclusions in the original article remain unaffected by these corrections. The structures with two deuterium atoms at the N-terminus show no negative difference density, neither in the traditional joint refinement nor in the quantum refinement. Removal of the D2 atom from the N-terminus gives rise to positive difference density in subunit B, although at a lower σ level than that reported initially (2.7–2.8σ compared to 3.2σ), but above the noise level of the nuclear density maps in that area (∼2.3σ). While the maps without the D2 atom at the N-terminus show closer resemblance to those reported by Bacik et al.,1 the structures with two deuterium atoms still fit better to the neutron data both in the traditional joint refinement and in the quantum refinement. Thus, the original conclusions about the protonation state of the N-terminus are unaffected by these corrections. The calculations suggest that the N-terminus is not deprotonated in the crystal structure.
The technical errors corrected above also do not affect the conclusions of the studies regarding the nature of the oxygen species and the mechanism of hydrogen peroxide formation by the AA10 LPMO presented in the original article.
Throughout the article, Glu-B65 was also consistently mislabeled as Glu-201.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
References
- J. Bacik, S. Mekasha, Z. Forsberg, A. Y. Kovalevsky, G. Vaaje-Kolstad, V. G. H. Eijsink, J. C. Nix, L. Coates, M. J. Cuneo, J. Unkefer and J. C. Chen, Biochemistry, 2017, 8–11 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2019 |