Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Donor–acceptor covalent organic frameworks for visible light induced free radical polymerization†
Pradip
Pachfule
*a,
Amitava
Acharjya
a,
Jérôme
Roeser
a,
Ramesh P.
Sivasankaran
b,
Meng-Yang
Ye
a,
Angelika
Brückner
 b,
Johannes
Schmidt
a and
Arne
Thomas
b,
Johannes
Schmidt
a and
Arne
Thomas
 *a
*a
aDepartment of Chemistry/Functional Materials, Technische Universität Berlin, Hardenbergstraße 40, 10623 Berlin, Germany. E-mail: pradip.pachfule@campus.tu-berlin.de; arne.thomas@tu-berlin.de
bLeibniz Institute for Catalysis, University of Rostock, Albert-Einstein-Str. 29a, 18059 Rostock, Germany
First published on 5th August 2019
Abstract
Covalent organic frameworks (COFs) are promising materials for applications in photocatalysis, due to their conjugated, porous and chemically stable architectures. Alternating electron donor–acceptor-type structures are known to enhance charge carrier transport mobility and stability in polymers and are therefore also interesting building units for COFs used as photocatalysts but also as photoinitiator. In this work, two donor–acceptor COFs using electron deficient 4,4′,4′′-(1,3,5-triazine-2,4,6-triyl)trianiline and electron rich thiophene-based thieno[3,2-b]thiophene-2,5-dicarbaldehyde or [2,2′-bithiophene]-5,5′-dicarbaldehyde linkers are presented. The resulting crystalline and porous COFs have been applied as photoinitiator for visible light induced free radical polymerization of methyl methacrylate (MMA) to poly-methyl methacrylate (PMMA). These results pave the way to the development of robust and heterogeneous systems for photochemistry that offers the transfer of radicals induced by visible light.
Covalent organic frameworks (COFs) have recently received increasing interest due to their intriguing properties such as low density, high porosity, good crystallinity and the possibility to introduce a range of organic functional moieties into chemically stable frameworks.1–5 In terms of photochemistry, this combination of π-conjugated rigid architectures with high porosities and tailorable chemical functionalities in the COF backbones can enable: (a) rapid diffusion of charge carriers in the framework and to the surface, (b) immobilization of organic functionalities to enhance the life-time of excited species by preventing the formation of dimers, and (c) a high interface between the solid COF and reactants, electrolytes, sacrificial donors and co-catalysts. In two-dimensional (2D) COFs, π-functional organic building blocks can be organized in particular stacking patterns, thus that charge carriers can be transported in discrete and periodic columns.6 These unidirectional charge carrier pathways can improve the charge carrier mobility and lifetime in 2D COFs.7,8 Due to these properties COFs have been described as highly promising materials for efficient and recyclable photocatalysis, recently,9–14 beside other applications, for example for gas adsorption and separation, heterogeneous catalysis, proton conductivity, optoelectronics and energy storage.15–23
To further improve the catalytic activities, strategies such as introduction of heteroatoms (N and S),10 increasing the catalyst wettability,24 immobilization of molecular co-catalysts25 and improving the conjugation have been endeavored.26 It has further been demonstrated that the precise combination of donor–acceptor moieties in organic materials and COFs can yield enhanced performance in solar cells and optoelectronic applications, respectively.27–34 Herein, two novel COFs are presented, whose structures were designed considering the above-mentioned criteria. These materials have been successfully applied as photoinitiators for efficient and recyclable radical polymerization, paving the way to the development of robust and heterogeneous systems for photochemistry that offers the transfer of radicals induced by visible light.
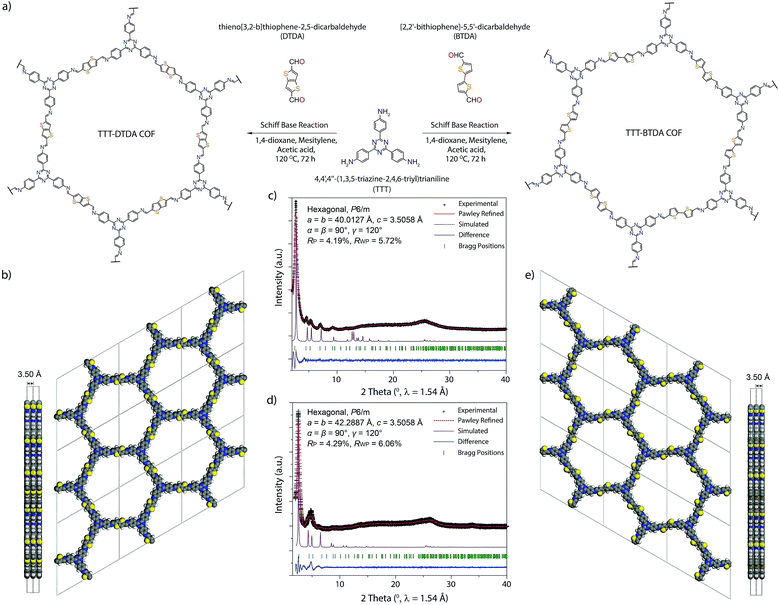
Considering the beneficial properties of donor–acceptor based materials for charge generation, mobility and stability, we have attempted the synthesis of donor–acceptor COFs as photoinitiators for visible light induced free radical polymerization (Fig. 1).35,36 TTT–DTDA and TTT–BTDA COFs have been synthesized by the solvothermal reaction of 4,4′,4′′-(1,3,5-triazine-2,4,6-triyl)trianiline (TTT, 106 mg, 0.3 mmol) with thieno[3,2-b]thiophene-2,5-dicarbaldehyde (DTDA, 89 mg, 0.45 mmol) or [2,2′-bithiophene]-5,5′-dicarbaldehyde (BTDA, 99 mg, 0.45 mmol), respectively, in presence of 6 M acetic acid (0.5 mL) and mesitylene/dioxane (3 mL) as the solvents [Section S2, ESI†]. In order to synthesize crystalline and porous COFs, a series of experiments was carried out to optimize the best solvent combinations (Tables S1 and S2, ESI†), showing that choosing the right solvent ratio plays a vital role in reaching optimum crystallinity and porosity. The as-synthesized COFs were further characterized by powder X-ray diffraction (PXRD), 13C solid-state cross-polarization magic angle spinning nuclear magnetic resonance (CP/MAS-NMR) spectroscopy, N2 sorption analyses, scanning electron microscopy (SEM), in situ as well as ex situ electron paramagnetic resonance (EPR) spectroscopy, and ultraviolet-visible spectroscopy (UV-vis) analyses.
Powder X-ray diffraction (PXRD) analyses with Cu Kα radiation (λ = 1.5418 Å) was performed to assess the crystallinity of the as-synthesized COFs. The PXRD patterns for TTT–DTDA and TTT–BTDA are dominated by an intense reflection in the low-angle region, at 2.68 and 2.52 2θ degrees for TTT–DTDA and TTT–BTDA, respectively, which can be assigned to the (100) facet of a primitive hexagonal lattice (Fig. 1c and d). Additionally, the presence of further weak reflections and a broad reflection at ∼25 2θ degrees for TTT–DTDA and TTT–BTDA COFs that can be assigned to the (001) facet, confirms the formation of two-dimensional (2D) COFs in a crystalline and π–π stacked form. The structural models for TTT–DTDA and TTT–BTDA were constructed by generating the probable 2D hexagonal layers with hcb topology with either eclipsed (AA) or staggered (AB) stacking arrangement (Fig. S13, S14 and Section S7, ESI†). After geometrical optimization of the models for TTT–DTDA and TTT–BTDA, the simulated PXRD patterns were calculated and compared with the corresponding experimentally measured patterns. A fully eclipsed AA-type stacking was found to be in agreement with the experimental PXRD patterns (Fig. 1c and d; violet curves). In contrast, AB stacking can be discarded on the account of a significant mismatch in intensities of the PXRD patterns (Fig. S15, ESI†). The final unit cell parameters were determined after Pawley refinement, which led to satisfactorily low residual values and acceptable profile differences (Fig. 1c and d).
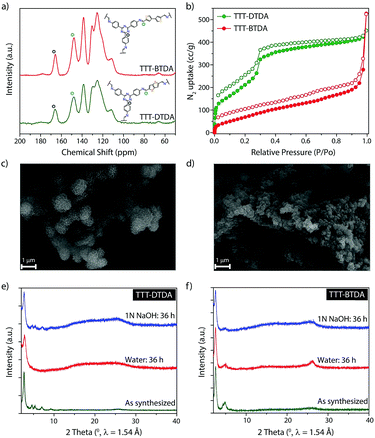
The chemical structure of TTT–DTDA and TTT–BTDA was further verified by solid-state NMR spectroscopy. Fig. 2a shows the 13C CP/MAS NMR spectrum for TTT–DTDA and TTT–BTDA, where a peak at ∼147 ppm can be assigned to the carbon atoms of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds, confirming the condensation reaction of aldehyde (TTT) and primary amines (DTDA or BTDA). The signals at ∼110, 125, 130 and 138 ppm can be ascribed to the carbon atoms of the phenyl groups, while the sharp peak at ∼165 ppm is attributed to the carbon atoms of core triazine ring from TTT linkers in the COFs, validating the presence of intact triazine moieties in the backbones. The formation of frameworks in TTT–DTDA and TTT–BTDA was further corroborated by Fourier transform infrared (FT-IR) spectroscopy. In the FT-IR spectra the presence of a strong vibration at 1582 cm−1 can be assigned to the stretching mode of the –C
N bonds, confirming the condensation reaction of aldehyde (TTT) and primary amines (DTDA or BTDA). The signals at ∼110, 125, 130 and 138 ppm can be ascribed to the carbon atoms of the phenyl groups, while the sharp peak at ∼165 ppm is attributed to the carbon atoms of core triazine ring from TTT linkers in the COFs, validating the presence of intact triazine moieties in the backbones. The formation of frameworks in TTT–DTDA and TTT–BTDA was further corroborated by Fourier transform infrared (FT-IR) spectroscopy. In the FT-IR spectra the presence of a strong vibration at 1582 cm−1 can be assigned to the stretching mode of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds (Fig. S4, ESI†), indicating the formation of imine bonds in COFs. Meanwhile, the aldehyde (∼1645 cm−1) and amino (∼3450 cm−1) stretching bands of respective starting materials completely disappeared after COF formation (Fig. S4, ESI†), which offers further evidence for the formation of imine bonds via the condensation of aldehyde and primary amines.
N bonds (Fig. S4, ESI†), indicating the formation of imine bonds in COFs. Meanwhile, the aldehyde (∼1645 cm−1) and amino (∼3450 cm−1) stretching bands of respective starting materials completely disappeared after COF formation (Fig. S4, ESI†), which offers further evidence for the formation of imine bonds via the condensation of aldehyde and primary amines.
The permanent porosity of TTT–DTDA and TTT–BTDA was evaluated by N2 adsorption–desorption isotherm measured at 77 K (Fig. 2b). The calculated Brunauer–Emmett–Teller (BET) and Langmuir surface area values for TTT–DTDA were 1012 and 2195 m2 g−1, respectively, whereas TTT–BTDA showed lower BET (302 m2 g−1) and Langmuir (440 m2 g−1) surface areas. In case of TTT–BTDA, most probably, due to the partial offset stacking of adjacent layers seen in the less defined PXRD pattern in comparison to TTT–DTDA, just moderate surface area values were observed (Fig. S3, ESI†).37–39 Scanning electron microscopy (SEM) analyses indicate that TTT–DTDA and TTT–BTDA crystallize in spherical and flower-like crystallites, respectively (Fig. 2c, d and S5, ESI†). The thermal stability of as-synthesized TTT–DTDA and TTT–BTDA was evaluated by thermogravimetric analyses, showing no weight loss under nitrogen atmosphere up to 450 °C (Fig. S5, ESI†). Further, to analyze the chemical stabilities of COFs, 50 mg of TTT–DTDA and TTT–BTDA were submerged in water and 1 N NaOH. The samples were kept immersed in the respective liquids for 36 h and after washing with water, their structure was analyzed by PXRD. Both COFs retained their crystalline structure in aqueous and alkaline media (Fig. 2e and f). Besides, the sample could be completely recovered in quantitative yield after the chemical treatment, thus showing no indication of decomposition, even though some loss in surface area was observed (Fig. S12, ESI†). Obviously, the stability of the COFs in water and alkaline media is an important prerequisite for their application in visible light induced radical polymerization that generally takes place in presence of tertiary amines as initiators.35,36,40,41
Photopolymerization, a process in which visible or ultraviolet light initiates and propagates a polymerization reaction to form linear or cross-linked polymer structures, is an important process in polymer chemistry due to its wide range of economic and ecological applications.42,43 This process has been widely used for coatings, adhesives, microelectronics, optical waveguides, etc.43,44 In order to meet the industrial requirements of products obtained from the photopolymerization process, a variety of photoinitiators have been established and applied successfully.45,46 However, most of these low molecular weight photoinitiators suffer from severe drawbacks such as migration from cured films, high volatility and strong odor. To address these concerns and achieve a higher durability of the catalysts, heterogeneous photoinitiators like polymeric carbon nitride,35 porous polymers36 metal organic frameworks (MOFs),47 onium salts,48 cross-linked polymers,49,50etc. have been exploited. Although these materials show promising results regarding recyclability, lower activities of these heterogeneous visible-light photoinitiators have been observed in comparison to their molecular counterparts, probably due to their low surface areas and/or less accessible reactive sites. Considering the significant porosity and high surface areas, and the presence of donor–acceptors moieties in the frameworks allowing facile migration of photogenerated charge carriers, the performance of TTT–DTDA and TTT–BTDA have been investigated for visible-light induced, heterogeneous free radical polymerization.
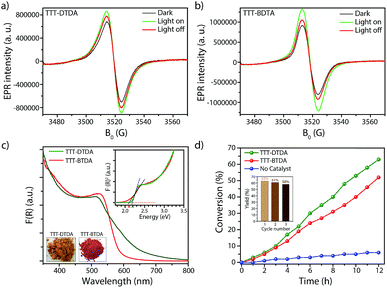
EPR spectra of both COFs have been recorded in the dark and under photocatalytic reaction conditions to visualize charge separation and transfer. Both COFs show a narrow isotropic singlet with Lorentzian line shape at g = 2.005. This signal increased when TTT–DTDA and TTT–BTDA samples were irradiated with light, since more electrons are excited from the valence to the conduction band, indicating electron–hole pair generation in the COF semiconductor (Fig. 3a and b, green spectra).51–53 After switching off the light, the EPR signal intensity decreased again, due to recombination of the CB-e− with holes (Fig. 3a and b, red spectra).54 The drop of the EPR signal is more pronounced for the less active sample TTT–BTDA, suggesting that undesired recombination of CB-e− with holes is faster than transfer of the latter to potential reactants.
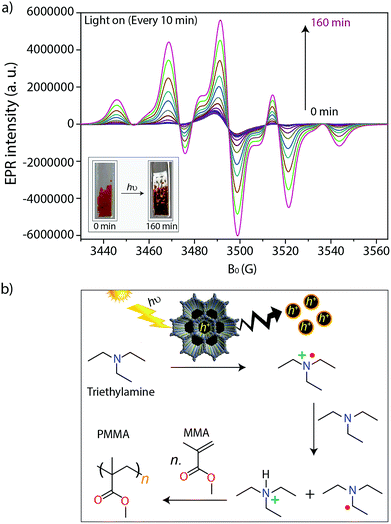
During irradiation with UV-vis light, a radical signal developed in the operando EPR spectra (exemplarily shown for TTT–DTDA in Fig. 4a, for comparison of both samples see Fig. S8, ESI†) and polymer formation was observed with time inside the EPR flat cell for both catalysts (Fig. S9, ESI†). This signal can be assigned to the propagating radical –CβH2–˙Cα(CH3)–COOCH3. The signal recorded after 160 min reaction time (Fig. 4a) could be satisfactorily reproduced by spectra simulation using g = 2.0048 and superhyperfine structure (shfs) constants for the coupling of the electron at Cα with the three CH3 protons and the two protons at Cβ, ACH3 = 22.2 G, AHβ1 = 14.7 G, AHβ2 = 7.9 (Fig. S9a, ESI†), which are in good agreement with literature data.55,56 This species might be produced upon reaction of MMA with initiating radicals formed by transfer of holes from COF to TEA (Fig. 4b). Notably, such TEA-derived radicals have not been seen in the operando EPR spectra, probably due to fast reaction with MMA and/or too low concentration.53 Diffuse reflectance UV-vis spectra of TTT-DTDA and TTT-BTDA exhibit a broad and strong absorbance peak in the visible region and an absorbance edge around 527 and 515 nm, respectively (Fig. 3c). As compared with TTT-BTDA, the absorbance tail for TTT-DTDA is extended beyond 750 nm, yielding enhanced light absorption. The calculated optical band gaps from Tauc plots are 2.10 and 2.19 eV, respectively, for TTT-BTDA and TTT-DTDA (Fig. 3c, inset).
Considering the light absorption and charge separation under visible light irradiation, visible light induced radical polymerization experiments using the as-synthesized COFs were attempted, using a water-cooled glass reactor equipped with stirring plate and external light source (λ > 420 nm) (Fig. S1, ESI†). A mixture of methyl methacrylate (MMA; 1 mL, 9.39 mmol) and triethylamine (TEA; 35 mg, 0.35 mmol) was irradiated with light in presence of the respective COFs (20 mg). TEA acts as co-initiators for radical polymerization reaction (Section S2 and Table S3, ESI†). During the whole process, the temperature was maintained at 25 °C and the reaction was performed under argon atmosphere. After performing the reaction for a given time, the reaction mixture was dissolved in THF, the COFs were separated by filtration and the remaining solutes were re-precipitated in methanol to obtain poly-methyl methacrylate (PMMA). The polymeric products were dried at room temperature. In order to analyze the performance of TTT–DTDA and TTT–BTDA COFs for radical polymerization, the MMA conversion vs. time was plotted (Fig. 3d). TTT–DTDA showed the highest conversion up to 63% after 12 h of reaction time, whereas TTT–BTDA showed a lower activity to yield ∼54% conversion. When the same reaction was carried out without COF as catalyst, just negligible conversions were observed (Fig. 3b). Notably, the COFs showed a better performance in radical polymerization of MMA to PMMA with higher molecular weight (Table 1), compared with carbon nitride,35 porous polymers36 MOFs,47 cross-linked polymers,49,50etc.,43 probably due to the combination of their high surface area and π-conjugated, donor–acceptor type backbones (Table S6, ESI†).
| COF | Conversion (%) | Molecular wt (Mn) (g mol−1) | DispersitybMw/Mn |
|---|---|---|---|
| a λ = 420–700 nm, time = 12 h, [MMA] = 9.39 mmol, [TEA] = 0.35 mmol, 20 mg of as-synthesized COF initiator. b Number-average molecular weight (Mn) and molecular weight distribution (Mw/Mn) determined by GPC. c Without any addition of COF initiator. | |||
| TTT–DTDA | 63% | 2.3950 × 105 | 2.2161 |
| TTT–BTDA | 54% | 2.4292 × 105 | 2.9246 |
| No catalystc | 5% | 0.9437 × 102 | 1.1328 |
Most importantly, due to the heterogeneous nature, the COF-based photoinitiators maintain its photoinitiation activity, thus can be easily separated and used for further polymerizations with nearly maintained activities (Fig. 3d, inset). After the photopolymerization experiments, TTT–DTDA and TTT–BTDA samples were recovered, thoroughly washed, and then again characterized by 13C CP-MAS solid-state NMR spectroscopy analyses, showing the preservation of the COF structure (Fig. S10, ESI†). The PXRD analyses of the recovered COFs showed that the materials maintained their crystallinity, confirming the robustness of COF-based photoinitiators for radical polymerization (Fig. S11, ESI†). In the control experiments, the reaction without initiator (COF) and co-initiators (TEA) yielded no or just a negligible amount of product, suggesting the necessity of both COF and TEA for radical polymerization (Table S3, ESI†). In addition, no polymer was obtained without light irradiation or in the presence of oxygen (O2) suggesting a light-driven process as well as involvement of a radical polymerization mechanism (Table S3, ESI†).42 In additional control experiments, the radical polymerization was carried out using an amorphous TTT–DTDA polymer, a simple mixture of the monomers, and another COF with comparable structure but without the strong electron donating thiophenes in the backbone (IISERP-COF4),57 which all yielded lower conversions of 42%, 37% and 7%, respectively (Fig. S16 and Table S3, ESI†). These results prove the importance of the open, crystalline, donor–acceptor type structure of the COF for enhancing its photoinitiating activity for free radical polymerization.
After the absorption of light in the visible region by the COFs, the photochemically formed holes can oxidize triethylamine to the corresponding radical cations, which abstract hydrogens from another amine molecule leading to the formation of amine radicals, which subsequently are starting the radical polymerization reaction with MMA (Fig. 4b).35,36,47,58 The formation of radicals and their relation to catalytic activity have been confirmed by in situ EPR analysis (Fig. 4a and S7, ESI†). In these experiments, we have observed that undesired electron–hole pair recombination is slower for catalyst TTT–DTDA, while the radical formation is most pronounced for this catalyst. Both aspects might be responsible for the higher catalytic activity of this COF in comparison to TTT–BTDA.
Conclusions
In summary, we have synthesized two donor–acceptor COFs using electron deficient triazine building block and electron rich thiophene-based linkers. The crystalline and highly porous COFs absorb light in the visible region. The as-synthesized COFs, having promising photochemical properties, have been utilized to initiate the free radical polymerization of methyl methacrylate (MMA) to poly-methyl methacrylate (PMMA), where COFs play a key role in the generation of holes that induce hydrogen abstraction from the amine co-initiator. The facile separation of these COF-photoinitiators from the MMA/PMMA mixture and their recyclability for multiple radical polymerization cycles together with their capability to initiate radical reactions under visible-light, make these materials very promising photoinitiators for a range of photochemical reactions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the support from the Cluster of Excellence UniSysCat (Unifying Systems in Catalysis) funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy – EXC 2008/1 (UniSysCat)–390540038 and further within the DFG project TH1463/12-1. We thank Mr Ivan Ilić and Ms. Marlies Graewert for their help in GPC analyses, and Mrs Christina Eichenauer and Ms. Maria Unterweger for their assistance. We thank Dr Jabor Rabeah for simulation of the EPR spectrum.References
- A. P. Cote, Science, 2005, 310, 1166–1170 CrossRef CAS PubMed.
- X. Feng, X. Ding and D. Jiang, Chem. Soc. Rev., 2012, 41, 6010–6022 RSC.
- C. S. Diercks and O. M. Yaghi, Science, 2017, 355, eaal1585 CrossRef PubMed.
- A. G. Slater and A. I. Cooper, Science, 2015, 348, 988 CrossRef CAS PubMed.
- J. Roeser, D. Prill, M. J. Bojdys, P. Fayon, A. Trewin, A. N. Fitch, M. U. Schmidt and A. Thomas, Nat. Chem., 2017, 9, 977–982 CrossRef CAS PubMed.
- D. D. Medina, T. Sick, T. Bein, D. D. Medina, T. Sick and T. Bein, Adv. Energy Mater., 2017, 7, 1700387 CrossRef.
- X. Zhan, Z. Chen and Q. Zhang, J. Mater. Chem. A, 2017, 5, 14463–14479 RSC.
- G. H. V Bertrand, V. K. Michaelis, T.-C. Ong, R. G. Griffin and M. Dinca, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 4923–4928 CrossRef PubMed.
- V. S. Vyas, V. W. H. Lau and B. V. Lotsch, Chem. Mater., 2016, 28, 5191–5204 CrossRef CAS.
- V. S. Vyas, F. Haase, L. Stegbauer, G. Savasci, F. Podjaski, C. Ochsenfeld and B. V. Lotsch, Nat. Commun., 2015, 6, 8508 CrossRef CAS PubMed.
- P. Pachfule, A. Acharjya, J. Roeser, T. Langenhahn, M. Schwarze, R. Schomäcker, A. Thomas and J. Schmidt, J. Am. Chem. Soc., 2018, 140, 1423–1427 CrossRef CAS PubMed.
- P. F. Wei, M. Z. Qi, Z. P. Wang, S. Y. Ding, W. Yu, Q. Liu, L. K. Wang, H. Z. Wang, W. K. An and W. Wang, J. Am. Chem. Soc., 2018, 140, 4623–4631 CrossRef CAS PubMed.
- S. Kuecken, A. Acharjya, L. Zhi, M. Schwarze, R. Schomäcker and A. Thomas, Chem. Commun., 2017, 53, 5854–5857 RSC.
- K. Wang, L. Yang, X. Wang, L. Guo, G. Cheng, C. Zhang, S. Jin, B. Tan and A. Cooper, Angew. Chem., Int. Ed., 2017, 56, 14149–14153 CrossRef CAS PubMed.
- S. Kandambeth, A. Mallick, B. Lukose, T. Heine, R. Banerjee and M. Mane, J. Am. Chem. Soc., 2012, 134, 19524 CrossRef CAS PubMed.
- D. B. Shinde, G. Sheng, X. Li, M. Ostwal, A.-H. Emwas, K.-W. Huang and Z. Lai, J. Am. Chem. Soc., 2018, 140, 14342–14349 CrossRef CAS PubMed.
- J. W. Colson and W. R. Dichtel, Nat. Chem., 2013, 5, 453–465 CrossRef CAS PubMed.
- Z. Li, N. Huang, K. H. Lee, Y. Feng, S. Tao, Q. Jiang, Y. Nagao, S. Irle and D. Jiang, J. Am. Chem. Soc., 2018, 140, 12374–12373 CrossRef CAS PubMed.
- X. Han, J. Huang, C. Yuan, Y. Liu and Y. Cui, J. Am. Chem. Soc., 2018, 140, 892–895 CrossRef CAS PubMed.
- Q. Fang, S. Gu, J. Zheng, Z. Zhuang, S. Qiu and Y. Yan, Angew. Chem., 2014, 126, 2922–2926 CrossRef.
- Q. Sun, B. Aguila, J. Perman, L. D. Earl, C. W. Abney, Y. Cheng, H. Wei, N. Nguyen, L. Wojtas and S. Ma, J. Am. Chem. Soc., 2017, 139, 2786–2793 CrossRef CAS PubMed.
- S. Wang, Q. Wang, P. Shao, Y. Han, X. Gao, L. Ma, S. Yuan, X. Ma, J. Zhou, X. Feng and B. Wang, J. Am. Chem. Soc., 2017, 139, 4258–4261 CrossRef CAS PubMed.
- S. H. Je, H. J. Kim, J. Kim, J. W. Choi and A. Coskun, Adv. Funct. Mater., 2017, 27, 1703947 CrossRef.
- X. Wang, L. Chen, S. Y. Chong, M. A. Little, Y. Wu, W.-H. Zhu, R. Clowes, Y. Yan, M. A. Zwijnenburg, R. S. Sprick and A. I. Cooper, Nat. Chem., 2018, 10, 1180–1189 CrossRef CAS PubMed.
- S. Yang, W. Hu, X. Zhang, P. He, B. Pattengale, C. Liu, M. Cendejas, I. Hermans, X. Zhang, J. Zhang and J. Huang, J. Am. Chem. Soc., 2018, 140, 14614–14618 CrossRef CAS PubMed.
- E. Jin, M. Asada, Q. Xu, S. Dalapati, M. A. Addicoat, M. A. Brady, H. Xu, T. Nakamura, T. Heine, Q. Chen and D. Jiang, Science, 2017, 676, 673–676 CrossRef PubMed.
- M. Calik, F. Auras, L. M. Salonen, K. Bader, I. Grill, M. Handloser, D. D. Medina, M. Dogru, F. Löbermann, D. Trauner, A. Hartschuh and T. Bein, J. Am. Chem. Soc., 2014, 136, 17802–17807 CrossRef CAS PubMed.
- S. Izawa, K. Nakano, K. Suzuki, K. Hashimoto and K. Tajima, Adv. Mater., 2015, 27, 3025–3031 CrossRef CAS PubMed.
- S. Jin, K. Furukawa, M. Addicoat, L. Chen, S. Takahashi, S. Irle, T. Nakamura and D. Jiang, Chem. Sci., 2013, 4, 4505 RSC.
- D. Bessinger, L. Ascherl, F. Auras and T. Bein, J. Am. Chem. Soc., 2017, 139, 12035–12042 CrossRef CAS PubMed.
- L. Yang and D. C. Wei, Chin. Chem. Lett., 2016, 27, 1395–1404 CrossRef CAS.
- Y. S. Kochergin, D. Schwarz, A. Acharjya, A. Ichangi, R. Kulkarni, P. Eliášová, J. Vacek, J. Schmidt, A. Thomas and M. J. Bojdys, Angew. Chem., Int. Ed., 2018, 57, 14188–14192 CrossRef CAS PubMed.
- K. Kailasam, M. B. Mesch, L. Möhlmann, M. Baar, S. Blechert, M. Schwarze, M. Schröder, R. Schomäcker, J. Senker and A. Thomas, Energy Technol., 2016, 4, 744–750 CrossRef CAS.
- J. Lee, O. Buyukcakir, T. Kwon and A. Coskun, J. Am. Chem. Soc., 2018, 140, 10937–10940 CrossRef CAS PubMed.
- B. Kiskan, J. Zhang, X. Wang, M. Antonietti and Y. Yagci, ACS Macro Lett., 2012, 1, 546–549 CrossRef CAS.
- S. Dadashi-Silab, H. Bildirir, R. Dawson, A. Thomas and Y. Yagci, Macromolecules, 2014, 47, 4607–4614 CrossRef CAS.
- P. Pachfule, A. Acharjya, J. Roeser, T. Langenhahn, M. Schwarze, R. Schomäcker, A. Thomas and J. Schmidt, J. Am. Chem. Soc., 2018, 140, 1423–1427 CrossRef CAS PubMed.
- E. L. Spitler, B. T. Koo, J. L. Novotney, J. W. Colson, F. J. Uribe-Romo, G. D. Gutierrez, P. Clancy and W. R. Dichtel, J. Am. Chem. Soc., 2011, 133, 19416–19421 CrossRef CAS PubMed.
- B. Lukose, A. Kuc and T. Heine, Chem.–Eur. J., 2011, 17, 2388–2392 CrossRef CAS PubMed.
- F. J. Uribe-Romo, J. R. Hunt, H. Furukawa, C. Klöck, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 4570–4571 CrossRef CAS PubMed.
- S.-Y. Ding, J. Gao, Q. Wang, Y. Zhang, W.-G. Song, C.-Y. Su and W. Wang, J. Am. Chem. Soc., 2011, 133, 19816–19822 CrossRef CAS PubMed.
- M. Kaur and A. K. Srivastava, J. Macromol. Sci., Part C: Polym. Rev., 2002, 42, 481–512 CrossRef.
- M. Chen, M. Zhong and J. A. Johnson, Chem. Rev., 2016, 116, 10167–10211 CrossRef CAS PubMed.
- J. Fouassier, X. Allonas and D. Burget, Prog. Org. Coat., 2003, 47, 16–36 CrossRef CAS.
- Y. Yagci, S. Jockusch and N. J. Turro, Macromolecules, 2010, 43, 6245–6260 CrossRef CAS.
- H. Sun and S. Kawata, in Adv. Polym. Sci., 2006, pp. 169–273 Search PubMed.
- H. L. Nguyen, F. Gándara, H. Furukawa, T. L. H. Doan, K. E. Cordova and O. M. Yaghi, J. Am. Chem. Soc., 2016, 138, 4330–4333 CrossRef CAS PubMed.
- S. Dadashi-Silab, C. Aydogan and Y. Yagci, Polym. Chem., 2015, 6, 6595–6615 RSC.
- G. Temel, B. Aydogan, N. Arsu and Y. Yagci, Macromolecules, 2009, 42, 6098–6106 CrossRef CAS.
- A. Ajayaghosh, R. Francis and S. Das, Eur. Polym. J., 1993, 29, 63–68 CrossRef CAS.
- J. Zhang, G. Zhang, X. Chen, S. Lin, L. Möhlmann, G. Dołęga, G. Lipner, M. Antonietti, S. Blechert and X. Wang, Angew. Chem., Int. Ed., 2012, 51, 3183–3187 CrossRef CAS PubMed.
- D. Hollmann, M. Karnahl, S. Tschierlei, K. Kailasam, M. Schneider, J. Radnik, K. Grabow, U. Bentrup, H. Junge, M. Beller, S. Lochbrunner, A. Thomas and A. Brückner, Chem. Mater., 2014, 26, 1727–1733 CrossRef CAS.
- D. Hollmann, F. Gärtner, R. Ludwig, E. Barsch, H. Junge, M. Blug, S. Hoch, M. Beller and A. Brückner, Angew. Chem., Int. Ed., 2011, 50, 10246–10250 CrossRef CAS PubMed.
- R. Sivasankaran, N. Rockstroh, D. Hollmann, C. Kreyenschulte, G. Agostini, H. Lund, A. Acharjya, J. Rabeah, U. Bentrup, H. Junge, A. Thomas and A. Brückner, Catalysts, 2018, 8, 52 CrossRef.
- M. Iwasaki and Y. Sakai, J. Polym. Sci., Part A-1: Polym. Chem., 1969, 7, 1537–1547 CrossRef CAS.
- A. Lund and M. Danilczuk, Spectrochim. Acta, Part A, 2012, 98, 367–377 CrossRef CAS PubMed.
- D. Mullangi, S. Shalini, S. Nandi, B. Choksi and R. Vaidhyanathan, J. Mater. Chem. A, 2017, 5, 8376–8384 RSC.
- R. Nomoto, Dent. Mater. J., 1997, 16, 60–73 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, synthetic procedures, radical polymerization details, structure modelling details, Tables S1–S6, and Fig. S1–S17. See DOI: 10.1039/c9sc02601k |
| This journal is © The Royal Society of Chemistry 2019 |