Open Access Article
Open Access ArticleA selective route to aryl-triphosphiranes and their titanocene-induced fragmentation†
André
Schumann
a,
Fabian
Reiß
 a,
Haijun
Jiao
a,
Haijun
Jiao
 a,
Jabor
Rabeah
a,
Jabor
Rabeah
 a,
Jan-Erik
Siewert
a,
Ivo
Krummenacher
bc,
Holger
Braunschweig
a,
Jan-Erik
Siewert
a,
Ivo
Krummenacher
bc,
Holger
Braunschweig
 bc and
Christian
Hering-Junghans
bc and
Christian
Hering-Junghans
 *a
*a
aLeibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany. E-mail: christian.hering-junghans@catalysis.de
bInstitut für Anorganische Chemie, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
cInstitute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
First published on 30th July 2019
Abstract
Triphosphiranes are three-membered phosphorus cycles and their fundamental reactivity has been studied in recent decades. We recently developed a high-yielding, selective synthesis for various aryl-substituted triphosphiranes. Variation of the reaction conditions in combination with theoretical studies helped to rationalize the formation of these homoleptic phosphorus ring systems and highly reactive intermediates could be isolated. In addition we showed that a titanocene synthon [Cp2Ti(btmsa)] facilitates the selective conversion of these triphosphiranes into titanocene diphosphene complexes. This unexpected reactivity mode was further studied theoretically and experimental evidence is presented for the proposed reaction mechanism.
Introduction
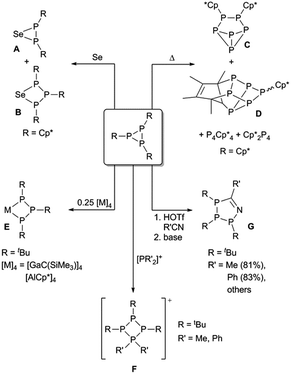
Triphosphiranes are three-membered cyclo-phosphines, which are promising synthons in inorganic chemistry (Scheme 1). As early as 1877 the first cyclic oligophosphine was synthesized by Köhler and Michaelis in an attempt to prepare a phosphorus analogue of azobenzene with a PP double bond.1 Almost 100 years later in 1964 the molecular structure of the product could be identified as P5Ph5 by X-ray crystal structure analysis.2 Although, Cowley et al. already mentioned the synthesis of P3(C2F5)3 in 1970,3 it was later discussed that in fact the tetramer and pentamer were formed under the reaction conditions described.4 The first stable triphosphirane P3tBu3 was reported by Baudler and co-workers in 1976,5,6 and various synthetic approaches towards triphosphiranes have since emerged.7 Reductive approaches starting from dihalophosphines RPX2 (X = Cl, Br) result in a mixture of oligophosphines of different ring sizes of PnRn (n = 3, 4, 5, 6) and are thus regarded as unspecific.8 The ratio of the different oligomers heavily depends on the steric demand of the substituent R.5,9 Cyclo-condensation reactions, which also allow the preparation of unsymmetrically substituted triphosphiranes, and cyclization by reductive dehalogenation of dihalotriphosphines have emerged as more selective synthetic pathways.10 Nevertheless, the presence of other cyclic oligophosphines as side products is often observed.Jutzi and co-workers have shown that selenium inserts into one P–P bond of P3Cp*3 (Cp* = pentamethylcyclopentadienyl), affording a mixture of cyclic selenotriphosphabutanes (Scheme 1, A) and cyclic selenodiphosphapropanes (Scheme 1, B).11 In contrast, thermolysis of P3Cp*3 in xylene resulted in the formation of different phosphorus clusters, some of which are structurally related to Hittorf's-phosphorus (Scheme 1, C and D).12 Ring expansion reactions were reported by Uhl and Benter by the insertion of Ga(I) into a P–P bond of P3tBu3, thus establishing a way to prepare cyclo-galliumtriphosphabutanes (Scheme 1, E, M = Ga).13 A similar reactivity is observed when Al(I) compound (AlCp*)4 reacts with P3tBu3 (Scheme 1, E, M = Al).14 In addition, the reaction of P3tBu3 with PMe2Cl or PPh2Cl in the presence of Me3SiOTf or GaCl3, respectively, resulted in the selective ring expansion with insertion of [PMe2]+ into the P–P bond between the two identical P atoms of P3tBu3 to afford [R2P(P3tBu3)]+ (Scheme 1, F; R = Me, Ph).15,16 More recently, Manners and co-workers showed the addition of P3tBu3 to organic nitriles after activation of the three-membered ring by electrophiles to yield differently substituted 1-aza-2,3,4-triphospholenes in a click-type reaction (Scheme 1, G),17,18 underlining the value of triphosphiranes as synthons in synthetic inorganic chemistry. Fragmentation of P3tBu3 was observed by Fenske and Ahlrichs in the reaction with Ni(CO)4, resulting in the formation of [Ni5(PtBu)3(P3tBu3)(CO)5] with μ4- and μ3-bridging PtBu ligands as well as a P3tBu3 chain, acting as a μ4(η2,η1′,η2′′) ligand to three Ni atoms of the cluster.19
To the best of our knowledge, only four aryl-substituted triphosphiranes are reported in the literature. P3Ph3 was described as early as 1973 as a labile solid with respect to P5Ph5,20 and it has been shown that this compound is part of an equilibrium mixture consisting of different oligomers with ring sizes of n = 3, 4, 5, 6.21 Tokitoh et al. synthesized (Anth = 9-anthryl, Bbt = 2,6-bis[bis-(trimethylsilyl)methyl]-4-[tris(trimethylsilyl)phenyl]) in good yield by heating a mixture of AnthP![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PBbt and nBu3P
PBbt and nBu3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Te.22 P3Tipp3 (Tipp = 2,4,6-iPr3C6H2) and P3Mes3 (Mes = 2,4,6-Me3C6H2) were described as one of a mixture of products when free phosphinidenes were generated by reductive dechlorination of RPCl2 (R = Tipp, Mes).23–25 Moreover, Gaspar and co-workers reported on the photochemical release of the triplet phosphinidene MesP from MesP(C2H4) in 1992.26 In the absence of a trapping reagent these triplet phosphinidenes oligomerize to give a mixture containing P3Mes3 and P4Mes4.
Te.22 P3Tipp3 (Tipp = 2,4,6-iPr3C6H2) and P3Mes3 (Mes = 2,4,6-Me3C6H2) were described as one of a mixture of products when free phosphinidenes were generated by reductive dechlorination of RPCl2 (R = Tipp, Mes).23–25 Moreover, Gaspar and co-workers reported on the photochemical release of the triplet phosphinidene MesP from MesP(C2H4) in 1992.26 In the absence of a trapping reagent these triplet phosphinidenes oligomerize to give a mixture containing P3Mes3 and P4Mes4.
Using [W(PMe3)6] as a reducing agent the quantitative coupling of RPCl2 (R = Mes* = 2,4,6-tBuC6H2; 2,4,6-(CF3)3C6H2) to the respective diphosphenes RP![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PR was detected. Starting from TippPCl2, the initial formation of the diphosphene is detected by 31P NMR spectroscopy, however, the reaction continues to produce Tipp3P3 as the final product, clearly pointing to the intermediacy of W
PR was detected. Starting from TippPCl2, the initial formation of the diphosphene is detected by 31P NMR spectroscopy, however, the reaction continues to produce Tipp3P3 as the final product, clearly pointing to the intermediacy of W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PR species.27 Moreover, it was shown that the reductive degradation of P4 with mesityl-radicals (generated from Mes-Br and Ti(III)-based chlorine atom abstracting reagent [Ti{N(tBu)(3,5-C6H3Me2)}3]) yields P3Mes3 as the main product in good isolated yields.28
PR species.27 Moreover, it was shown that the reductive degradation of P4 with mesityl-radicals (generated from Mes-Br and Ti(III)-based chlorine atom abstracting reagent [Ti{N(tBu)(3,5-C6H3Me2)}3]) yields P3Mes3 as the main product in good isolated yields.28
In 1998 Shah and Protasiewicz reported the formation of the triphosphirane P3Tipp3 (1a) by treatment of TippPCl2 with PMe3 and Zn and subsequent reaction with benzaldehyde (Scheme 2).29 This so-called phospha-Wittig reaction afforded a mixture of P3Tipp3 and traces of the desired phosphaalkene Ph(H)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PTipp.
PTipp.
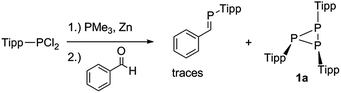 | ||
Scheme 2 Formation of Tipp3P3 (1a) and trace amounts of phosphaalkene H(Ph)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PTipp in a so-called phospha-Wittig protocol. PTipp in a so-called phospha-Wittig protocol. | ||
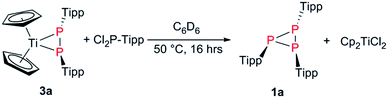
In this contribution, we report on the synthesis of aryl substituted triphosphiranes using a modified synthesis on the basis of the studies by Protasiewicz et al. Furthermore, we report on the selective degradation of these P3Ar3 systems using [Cp2Ti(btmsa)] (Cp = cyclopentadienyl, btmsa = C2(SiMe3)2) as a Ti(II) synthon.
Results
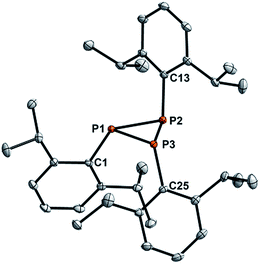
In an attempt to prepare new variants of pyridinephosphaalkenes,30 we utilized the phospha-Wittig protocol described by Protasiewicz et al. with DippPCl2 (Dipp = 2,6-iPr2C6H3), PMe3 and excess of Zn powder in a strict low-temperature regime (−78 °C); after subsequent treatment with pyridine-2-carbaldehyde at that temperature and warming to room temperature the formation of the respective phosphaalkene was not observed. The 31P NMR spectrum of the reaction mixture displayed a major product with an A2B spin system with a doublet at −99.47 ppm and a triplet at −132.90 ppm with a coupling constant of 178.5 Hz, which was identified as P3Dipp3 (1b), in line with attempted synthesis of the phospha-Wittig reagent TippPPMe3 as discussed before.29 X-ray quality crystals of 1b were grown from a saturated n-hexane solution at 5 °C (Fig. 1). 1b crystallises in the monoclinic space group P21/c with four molecules in the unit cell. The molecular structure of 1b shows the expected down-down-up orientation of the Dipp groups with respect to the central P3 plane, with a minimally distorted central P3-ring [P1–P2 2.1991(4), P2–P3 2.2440(4); P1–P3 2.2124(3) Å] (Fig. 1). These metric parameters are in line with those detected for 1a and 1c (Table S1†),31 of which the molecular structures have been reported previously.32,33We then utilized the sterically more demanding PEt3 to better stabilize the reactive phosphanylidenephosphorane intermediate TippP![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PEt3. Phosphanylidenephosphoranes have been identified as a source of the triplet phoshinidenes Ar–P.34
PEt3. Phosphanylidenephosphoranes have been identified as a source of the triplet phoshinidenes Ar–P.34
Additionally, we switched to TippPBr2, as its reduction should be more facile. TippPBr2, PEt3 (1.2 equiv.) and Zn (3 equiv.) were combined in THF at −78 °C and the formation of a deep yellow to orange suspension was observed, which again showed P3Tipp3 (1a) as the major species in the 31P NMR spectrum.
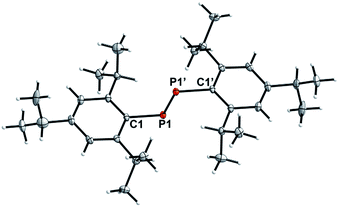
After removal of the solvent and extraction with n-hexane minimal amounts (<0.01 g) of yellow needles suitable for single crystal X-ray analysis were obtained and identified as the elusive diphosphene P2Tipp2 (2) (Fig. 2), which has only been observed in solution in the [W(PMe3)6] mediated coupling of ArPCl2 (Ar = Tipp, Mes*, 2,4,6-(CF3)3C6H2) by 31P NMR experiments to date.27 The 31P NMR spectrum of isolated 2 showed P2Tipp2 (δ(31P) = 517.4 ppm) to be the major species, whereas minor amounts of P3Tipp3 and P4Tipp4 were also detected. Monitoring a C6D6 solution of 2 over time at room temperature revealed that P2Tipp2 slowly coverts into P3Tipp3 and its dimer P4Tipp4, vide infra.312 crystallises as its trans-conformer in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with one molecule in the unit cell. The P1–P1′ distance [2.0290(5) Å] (cf. d(P
with one molecule in the unit cell. The P1–P1′ distance [2.0290(5) Å] (cf. d(P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P) P2Mes*2 2.034(2);35 P2Ter2 2.029(1);36 P2Bbt2 2.043(1)37) is in the expected range for a diphosphene (∑rcov(P
P) P2Mes*2 2.034(2);35 P2Ter2 2.029(1);36 P2Bbt2 2.043(1)37) is in the expected range for a diphosphene (∑rcov(P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P) = 2.04 Å),38 and rather acute C–P–P′ [99.61(3)°] angles at the dicoordinate P center are detected.
P) = 2.04 Å),38 and rather acute C–P–P′ [99.61(3)°] angles at the dicoordinate P center are detected.
Theoretical investigations at the M062X/TZVP level of density functional theory were carried out, assuming that transient phosphinidenes are formed. The gas-phase trimerization of Dipp-P with a triplet ground state (the corresponding singlet state is less stable by 26.01 kcal mol−1) is exergonic (−91.39 kcal mol−1). In addition, we computed the transfer reaction of a Dipp-P fragment (which may be formed intermediately at low temperatures) via DippPPMe3 to P2Dipp2 and found this reaction to be exergonic by −15.74 kcal mol−1 (energy barriers were not calculated). This is in line with the isolation of 2.
Since there are only few high-yielding, selective methods for the preparation of aryl-substituted triphosphiranes outlined in the literature, we decided to take a closer look at this synthetic approach. We therefore tested different aryl(dichloro)phosphines ArPCl2 (Ar = Mes, Dipp, Tipp) to elucidate whether treatment with PR3 (R = Me, Et) and Zn gives general access to aryl-substituted triphosphiranes (Scheme 3).
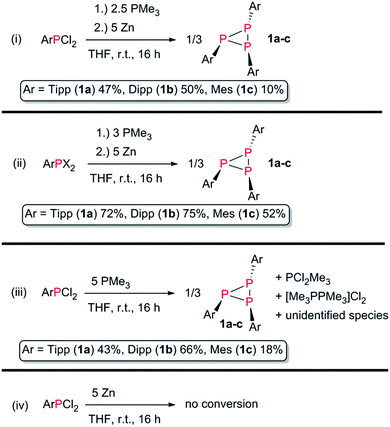 | ||
| Scheme 3 (i and ii) General procedure for the preparation of 1a–c; (iii) identification of PMe3 as the active reductant; (iv) Zn can be excluded as active reductant. | ||
The reaction of ArPCl2 with PMe3 (2.5 equiv.) and an excess of Zn (5 equiv.) in anhydrous THF afforded P3Ar3 (Ar = Tipp (1a), Dipp (1b), Mes (1c)) as expected (Scheme 3, reaction (i)).
Purification by recrystallisation from a saturated n-hexane solution at 5 °C yielded 1a–c as colourless crystalline solids in 47, 50 and 10% isolated yield, respectively.
Starting from the easily accessible mixed dihalophosphines ArPX2 (Ar = Tipp, Dipp, Mes; X = Cl, Br; obtained through treatment of ArMgBr with PCl3),39 with PMe3 and Zn in a 1/2/2.5 molar ratio in THF at room temperature (Scheme 3, reaction (ii)), 1a, 1b and 1c could be obtained in up to 72%, 75% and 52% isolated yield, respectively, after extraction with benzene or Et2O in case of 1c. 1a–c show good thermal stability with melting points of higher than 167 °C.31 Heating a solution of 1a in C6D6 for 36 h at 80 °C showed no decomposition or rearrangement products in the 31P NMR spectrum.
Since either PMe3 or Zn can act as reducing agents, we reduced TippPCl2 with each reductant separately (Scheme 3(iii) and (iv)). While there is no reaction observed, when TippPCl2 or TippPBr2 are stirred with an excess of Zn in THF over a period of 24 h, treatment of ArPCl2 with a fivefold excess of PMe3 afforded 1a–c in 43, 66 and 18% isolated yield, respectively. The potential of PMe3 to act as a chlorine abstracting reagent is documented in the literature and results in oxidation to the respective dichlorophosphorane,40,41 or the homoleptic dication salt [Me3PPMe3]2Cl2. This concept has been used to access cyclo-tetra(stibinophosphonium) triflate salts of the type [Sb4(PR3)4][OTf]4 (R = Me, Et, Pr, Bu), cationic antimony compounds related to the cyclic oligophosphines.42 To shed light on this proposition, we independently synthesized PMe3Cl2 and treated it with an excess of zinc dust in the presence of TippPCl2 in a mixture of MeCN/THF (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) over 24 h. A 31P NMR spectrum of the reaction mixture indeed showed 1a to be the main product of this reaction.31 It can thus be concluded that PMe3Cl2 is a plausible by-product of the reduction with PMe3 and zinc can reduce it back to PMe3, vide infra. This opens the pathway for potential catalytic reduction of ArPCl2 with PMe3 and Zn as a sacrificial reductant. In another experiment DippPCl2 was reduced with an excess of PMe3 and the white precipitate was carefully washed with benzene and n-hexane. Subsequently, the precipitate was treated with AgOTf in CH2Cl2. After filtration a colourless solid was obtained, which was dissolved in CD3CN, allowing to unambiguously identify [Me3PCl]OTf (δ31P{1H} = 93.6 ppm),43 and [Me3P–PMe3][OTf]2 (δ31P{1H} = 28.4 ppm)44 among three unidentified PMe3 containing species (Scheme 3(iii)).31
1) over 24 h. A 31P NMR spectrum of the reaction mixture indeed showed 1a to be the main product of this reaction.31 It can thus be concluded that PMe3Cl2 is a plausible by-product of the reduction with PMe3 and zinc can reduce it back to PMe3, vide infra. This opens the pathway for potential catalytic reduction of ArPCl2 with PMe3 and Zn as a sacrificial reductant. In another experiment DippPCl2 was reduced with an excess of PMe3 and the white precipitate was carefully washed with benzene and n-hexane. Subsequently, the precipitate was treated with AgOTf in CH2Cl2. After filtration a colourless solid was obtained, which was dissolved in CD3CN, allowing to unambiguously identify [Me3PCl]OTf (δ31P{1H} = 93.6 ppm),43 and [Me3P–PMe3][OTf]2 (δ31P{1H} = 28.4 ppm)44 among three unidentified PMe3 containing species (Scheme 3(iii)).31
The synthetic approach using Zn/PMe3 showed a high selectivity towards the respective triphosphiranes. In the case of 1a and 1b just little amounts of the corresponding cyclic tetraphosphines P4Ar4 were detected as side products by 31P NMR spectroscopy of the reaction mixture. When MesPCl2 is applied in our approach, the selectivity decreases and the formation of little amounts of the cyclic tetraphosphine P4Mes4, and the cyclic pentaphosphine P5Mes5 species can be detected. We conclude that this is due to lesser steric bulk imposed by the mesityl substituent. The sterically more demanding substituents Tipp and Dipp promote the formation of the three-membered phosphorus ring more effectively.7
Having prepared 1a–c we wanted to explore their reactivity with the titanocene synthon [Cp2Ti(btmsa)] in order to access titanium phosphinidene complexes.
Titanocene-induced degradation of R3P3
Stephan and co-workers have shown the phospha-Wittig-type phosphinidene transfer for [Cp2Zr![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PMes*(PMe3)] resulting in the formation of phosphaalkenes in the reaction with aldehydes along with the formation of [Cp2ZrO]n.45 Similar reactivity was observed by Cummins and Schrock for the terminal tantalum phosphinidene complexes, [(N3N)Ta
PMes*(PMe3)] resulting in the formation of phosphaalkenes in the reaction with aldehydes along with the formation of [Cp2ZrO]n.45 Similar reactivity was observed by Cummins and Schrock for the terminal tantalum phosphinidene complexes, [(N3N)Ta![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PR] (N3N = (Me3Si–NCH2CH2)3N).46
PR] (N3N = (Me3Si–NCH2CH2)3N).46
With the series of triphosphiranes 1a–c synthesized, we wanted to investigate the propensity to access monomeric, terminal Cp2Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PR complexes, by reaction of 1 with the titanocene synthon [Cp2Ti(btmsa)]. Cp2Ti
PR complexes, by reaction of 1 with the titanocene synthon [Cp2Ti(btmsa)]. Cp2Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PR has not been described in the literature. There are reports of neutral and zwitterionic terminal titanium phosphinidene complexes of the type [(ArNacnac)Ti
PR has not been described in the literature. There are reports of neutral and zwitterionic terminal titanium phosphinidene complexes of the type [(ArNacnac)Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PAr′(R)] (Ar′ = Tipp, Mes*; R = CH2tBu, CH3, CH3[B(C6F5)3]) by Mindiola and co-workers with a bulky β-diketiminate ligand (ArNacnac
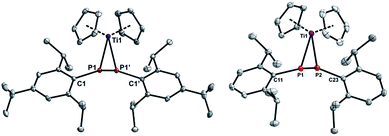
PAr′(R)] (Ar′ = Tipp, Mes*; R = CH2tBu, CH3, CH3[B(C6F5)3]) by Mindiola and co-workers with a bulky β-diketiminate ligand (ArNacnac![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) [Ar]NC(Me)CHC(Me)N[Ar], Ar = Dipp) on titanium.47,48 [Cp2Ti(btmsa)] is obtained by reduction of Cp2TiCl2 in the presence of btmsa. In these complexes btmsa acts as a spectator ligand and its facile release under the respective reaction conditions generates the highly reactive 14-electron [Cp2Ti] fragment in situ.49 Combination of three equivalents [Cp2Ti(btmsa)] with 1b in C6D6 at room temperature and monitoring by 31P NMR spectroscopy revealed slow, but selective, conversion into a phosphorus-containing species with a singlet resonance at 283.8 ppm. Heating this reaction mixture to 80 °C over a period of 16 h in a sealed NMR tube resulted in consumption of [Cp2Ti(btmsa)] according to 1H NMR spectroscopy. However, unreacted P3Ar3 remained in the reaction mixture and thus, more [Cp2Ti(btmsa)] was added to the reaction mixture and heating to 80 °C was continued. Fractional crystallisation from C6D6 and determination of the molecular structure by single crystal X-ray analysis revealed the formation of the η2-diphosphene complex [Cp2Ti(P2Dipp2)] (3b) (Fig. 3, right). Consequently, the reaction was repeated in the correct stoichiometry with [Cp2Ti(btmsa)] and 1b in a 3
[Ar]NC(Me)CHC(Me)N[Ar], Ar = Dipp) on titanium.47,48 [Cp2Ti(btmsa)] is obtained by reduction of Cp2TiCl2 in the presence of btmsa. In these complexes btmsa acts as a spectator ligand and its facile release under the respective reaction conditions generates the highly reactive 14-electron [Cp2Ti] fragment in situ.49 Combination of three equivalents [Cp2Ti(btmsa)] with 1b in C6D6 at room temperature and monitoring by 31P NMR spectroscopy revealed slow, but selective, conversion into a phosphorus-containing species with a singlet resonance at 283.8 ppm. Heating this reaction mixture to 80 °C over a period of 16 h in a sealed NMR tube resulted in consumption of [Cp2Ti(btmsa)] according to 1H NMR spectroscopy. However, unreacted P3Ar3 remained in the reaction mixture and thus, more [Cp2Ti(btmsa)] was added to the reaction mixture and heating to 80 °C was continued. Fractional crystallisation from C6D6 and determination of the molecular structure by single crystal X-ray analysis revealed the formation of the η2-diphosphene complex [Cp2Ti(P2Dipp2)] (3b) (Fig. 3, right). Consequently, the reaction was repeated in the correct stoichiometry with [Cp2Ti(btmsa)] and 1b in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio in benzene, which allowed for full conversion into 3b after stirring at 80 °C over a period of 16 h. In analogy, 1a and 1c were converted into the respective titanocene diphosphene complexes [Cp2Ti(P2Tipp2)] (3a, Fig. 3, left) and [Cp2Ti(P2Mes2)] (3c) (Scheme 4). Filtration and subsequent concentration of the reaction mixtures and standing overnight at 5 °C resulted in the formation of deep yellow crystals of 3a suitable for X-ray analysis, whereas formation of 3c was authenticated by NMR spectroscopy, elemental analysis and HR-MS studies.31 Interestingly, in the 1H NMR spectrum three or two independent septets are detected for 3a and 3b, respectively. This indicates hindered rotation about the P–CAr bond and the Me group of the isopropyl moiety in close proximity to the Cp2Ti-fragment is significantly upfield-shifted, resonating at −0.99 ppm in 3a and 3b. This hindered rotation is also evident in 3c, in which three 1H NMR signals are detected for the Me groups of the Mes moiety.
2 molar ratio in benzene, which allowed for full conversion into 3b after stirring at 80 °C over a period of 16 h. In analogy, 1a and 1c were converted into the respective titanocene diphosphene complexes [Cp2Ti(P2Tipp2)] (3a, Fig. 3, left) and [Cp2Ti(P2Mes2)] (3c) (Scheme 4). Filtration and subsequent concentration of the reaction mixtures and standing overnight at 5 °C resulted in the formation of deep yellow crystals of 3a suitable for X-ray analysis, whereas formation of 3c was authenticated by NMR spectroscopy, elemental analysis and HR-MS studies.31 Interestingly, in the 1H NMR spectrum three or two independent septets are detected for 3a and 3b, respectively. This indicates hindered rotation about the P–CAr bond and the Me group of the isopropyl moiety in close proximity to the Cp2Ti-fragment is significantly upfield-shifted, resonating at −0.99 ppm in 3a and 3b. This hindered rotation is also evident in 3c, in which three 1H NMR signals are detected for the Me groups of the Mes moiety.
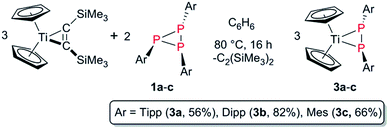 | ||
| Scheme 4 Selective degradation of P3Ar3 (1a–c) into [Cp2Ti(P2Ar2)] (3a–c) complexes using [Cp2Ti(btmsa)] as a synthon for [Cp2Ti]. | ||
3a crystallises in the monoclinic space group C2/c with four molecules in the unit cell as a benzene solvate. 3b crystallises in the monoclinic space group P21/c with four molecules of 3b and four C6D6 molecules in the unit cell. 3a is located on a special position and thus shows C2 symmetry in the solid state. The P–P distances in 3a [2.1826(7) Å] and 3b [2.1699(5) Å] are intermediate between a P–P single and double bond (∑rcov(P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P) = 2.04 Å; (P–P) 2.22 Å)38 and are in line with the P–P distance [2.173(4) Å] in [rac-(EBTHI)Ti(P2Ph2)] (ETBHI = ethylene-1,2-bis(5-4,5,6,7-tetrahydro-1-indenyl)), the only titanium diphosphene complex known to date.50 It is worth noting that green [rac-(EBTHI)Ti(P2Ph2)] is insoluble in common non-halogenated organic solvents and thus, NMR data was not obtained. It is formed through the dehydrocoupling of PhPH2 in the presence of the Ti(III)-hydride dimer [rac-(EBTHI)-TiH]2.51 η2-Diphosphene complexes of various transition metals have been known and were thoroughly reviewed by Weber.52 Noteworthy, is the formation of [(Ph3P)2M(P2{C6F5}2)] with an E-configured diphosphene ligand by the degradation of cyclic tetraphosphine P4(C6F5)4 in the presence of M(PPh3)4 (M = Pt,53 Pd54). Other known diphosphene complexes of group 4 include the anionic species [Cp2Zr(PPh)2Br]− with a P–P distance [2.145(3) Å] shorter than in 3a and 3b,55 and the related Mes-substituted complex [Cp2Zr(P2Mes2)] with a similar P–P distance [2.188(3) Å].56 The Ti–P distances in 3a [2.5425(5), 2.5230(5) Å] and 3b [2.5329(5)) Å], as well as the P–Ti–P angles (3a 50.725(12)°; 3b 51.042(17)°), are similar to that in [Cp2Zr(PPh)2Br]− [d(Ti–P) 2.525(2) Å; <(P–Ti–P) 51.00(6)°] and point to a Ti(IV) center and an overall titana-cyclo-propane, rather than a titana-cyclo-propene type structure.
P) = 2.04 Å; (P–P) 2.22 Å)38 and are in line with the P–P distance [2.173(4) Å] in [rac-(EBTHI)Ti(P2Ph2)] (ETBHI = ethylene-1,2-bis(5-4,5,6,7-tetrahydro-1-indenyl)), the only titanium diphosphene complex known to date.50 It is worth noting that green [rac-(EBTHI)Ti(P2Ph2)] is insoluble in common non-halogenated organic solvents and thus, NMR data was not obtained. It is formed through the dehydrocoupling of PhPH2 in the presence of the Ti(III)-hydride dimer [rac-(EBTHI)-TiH]2.51 η2-Diphosphene complexes of various transition metals have been known and were thoroughly reviewed by Weber.52 Noteworthy, is the formation of [(Ph3P)2M(P2{C6F5}2)] with an E-configured diphosphene ligand by the degradation of cyclic tetraphosphine P4(C6F5)4 in the presence of M(PPh3)4 (M = Pt,53 Pd54). Other known diphosphene complexes of group 4 include the anionic species [Cp2Zr(PPh)2Br]− with a P–P distance [2.145(3) Å] shorter than in 3a and 3b,55 and the related Mes-substituted complex [Cp2Zr(P2Mes2)] with a similar P–P distance [2.188(3) Å].56 The Ti–P distances in 3a [2.5425(5), 2.5230(5) Å] and 3b [2.5329(5)) Å], as well as the P–Ti–P angles (3a 50.725(12)°; 3b 51.042(17)°), are similar to that in [Cp2Zr(PPh)2Br]− [d(Ti–P) 2.525(2) Å; <(P–Ti–P) 51.00(6)°] and point to a Ti(IV) center and an overall titana-cyclo-propane, rather than a titana-cyclo-propene type structure.
The surprising selective formation of the titanocene diphosphene species 3, prompted us to study the reactivity by DFT calculations on the M062X/TZVP level of theory. The calculated gas phase structure of 1b and 3b and the metric parameters derived from X-ray crystallography are in good agreement. In a next step the reaction of [Cp2Ti(btmsa)] with 1b in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio was investigated. It is found that the gas phase reaction is exergonic by −15.93 kcal mol−1, indicating that the reaction is accessible thermodynamically, even though energy barriers for this transformation could not be determined (Scheme 5(i)). Using the truncated model compound P3Ph3 (1Ph) the same exergonic character was calculated (ΔG = −18.32 kcal mol−1) for this transformation. Additionally, we were interested to determine whether the free trans-diphosphenes P2Dipp2 and P2Ph2 can displace the btmsa molecule in [Cp2Ti(btmsa)] to afford complexes 3b and [Cp2Ti(P2Ph2)] (3Ph), respectively (Scheme 7, bottom). Interestingly, this reaction is also exergonic for P2Dipp2 and P2Ph2 by −10.69 and −20.87 kcal mol−1, respectively, illustrating that diphosphenes are potential intermediates along the reaction pathway (Scheme 5(iv)).
2 ratio was investigated. It is found that the gas phase reaction is exergonic by −15.93 kcal mol−1, indicating that the reaction is accessible thermodynamically, even though energy barriers for this transformation could not be determined (Scheme 5(i)). Using the truncated model compound P3Ph3 (1Ph) the same exergonic character was calculated (ΔG = −18.32 kcal mol−1) for this transformation. Additionally, we were interested to determine whether the free trans-diphosphenes P2Dipp2 and P2Ph2 can displace the btmsa molecule in [Cp2Ti(btmsa)] to afford complexes 3b and [Cp2Ti(P2Ph2)] (3Ph), respectively (Scheme 7, bottom). Interestingly, this reaction is also exergonic for P2Dipp2 and P2Ph2 by −10.69 and −20.87 kcal mol−1, respectively, illustrating that diphosphenes are potential intermediates along the reaction pathway (Scheme 5(iv)).
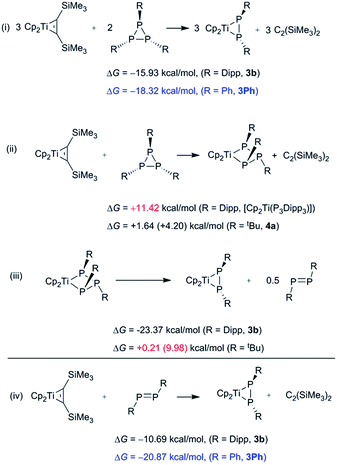 | ||
| Scheme 5 M062X/TZVP (i and iv) and BP86/TZVP ((ii and iii) (M062X) for R = tBu) computed reaction free energies for possible paths of formation of [Cp2Ti(P2R2)] in the gas phase. | ||
With minimal amounts of the free diphosphene Tipp2P2 (2) in hand, we treated 2 with [Cp2Ti(btmsa)] in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio at room temperature in C6D6. Having shown that the reaction of 1a with [Cp2Ti(btmsa)] is slow at room temperature and full conversion is only achieved at 80 °C, we were delighted to see the disappearance of the diagnostic diphosphene signal at 517.4 ppm and formation of 3a with a characteristic 31P NMR shift of 290.7 ppm. This clearly shows, that diphosphenes are potential intermediates in the reaction of 1 with [Cp2Ti(btmsa)]. Furthermore, this shows the drastic influences of the sterically demanding groups attached to phosphorus, as the diphosphene P2Mes*2 was shown to not afford the respective diphosphene complex in the reaction with [Cp2Ti(btmsa)].57
1 ratio at room temperature in C6D6. Having shown that the reaction of 1a with [Cp2Ti(btmsa)] is slow at room temperature and full conversion is only achieved at 80 °C, we were delighted to see the disappearance of the diagnostic diphosphene signal at 517.4 ppm and formation of 3a with a characteristic 31P NMR shift of 290.7 ppm. This clearly shows, that diphosphenes are potential intermediates in the reaction of 1 with [Cp2Ti(btmsa)]. Furthermore, this shows the drastic influences of the sterically demanding groups attached to phosphorus, as the diphosphene P2Mes*2 was shown to not afford the respective diphosphene complex in the reaction with [Cp2Ti(btmsa)].57
To compare the reactivity of the aryl-substituted triphosphiranes with alkyl-substituted derivatives we treated [Cp2Ti(btmsa)] with the known triphosphiranes P3tBu3 (1d) and P3Ad3 (Ad = adamantyl),58,59 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
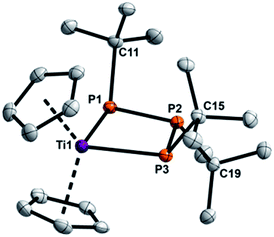
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in benzene at 80 °C in C6D6 (Scheme 6). Interestingly, in the case of 1d full consumption of both starting materials was noted, with a new characteristic A2B spin system in the 31P NMR spectrum. 1e also cleanly reacted in similar fashion, however full consumption was not achieved due to the poor solubility of 1e. Compared to 1d and 1e the A2-part of the 31P NMR signal is downfield-shifted, thus indicating selective insertion into the P–P bond with the two identical P atoms and the formation of the triphosphanato-complexes [Cp2Ti(P3tBu3)] (4a) and [Cp2Ti(P3Ad3)] (4b).31 Complex 4a among other [Cp2Ti(P3R3)] species has been described before by Köpf and co-workers in the reaction of Cp2TiCl2 with the salt K2[P4tBu4] in a salt elimination reaction on the basis of NMR experiments.60 Extraction of the reaction mixture with Et2O, concentration to incipient crystallisation and standing at 5 °C overnight, afforded deeply coloured brown crystals of 4a suitable for X-ray analysis (Fig. 4) in 64% yield. To the best of our knowledge this is the first structural characterization of a cyclo-titanatriphosphine.
1 ratio in benzene at 80 °C in C6D6 (Scheme 6). Interestingly, in the case of 1d full consumption of both starting materials was noted, with a new characteristic A2B spin system in the 31P NMR spectrum. 1e also cleanly reacted in similar fashion, however full consumption was not achieved due to the poor solubility of 1e. Compared to 1d and 1e the A2-part of the 31P NMR signal is downfield-shifted, thus indicating selective insertion into the P–P bond with the two identical P atoms and the formation of the triphosphanato-complexes [Cp2Ti(P3tBu3)] (4a) and [Cp2Ti(P3Ad3)] (4b).31 Complex 4a among other [Cp2Ti(P3R3)] species has been described before by Köpf and co-workers in the reaction of Cp2TiCl2 with the salt K2[P4tBu4] in a salt elimination reaction on the basis of NMR experiments.60 Extraction of the reaction mixture with Et2O, concentration to incipient crystallisation and standing at 5 °C overnight, afforded deeply coloured brown crystals of 4a suitable for X-ray analysis (Fig. 4) in 64% yield. To the best of our knowledge this is the first structural characterization of a cyclo-titanatriphosphine.
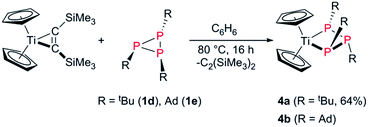 | ||
| Scheme 6 Formation of the cyclo-titanatriphosphabutanes [Cp2Ti(P3R3)] (R = tBu (4a), Ad (4b)) starting from [Cp2Ti(btmsa)] and triphosphiranes 1d and 1e. | ||
4a crystallises in the orthorhombic space group P212121 with four molecules in the unit cell. The P–P distances [P1–P2 2.1953(8), P2–P3 2.1840(8)] are shorter than a P–P single bond (∑rcov(P–P) = 2.22 Å)38 and the P–Ti–P angle [90.34(2)°] is wider than in 3a and 3b and compares nicely with the P–Zr–P angle [89.8(2)°] found in the related compound [Cp2Zr(P3Ph3)].55
To rationalize the contrasting reactivity of alkyl- and aryl-substituted triphosphiranes noted in this study, we calculated the free enthalpies for the gas phase reaction of [Cp2Ti(btmsa)] with Dipp3P3 to afford the insertion product [Cp2Ti(P3Dipp3)] under liberation of btmsa at the BP86//TZVP/LANL2DZ level of theory.31 This transformation was found to be endergonic by 11.41 kcal mol−1, whereas this insertion process was computed to be almost thermo-neutral for P3tBu3 (+1.64 (+4.20 M062X) kcal mol−1) to give 4a (Scheme 5(ii)). The selective degradation of [Cp2Ti(P3Dipp3)] to yield 3b and half an equivalent of P2Dipp2 was also considered and is shown to be exergonic by −23.27 kcal mol−1, whereas the same process is endergonic by +0.21 (+9.97 M062X) kcal mol−1 for 4a (Scheme 5(iii)). These results are in line with the observed difference in reactivity of alkyl- and aryl-substituted triphosphiranes and that the reactions only take place at elevated temperatures. We then wanted to determine whether single electron transfer (SET) is preferred over reduction of the cyclo-P3R3 in two electron steps by comparison of the free energies of the reduction products. It is noted from successive theoretical one-electron addition to triphosphiranes P3R3 that the single-electron transfer step is exergonic and favoured thermodynamically, while the two-electron transfer process is endergonic and thermodynamically not favored.31
On the basis of these results, one can expect a stepwise reaction mechanism for the electron transfer reactions. Furthermore one of the P–P bonds in the radical anion species [P3R3]˙− is considerably elongated [2.814 (R = Dipp), 2.973 Å (R = Ph)], which would allow for the liberation of a phosphinidene fragment or the recombination of two radical anions, under formal exchange of P–R groups. If arylphosphinidenes were formed in this transformation these would be triplet species, with the triplet state being thermodynamically favored by −26.01 (R = Dipp) and −33.71 kcal mol−1 (R = Ph), respectively. With these insights we set out to generate experimental evidence for these assumptions.
On the basis of these results, one can expect a stepwise reaction mechanism for the electron transfer reactions and the possible intermediary formation of a titanocene phosphinidene species. Electrochemical studies revealed an electrochemically irreversible reduction of 1b in THF at a potential of −3.09 V (vs. Fc/Fc+), which is in line with degradation of the aryl-substituted triphosphiranes into diphosphene fragments upon treatment with [Cp2Ti(btmsa)]. Investigation of the reaction mixture of [Cp2Ti(btmsa)] and 1a (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
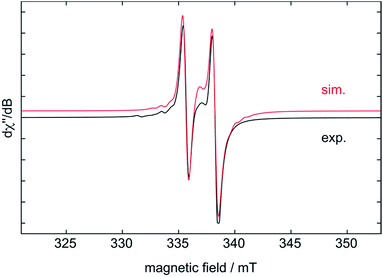
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio, after heating to 80 °C for 1 h) at room temperature by electron paramagnetic resonance (EPR) spectroscopy revealed the occurrence of an EPR-active intermediate (Fig. 5) with an isotropic g-factor of 1.978. This doublet signal shows strong coupling to one 31P nucleus with a(31P) = 72 MHz and hyperfine coupling to titanium a(49/47Ti) = 22 MHz. The rather large g-value and small hyperfine coupling to Ti indicates a species with a high spin density on phosphorus, in which only one phosphorus is attached to titanium, as a more complex EPR-signal would be expected otherwise.61 In addition, there is an underlying signal stemming from [Cp2Ti(btmsa)], which could be fitted to a species with giso = 1.973 and a(1H) = 32 MHz.62 This could indicate a hydridic species such as [Cp2Ti(III)–H], which has been discussed as resting state of [Cp2Ti] in solution. In this case hydrogen release would generate the free titanocene and subsequent addition of H2 regenerates the [Cp2TiH] species.63
2 ratio, after heating to 80 °C for 1 h) at room temperature by electron paramagnetic resonance (EPR) spectroscopy revealed the occurrence of an EPR-active intermediate (Fig. 5) with an isotropic g-factor of 1.978. This doublet signal shows strong coupling to one 31P nucleus with a(31P) = 72 MHz and hyperfine coupling to titanium a(49/47Ti) = 22 MHz. The rather large g-value and small hyperfine coupling to Ti indicates a species with a high spin density on phosphorus, in which only one phosphorus is attached to titanium, as a more complex EPR-signal would be expected otherwise.61 In addition, there is an underlying signal stemming from [Cp2Ti(btmsa)], which could be fitted to a species with giso = 1.973 and a(1H) = 32 MHz.62 This could indicate a hydridic species such as [Cp2Ti(III)–H], which has been discussed as resting state of [Cp2Ti] in solution. In this case hydrogen release would generate the free titanocene and subsequent addition of H2 regenerates the [Cp2TiH] species.63
We then wanted to generate more evidence for the end group liberation and formation of free phosphinidenes during the reaction. If this is the case, starting from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of differently substituted triphosphiranes P3Ar3 and P3Ar′3 should result in the formation of the mixed diphosphene complex [Cp2TiP2ArAr′] (from recombination of differently substituted phosphinidenes) along with [Cp2TiP2Ar2] and [Cp2TiP2Ar′2]. Therefore, a 1
1 mixture of differently substituted triphosphiranes P3Ar3 and P3Ar′3 should result in the formation of the mixed diphosphene complex [Cp2TiP2ArAr′] (from recombination of differently substituted phosphinidenes) along with [Cp2TiP2Ar2] and [Cp2TiP2Ar′2]. Therefore, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 1a and 1b (1 equiv.) was mixed with 1.5 equiv. of [Cp2Ti(btmsa)] in C6D6 in an NMR scale reaction.
1 mixture of 1a and 1b (1 equiv.) was mixed with 1.5 equiv. of [Cp2Ti(btmsa)] in C6D6 in an NMR scale reaction.
The 31P NMR spectrum of the resulting product solution is shown in Fig. 6. For comparison the spectra of the pure compounds 3a and 3b are depicted as well. In the spectrum of the product mixture the singlet signals of the symmetric compounds 3a and 3b can be seen clearly at 283.8 and 290.7 ppm, respectively. Additionally, there are two doublets, indicating the formation of the mixed diphosphene complex [Cp2Ti(P2DippTipp)] (3ab). We conclude from this experiment that an exchange of P–R end groups or the intermediacy of phospinidenes P–R are likely in the course of the reaction.
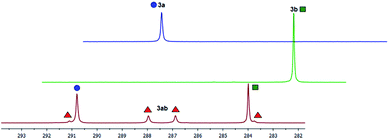 | ||
Fig. 6 Formation of the mixed diphosphene complex 3ab in a scrambling experiment utilizing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 3a and 3b in the presence of 1.5 equiv. [Cp2Ti(btmsa)]. 1 mixture of 3a and 3b in the presence of 1.5 equiv. [Cp2Ti(btmsa)]. | ||
Moreover, titana- and zirconacycles are regularly applied in the formation of main group element substituted heterocycles.64,65 We wanted to probe this reactivity by treating isolated 3a with TippPCl2 and found 1a as the product along with the formation of Cp2TiCl2 (Scheme 7),31 which clearly shows the potential of complexes 3 for the formation of small inorganic ring systems.
Conclusions
We have shown in here a simple and selective synthetic protocol for the formation of aryl-substituted triphosphiranes 1 of the type P3Ar3 and identified PMe3 as the active reductant. These findings open the way for future studies to render these transformations catalytic with respect to PMe3. Moreover, we have shown that the Ti(II) synthon [Cp2Ti(btmsa)] reacts with 1 to yield the respective titanocene diphosphene complexes 3 in straightforward fashion. Combined theoretical and experimental studies suggest the intermediate formation of a paramagnetic titanium phosphorus species, indicating single electron transfer steps. Moreover, experimental evidence is presented for the intermediacy of free diphosphenes, authenticated by reaction of the elusive diphosphene P2Tipp2 (2) with [Cp2Ti(btmsa)]. In first reactivity studies we have shown that 3 can be utilized as a P2R2-transfer reagent in transmetalation protocols using TippPCl2. This opens the pathway to generate new P2R2-containing heterocycles.Studies to further elucidate the reaction mechanism of the P3Ar3 degradation reaction are ongoing, to further investigate the nature of the paramagnetic intermediate. Additionally, application of the P3Ar3 systems in phosphinidene transfer reactions will be investigated.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
C. H.-J. thanks Prof. M. Beller for his support, the European Union for funding (H2020-MSCA-IF-2017 792177) and the Max Buchner-Foundation for a Scientific Fellowship. The CV studies were co-funded through the Leibniz Science Campus Phosphorous Research Rostock and the FCI (SK 202/22). We thank our technical and analytical staff for assistance, especially Dr Anke Spannenberg for her support regarding X-ray analysis. Dr Jonas Bresien is kindly acknowledged for help with vibrational spectroscopy.Notes and references
- H. Köhler and A. Michaelis, Ber. Dtsch. Chem. Ges., 1877, 10, 807 CrossRef
.
- J. J. Daly and L. Maier, Nature, 1964, 203, 1167 CrossRef CAS
.
- A. H. Cowley, T. A. Furtsch and D. S. Dierdorf, J. Chem. Soc., Chem. Commun., 1970, 523 RSC
.
- P. S. Elmes, M. E. Redwood and B. O. West, J. Chem. Soc. D, 1970, 1120 RSC
.
- M. Baudler, B. Carlsohn, W. Böhm and G. Reuschenbach, Z. Naturforsch., B: Anorg. Chem., Org. Chem., 1976, 31, 558 Search PubMed
.
- M. Baudler, B. Carlsohn, B. Kloth and D. Koch, Z. Anorg. Allg. Chem., 1977, 432, 67 CrossRef CAS
.
- M. Baudler and K. Glinka, Chem. Rev., 1993, 93, 1623 CrossRef CAS
.
- W. Mahler and A. B. Burg, J. Am. Chem. Soc., 1957, 79, 251 CrossRef CAS
.
- M. Baudler, J. Hahn and E. Clef, Z. Naturforsch., B: Anorg. Chem., Org. Chem., 1984, 39, 438 Search PubMed
.
- M. Baudler and J. Hellmann, Z. Naturforsch., B: Anorg. Chem., Org. Chem., 1981, 36, 266 Search PubMed
.
- P. Jutzi, N. Brusdeilins, H.-G. Stammler and B. Neumann, Chem. Ber., 1994, 127, 997 CrossRef CAS
.
- P. Jutzi and N. Brusdeilins, Z. Anorg. Allg. Chem., 1994, 620, 1375 CrossRef CAS
.
- W. Uhl and M. Benter, J. Chem. Soc., Dalton Trans., 2000, 3133 RSC
.
- C. Üffing, C. v. Hänisch and H. Schnöckel, Z. Anorg. Allg. Chem., 2000, 626, 1557 CrossRef
.
- C. A. Dyker, N. Burford, G. Menard, M. D. Lumsden and A. Decken, Inorg. Chem., 2007, 46, 4277 CrossRef CAS PubMed
.
- M. H. Holthausen, D. Knackstedt, N. Burford and J. J. Weigand, Aust. J. Chem., 2013, 66, 1155 CrossRef CAS
.
- S. S. Chitnis, R. A. Musgrave, H. A. Sparkes, N. E. Pridmore, V. T. Annibale and I. Manners, Inorg. Chem., 2017, 56, 4521 CrossRef CAS PubMed
.
- S. S. Chitnis, H. A. Sparkes, V. T. Annibale, N. E. Pridmore, A. M. Oliver and I. Manners, Angew. Chem., Int. Ed., 2017, 56, 9536 CrossRef CAS PubMed
.
- R. Ahlrichs, D. Fenske, H. Oesen and U. Schneider, Angew. Chem., Int. Ed. Engl., 1992, 31, 323–326 CrossRef
.
- M. Baudler and M. Bock, Z. Anorg. Allg. Chem., 1973, 395, 37 CrossRef
.
- K. Schwedtmann, R. Schoemaker, F. Hennersdorf, A. Bauzá, A. Frontera, R. Weiss and J. J. Weigand, Dalton Trans., 2016, 45, 11384 RSC
.
- N. Tokitoh, A. Tsurusaki and T. Sasamori, Phosphorus, Sulfur Silicon Relat. Elem., 2009, 184, 979 CrossRef CAS
.
- C. N. Smit, T. A. van der Knaap and F. Bickelhaupt, Tetrahedron Lett., 1983, 24, 2031 CrossRef CAS
.
- L. Weber, D. Bungardt, R. Boese and D. Bläser, Chem. Ber., 1988, 121, 1033 CrossRef CAS
.
- D. G. Yakhvarov, E. Hey-Hawkins, R. M. Kagirov, Y. H. Budnikova, Y. S. Ganushevich and O. G. Sinyashin, Russ. Chem. Bull., 2007, 56, 935 CrossRef CAS
.
- X. Li, D. Lei, M. Y. Chiang and P. P. Gaspar, J. Am. Chem. Soc., 1992, 114, 8526 CrossRef CAS
.
- K. B. Dillon, V. C. Gibson and L. J. Sequeira, J. Chem. Soc., Chem. Commun., 1995, 2429 RSC
.
- B. M. Cossairt and C. C. Cummins, New J. Chem., 2010, 34, 1533 RSC
.
- S. Shah and J. D. Protasiewicz, Chem. Commun., 1998, 1585 RSC
.
- P. Le Floch, Coord. Chem. Rev., 2006, 250, 627 CrossRef CAS
.
- Experimental and computational details, and details on the X-ray diffraction studies are included in the ESI.†.
- K. S. A. Motolko, D. J. H. Emslie, H. A. Jenkins and J. F. Britten, Eur. J. Inorg. Chem., 2017, 2920 CrossRef CAS
.
- C. Frenzel and E. Hey-Hawkins, Phosphorus, Sulfur Silicon Relat. Elem., 1998, 143, 1 CrossRef CAS
.
- J. D. Protasiewicz, Eur. J. Inorg. Chem., 2012, 4539 CrossRef CAS
.
- M. Yoshifuji, I. Shima, N. Inamoto, K. Hirotsu and T. Higuchi, J. Am. Chem. Soc., 1981, 103, 4587 CrossRef CAS
.
- J. D. Protasiewicz, M. P. Washington, V. B. Gudimetla, J. L. Payton and M. C. Simpson, Inorg. Chim. Acta, 2010, 364, 39 CrossRef CAS
.
- T. Sasamori, N. Takeda and N. Tokitoh, J. Phys. Org. Chem., 2003, 16, 450 CrossRef CAS
.
- P. Pyykkö and M. Atsumi, Chem.–Eur. J., 2009, 15, 12770 CrossRef PubMed
.
- D. Dhara, D. Mandal, A. Maiti, C. B. Yildiz, P. Kalita, N. Chrysochos, C. Schulzke, V. Chandrasekhar and A. Jana, Dalton Trans., 2016, 45, 19290 RSC
.
- A. Aistars, R. J. Doedens and N. M. Doherty, Inorg. Chem., 1994, 33, 4360 CrossRef CAS
.
- R. Appel and H. Schöler, Chem. Ber., 1977, 110, 2382 CrossRef CAS
.
- S. S. Chitnis, A. P. M. Robertson, N. Burford, J. J. Weigand and R. Fischer, Chem. Sci., 2015, 6, 2559 RSC
.
- J. J. Weigand, N. Burford, A. Decken and A. Schulz, Eur. J. Inorg. Chem., 2007, 4868 CrossRef CAS
.
- A. P. M. Robertson, S. S. Chitnis, H. A. Jenkins, R. McDonal, M. J. Ferguson and N. Burford, Chem.–Eur. J., 2015, 21, 7902 CrossRef CAS PubMed
.
- T. L. Breen and D. W. Stephan, J. Am. Chem. Soc., 1995, 117, 11914 CrossRef CAS
.
- C. C. Cummins, R. R. Schrock and W. M. Davis, Angew. Chem., Int. Ed. Engl., 1993, 32, 756 CrossRef
.
- F. Basuli, J. Tomaszewski, J. C. Huffman and D. J. Mindiola, J. Am. Chem. Soc., 2003, 125, 10170 CrossRef CAS PubMed
.
- G. Zhao, F. Basuli, U. J. Kilgore, H. Fan, H. Aneetha, J. C. Huffman, G. Wu and D. J. Mindiola, J. Am. Chem. Soc., 2006, 128, 13575 CrossRef CAS PubMed
.
- T. Beweries, M. Haehnel and U. Rosenthal, Catal. Sci. Technol., 2013, 3, 18 RSC
.
- S. Xin, H. G. Woo, J. F. Harrod, E. Samuel and A.-M. Lebuis, J. Am. Chem. Soc., 1997, 119, 5307 CrossRef CAS
.
- S. Xin, J. F. Harrod and E. Samuel, J. Am. Chem. Soc., 1994, 116, 11562 CrossRef CAS
.
- L. Weber, Chem. Rev., 1992, 92, 1839 CrossRef CAS
.
- P. S. Elmes, M. L. Scudder and B. O. West, J. Organomet. Chem., 1976, 122, 281 CrossRef CAS
.
- J. Chatt, P. B. Hitchcock, A. Pidcock, C. P. Warrens and K. R. Dixon, J. Chem. Soc., Dalton Trans., 1984, 2237 RSC
.
- J. Ho, T. L. Breen, A. Ozarowski and D. W. Stephan, Inorg. Chem., 1994, 33, 865 CrossRef CAS
.
- S. Kurz and E. Hey-Hawkins, J. Organomet. Chem., 1993, 462, 203 CrossRef CAS
.
- M. Schaffrath, A. Villinger, D. Michalik, U. Rosenthal and A. Schulz, Organometallics, 2008, 27, 1393 CrossRef CAS
.
-
M. Baudler, K. Glinka, A. H. Cowley and M. Pakulski, Organocyclophosphanes, in Inorg. Synth., ed. H. R. Allcock, 2007 Search PubMed
.
- J. R. Goehrlich and R. Schmutzler, Z. Anorg. Allg. Chem., 1994, 620, 173 CrossRef
.
- H. Köpf and R. Voigtländer, Chem. Ber., 1981, 114, 2731 CrossRef
.
- A. T. Normand, Q. Bonnin, S. Brandès, P. Richard, P. Fleurat-Lessard, C. H. Devillers, C. Balan, P. Le Gendre, G. Kehr and G. Erker, Chem.–Eur. J., 2019, 25, 2803 CrossRef CAS PubMed
.
- J. Pinkas, R. Gyepes, I. Císařová, J. Kubišta, M. Horáček, N. Žilkováa and K. Mach, Dalton Trans., 2018, 47, 8921 RSC
.
- M. Polášek and J. Kubišta, J. Organomet. Chem., 2007, 692, 4073 CrossRef
.
- X. Yan and C. Xi, Acc. Chem. Res., 2015, 48, 935 CrossRef CAS PubMed
.
- T. Baumgartner and R. Réau, Chem. Rev., 2006, 106, 4681 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of compounds, NMR spectra, crystallographic, EPR and computational details. CCDC 1915056–1915060. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc02322d |
| This journal is © The Royal Society of Chemistry 2019 |