Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Bis(imino)pyridine iron complexes for catalytic carbene transfer reactions†
Ban
Wang
,
Isaac G.
Howard
,
Jackson W.
Pope
,
Eric D.
Conte
and
Yongming
Deng
 *
*
Chemistry Department, Western Kentucky University, 1906 College Heights Boulevard, Bowling Green, Kentucky 42101, USA. E-mail: yongming.deng@wku.edu
First published on 2nd July 2019
Abstract
The bis(imino)pyridine iron complex, for the first time, is developed as an effective metal carbene catalyst for carbene transfer reactions of donor–acceptor diazo compounds. Its broad catalytic capability is demonstrated by a range of metal carbene reactions, from cyclopropanation, cyclopropenation, epoxidation, and Doyle–Kirmse reaction to O–H insertion, N–H insertion, and C–H insertion reactions. The asymmetric cyclopropanation of styrene and methyl phenyldiazoacetate was successfully achieved by the new chiral bis(imino)pyridine iron catalyst, which delivers a new gateway for the development of chiral iron catalysis for metal carbene reactions.
Introduction
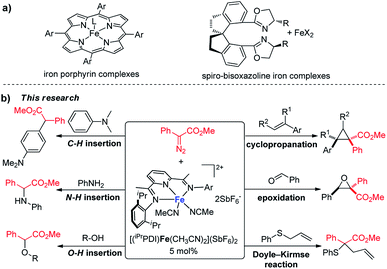
Transition-metal-catalyzed carbene transfer reactions occurring through metal carbene intermediates encompass a vast array of reactants and catalysts to achieve novel and selective strategies for organic synthesis.1 The reactive carbenoid intermediates can be catalytically generated from diazo compounds by metal-catalyzed dinitrogen extrusion,2 and their reactions extend from addition and insertion to cycloaddition and ylide formation.3 Dirhodium complexes have been established as the most successful catalysts for carbene transfer reactions of diazo compounds;4 great achievements have also been accomplished recently by copper and other precious metal catalysts (e.g. ruthenium, palladium, gold).5 Iron, the second most abundant metal, with its particular biological relevance, is emerging as an important metal for catalytic metal carbene reactions.6 However, iron catalysis is comparatively underdeveloped, with the enduring dominance of precious metal catalysis in metal carbene chemistry.Since the launch of iron porphyrin-catalyzed cyclopropanation by Woo,7 various carbene transfer processes of diazo compounds, including cyclopropanation, heteroatom–hydrogen bond insertions, and intramolecular C–H inversion, have been achieved by porphyrin and related macrocyclic iron complexes; however, these generally occur with active α-hydrogen-diazocarbonyl compounds, diazoalkanes, or the corresponding precursors.8 The spiro-bisoxazoline iron complexes developed by Zhou's group have exhibited high catalytic activities and selectivities for heteroatom–hydrogen bond insertions and intramolecular cyclopropanation reactions of α-diazoesters.9 Despite these achievements, iron has not been developed as a catalyst to the same extent as other late transition metals, particularly for usage in metal carbene reactions. The advancement of iron catalysis for general carbene transfer reactions with broad substrate schemes, especially asymmetric processes and under mild reaction conditions, remains a wide-open field for discovery and innovation. We report here, for the first time, bis(imino)pyridine iron complexes serving as effective catalysts for a range of metal carbene reactions under mild reaction conditions (at room temperature or 40 °C), including cyclopropanation/cyclopropenation, epoxidation, Doyle–Kirmse reaction, O–H insertion, N–H insertion, and C–H insertion (Scheme 1). To the best of our knowledge, this bis(imino)pyridine iron catalyst represents the most broad-ranging catalytic activity towards metal carbene reactions of diazo compounds over the previously reported iron catalysis system.6 The bis(imino)pyridine iron-catalyzed cyclopropanation proceeds on a wide range of aryldiazoacetates, vinyldiazoacetates, styrenes and phenylacetylene. Notably, a new chiral bis(imino)pyridine ligand derivatized from L-valine methyl ester has been synthesized, and the corresponding enantiopure, C1-symmetric iron catalyst enabled the asymmetric cyclopropanation of styrene and phenyl diazoacetate.
 | ||
| Scheme 1 (a) Selected iron catalysis for metal carbene reactions. (b) This research: bis(imino)pyridine iron catalyzed metal carbene reactions. | ||
In the past decade, bis(imino)pyridine chelated iron complexes have emerged as an effective class of catalysts for ethylene polymerization, olefin hydrogenation, hydrosilation, and [2π + 2π]-cycloaddition reactions.10 Owing to its ease of preparation, the bis(imino)pyridine ligand is easily modifiable, allowing versatility in ligand design, synthesis, and screening.10a,b However, catalytic metal carbene reactions by bis(imino)pyridine iron complexes have not been achieved. Recently, Chirik reported the formation of a bis(imino)pyridine iron carbene complex B from a stoichiometric amount of bis(imino)pyridine iron dinitrogen complex A and diphenyldiazomethane by dinitrogen extrusion (Fig. 1).11 However, the attempts towards metal carbene reactions, such as cyclopropanation and C–H insertion, were unsuccessful with this bis(imino)pyridine iron carbene complex.11,12
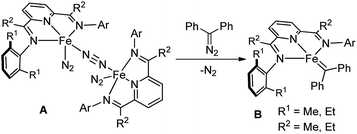 | ||
| Fig. 1 Formation of a bis(imino)pyridine iron carbene from bis(arylimino)pyridine iron dinitrogen complexes and diphenyldiazomethane.11 | ||
We hypothesized that one reason for the lack of reactivity for bis(imino)pyridine iron carbene complex B in the carbene transfer process is due to the charge delocalization induced by the diphenyl group. To address this issue, we predicted that augmenting the electrophilicity of the disubstituted diazo compound would increase the reactivity of the corresponding iron carbene; thus, it could more readily engage in carbene transfer reactions.2b,c It has been documented that the donor–acceptor metal carbene, which can be produced from donor–acceptor diazo compound by metal-catalyzed dinitrogen extrusion, exhibited higher reactivity than the one from diphenyldiazomethane due to its stronger electrophilicity.1c,3c,4a Herein, a donor–acceptor diazo compound, aryldiazoacetate, was selected as the carbene precursor to investigate the bis(imino)pyridine iron-catalyzed metal carbene reactions. Additionally, recent computational studies of bis(imino)pyridine iron complexes for C–H functionalization of donor–acceptor diazo compound also suggest feasibility.13
The catalytic cycle for the conversion of a diazo compound to a metal-stabilized carbene intermediate is initiated from the metal-catalyzed dinitrogen extrusion of nucleophilic diazo compound. We predicted that compared to the formal iron(0) complex A, the more electrophilic bis(imino)pyridine iron(II) complexes would exhibit higher reactivity towards the nucleophilic diazo compound and facilitate the subsequent metal carbene transfer. Therefore, we aimed to electronically and sterically tune the bis(imino)pyridine iron(II) complexes to achieve the carbene transfer reactions of the donor–acceptor diazo compound under mild reaction conditions.
Results and discussion
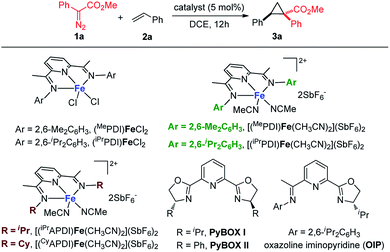
As a starting point, we focused on evaluating a series of bis(imino)pyridine iron(II) catalysts for the cyclopropanation reaction of styrene 2a with methyl phenyldiazoacetate 1a (Table 1). As proposed, in the presence of 5 mol% of bis(arylimino)pyridine iron(II) dichloride complexes (entries 1 and 2), the reaction of 2a and phenyldiazoacetate 1a afforded the cyclopropanation product 3a, however, in low yields with predominately recovered starting material. To improve the catalytic activity of the iron complexes, examination of the noncoordinating counterions was performed. The employment of more electrophilic iron complexes with hexafluoroantimonate (SbF6−) as counterions (entries 3 and 4) led to a marked increase in yield. The combination of (iPrPDI)FeCl2 and NaBArF4 also delivered 3a with enhanced yield (48%, Table S1†). [(iPrPDI)Fe(CH3CN)2](SbF6)2 bearing bulky 2,6-diisopropylphenyl substituents was identified as the best catalyst,14 which catalyzed the cyclopropanation under room temperature, generating 3a in 86% yield with excellent diastereoselectivity (dr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). A lower yield of 3a (entry 5, 68%) was obtained when the reaction was performed with 1a
1). A lower yield of 3a (entry 5, 68%) was obtained when the reaction was performed with 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a = 1
2a = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the presence of [(iPrPDI)Fe(CH3CN)2](SbF6)2. To reveal the imino-substituents' effect on the catalyst, iron complexes containing N-alkyl substituents were examined. However, they resulted in low catalytic activity with recovery of starting material (entries 6 and 7), which could be due to electronic and/or steric constraints from the imino-alkyl groups. Additionally, neither iron complexes of pyridine bis(oxazoline) ligands nor oxazoline iminopyridine iron complexes were effective catalysts for this transformation (entries 8 to 10). These results demonstrate the indispensability of the imino-aryl substituent in the ligand frame to conduct active iron catalysis in metal carbene reactions of donor–acceptor diazo compound.
1 in the presence of [(iPrPDI)Fe(CH3CN)2](SbF6)2. To reveal the imino-substituents' effect on the catalyst, iron complexes containing N-alkyl substituents were examined. However, they resulted in low catalytic activity with recovery of starting material (entries 6 and 7), which could be due to electronic and/or steric constraints from the imino-alkyl groups. Additionally, neither iron complexes of pyridine bis(oxazoline) ligands nor oxazoline iminopyridine iron complexes were effective catalysts for this transformation (entries 8 to 10). These results demonstrate the indispensability of the imino-aryl substituent in the ligand frame to conduct active iron catalysis in metal carbene reactions of donor–acceptor diazo compound.
| Entry | Catalyst | T (°C) | Yieldb |
|---|---|---|---|
a Reaction condition unless otherwise noted: 1a (0.20 mmol, 1.0 equiv.) in dry DCE (1.0 ml) was added to a 1.0 mL DCE solution of 2a (1.0 mmol, 5.0 equiv.) and catalyst (0.01 mmol) under N2 within 1 hour.
b Yield of isolated product 3a based on the limiting reagent 1a.
c The reaction was performed with 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a = 1 2a = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (1,2-dichloroethane = DCE). 1 (1,2-dichloroethane = DCE).
|
|||
| 1 | (MePDI)FeCl2 | 50 | 14 |
| 2 | (iPrPDI)FeCl2 | 50 | 18 |
| 3 | [(MePDI)Fe(CH3CN)2](SbF6)2 | rt | 65 |
| 4 | [( iPr PDI)Fe(CH 3 CN) 2 ](SbF 6 ) 2 | rt | 86 |
| 5c | [(iPrPDI)Fe(CH3CN)2](SbF6)2 | rt | 68 |
| 6 | [(iPrAPDI)Fe(CH3CN)2](SbF6)2 | 50 | 8 |
| 7 | [(CyAPDI)Fe(CH3CN)2](SbF6)2 | 50 | <5 |
| 8 | FeCl2/PyBOX(I)/AgSbF6 | 50 | <5 |
| 9 | FeCl2/PyBOX(II)/AgSbF6 | 50 | <5 |
| 10 | FeCl2/OIP/AgSbF6 | 50 | 9 |
Under the optimized condition, we investigated the scope of this bis(arylimino)pyridine iron-catalyzed cyclopropanation across a range of aryldiazoacetates and styrene derivatives (Table 2). As indicated by entries 1 to 5, aryldiazoacetates with electron-rich, halogen para-substituents and 2-naphthyl group all reacted smoothly with styrene, generating the corresponding cyclopropanes in good yields (81–88%, 3b–3f) with excellent diastereoselectivities (dr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). However, no reaction occurred with the electron-deficient system, even at 40 °C (1g, entry 6). Reactions of aryldiazoacetates 1h and 1j bearing ortho-substituents on the aromatic ring resulted in lower yields (entries 7 and 8). We rationalize that such lower reactivity can be attributed to a higher kinetic barrier for the generation of corresponding iron carbene intermediate, which is caused by the increased steric hindrance between the ortho-substituent and the bulky bis(imino)pyridine ligand frame. The cyclopropanes 3j–3l derived from styrene derivatives 2b–2d were obtained in yields ranging from 88 to 91%, whereas moderate yield (67%, entry 12) was obtained with 4-(trifluoromethyl)styrene 2e. Disubstituted styrenes, including α-phenylstyrene 2f and trans-β-methylstyrene 2g, were also ideal reagents for this iron-catalyzed cyclopropanation, producing products 3n and 3o in good yields.
1). However, no reaction occurred with the electron-deficient system, even at 40 °C (1g, entry 6). Reactions of aryldiazoacetates 1h and 1j bearing ortho-substituents on the aromatic ring resulted in lower yields (entries 7 and 8). We rationalize that such lower reactivity can be attributed to a higher kinetic barrier for the generation of corresponding iron carbene intermediate, which is caused by the increased steric hindrance between the ortho-substituent and the bulky bis(imino)pyridine ligand frame. The cyclopropanes 3j–3l derived from styrene derivatives 2b–2d were obtained in yields ranging from 88 to 91%, whereas moderate yield (67%, entry 12) was obtained with 4-(trifluoromethyl)styrene 2e. Disubstituted styrenes, including α-phenylstyrene 2f and trans-β-methylstyrene 2g, were also ideal reagents for this iron-catalyzed cyclopropanation, producing products 3n and 3o in good yields.
| Entry | 1 | 2 | Yieldb |
|---|---|---|---|
| a For experimental details, see ESI. b Isolated yield. c Reactions were performed at 40 °C. | |||
| 1 | 1b, 4-MeC6H4 | 2a, Ph, H, H | 3b, 81 |
| 2 | 1c, 4-MeOC6H4 | 2a, Ph, H, H | 3c, 83 |
| 3 | 1d, 4-ClC6H4 | 2a, Ph, H, H | 3d, 88 |
| 4 | 1e, 4-BrC6H4 | 2a, Ph, H, H | 3e, 83 |
| 5 | 1f, 2-naphthyl | 2a, Ph, H, H | 3f, 81 |
| 6c | 1g, 4-NO2C6H4 | 2a, Ph, H, H | 3g, <5 |
| 7 | 1h, 2-MeOC6H4 | 2a, Ph, H, H | 3h, 52 |
| 8 | 1i, 2-ClCH6H4 | 2a, Ph, H, H | 3i, 58 |
| 9 | 1a, Ph | 2b, 4-MeC6H4, H, H | 3j, 91 |
| 10 | 1a, Ph | 2c, 4-MeOC6H4, H, H | 3k, 88 |
| 11 | 1a, Ph | 2d, 4-ClC6H4, H, H | 3l, 90 |
| 12 | 1a, Ph | 2e, 4-CF3C6H4, H, H | 3m, 67 |
| 13c | 1a, Ph | 2f, Ph, Ph, H | 3n, 73 |
| 14c | 1a, Ph | 2g, Ph, H, CH3 | 3o, 70 |
In addition to the styrene derivatives, the reaction of 1,3-cyclohexadiene and 1a was also effectively catalyzed by [(iPrPDI)Fe(CH3CN)2](SbF6)2, affording the cyclopropane product 3p in 80% yield with dr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (eqn (1)). Furthermore, as shown in eqn (2), [(iPrPDI)Fe(CH3CN)2](SbF6)2 catalyzed the cyclopropanation of cyclohexene, and 1a was also successfully achieved, affording the desired product 3q in 72% yield. To further probe the diazo substrate generality, vinyl-diazoacetate 1j was subjected to bis(imino)pyridine iron-catalyzed cyclopropanation with styrene (eqn (3)). Gratifyingly, the cyclopropane product 3r was obtained in 84% yield, which demonstrates the catalytic capability of bis(imino)pyridine iron for a broader scope of donor–acceptor diazo compounds. Remarkably, [(iPrPDI)Fe(CH3CN)2](SbF6)2 was also capable of catalyzing the cyclopropenation of 1a and phenylacetylene, furnishing the product 3s in 61% yield at 40 °C (eqn (4)), which has not been achieved by other reported iron catalysts.
1 (eqn (1)). Furthermore, as shown in eqn (2), [(iPrPDI)Fe(CH3CN)2](SbF6)2 catalyzed the cyclopropanation of cyclohexene, and 1a was also successfully achieved, affording the desired product 3q in 72% yield. To further probe the diazo substrate generality, vinyl-diazoacetate 1j was subjected to bis(imino)pyridine iron-catalyzed cyclopropanation with styrene (eqn (3)). Gratifyingly, the cyclopropane product 3r was obtained in 84% yield, which demonstrates the catalytic capability of bis(imino)pyridine iron for a broader scope of donor–acceptor diazo compounds. Remarkably, [(iPrPDI)Fe(CH3CN)2](SbF6)2 was also capable of catalyzing the cyclopropenation of 1a and phenylacetylene, furnishing the product 3s in 61% yield at 40 °C (eqn (4)), which has not been achieved by other reported iron catalysts.
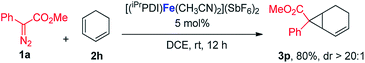 | (1) |
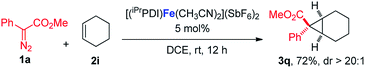 | (2) |
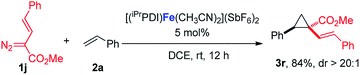 | (3) |
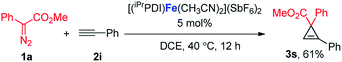 | (4) |
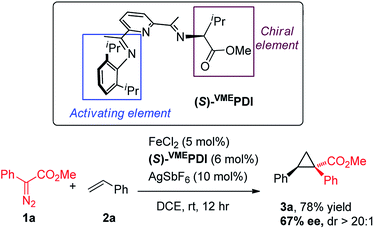
With the accomplishment of achiral bis(arylimino)pyridine iron-catalyzed cyclopropanation, we have sought to modify the ligand architecture to generate a chiral iron catalyst for asymmetric cyclopropanation. Our catalyst screening (Table 1) indicated that the N-aryl substituent in bis(imino)pyridine ligand is indispensable for the effective catalytic activity of iron complexes. Guided by these experimental results and Bianchini's original design of chiral bis(imino)pyridine ligand,15 we synthesized an enantiopure, C1-symmetric chiral bis(imino)pyridine ligand [(S)-VMEPDI] (Scheme 2), in which one imine is “anchored” by a 2,6-diisopropylphenyl group (activating element) and the other is prepared from L-valine methyl ester (chiral element). To our delight, the asymmetric cyclopropanation reaction of 1a and styrene was successfully achieved by in situ prepared chiral iron catalyst from (S)-VMEPDI, FeCl2, and AgSbF6 at room temperature. The cyclopropane product 1a was isolated in 78% yield with 67% enantiomeric excess.16 Although with moderate enantioselectivity, the success of this asymmetric cyclopropanation reaction provides a strong basis for the development of a new chiral bis(imino)pyridine iron catalyst for metal carbene reactions.
Encouraged by the success of bis(arylimino)pyridine iron(II)-catalyzed cyclopropanation, we then sought to examine the generality of this iron catalyst for metal carbene reactions. As depicted in Scheme 3, a range of metal carbene reactions of phenyldiazoacetate 1a, including epoxidation, Doyle–Kirmse reaction, N–H insertion, C–H insertion, and O–H insertion, were all successfully catalyzed by [(iPrPDI)Fe(CH3CN)2](SbF6)2. The bis(arylimino)pyridine iron-catalyzed reaction of 1a and benzaldehyde yielded the epoxide product 4 in 80% yield with excellent diastereoselectivity at room temperature (Scheme 3a). Under the same condition (Scheme 3b), allyl phenyl sulfide reacted with 1a smoothly to form the Doyle–Kirmse product 5 in 91% yield. [(iPrPDI)Fe(CH3CN)2](SbF6)2 was also able to catalyze the N–H insertion of aniline and C–H insertion of N,N-dimethylaniline, although higher reaction temperatures were required (Scheme 3c and d). Furthermore, in the presence of 5 mol% [(iPrPDI)Fe(CH3CN)2](SbF6)2, O–H insertion reactions of 1a with methanol, n-butanol, and water were achieved, furnishing the corresponding products 8a–8c in good to moderate yields (Scheme 3e).
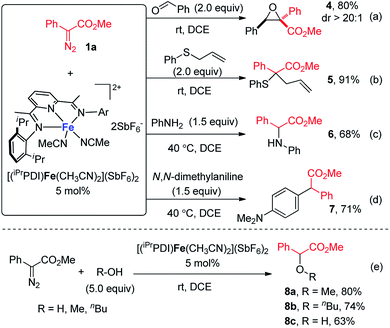 | ||
| Scheme 3 Bis(arylimino)pyridine iron-catalyzed (a) epoxidation; (b) Doyle–Kirmse reaction; (c) N–H insertion; (d) C–H insertion; and (e) O–H insertion. | ||
As documented, bis(imino)pyridines have been recognized as radical-based, redox non-innocent ligands that can directly participate in the electronic structure of metal complexes.10d,f,17 Chirik's study demonstrated that a carbene radical is engaged in bis(imino)pyridine iron carbene complex A, which is obtained from a formal iron(0) complex (Scheme 2).11 Therefore, considering the redox activity of the bis(imino)pyridine ligand, radical tapping experiments were conducted to address whether a radical carbene involved in this bis(arylimino)pyridine iron(II) catalyzes carbene transfer reactions.18 As shown in Scheme 4a, the addition of the radical scavenger TEMPO (2,2,6,6-tetramethylpiperidine N-oxide) did not harm the [(iPrPDI)Fe(CH3CN)2](SbF6)2-catalyzed cyclopropanation reactions of 1a or vinyl-diazoacetate 1j, and the corresponding products were isolated with similar yields to those from the reactions in the absence of TEMPO. These results reveal the unlikely involvement of the carbene radical intermediate in [(iPrPDI)Fe(CH3CN)2](SbF6)2-catalyzed cyclopropanation reactions. Moreover, the achievement of C–H insertion reaction of 1a with N,N-dimethylaniline (Scheme 3d) implies the likely generation of donor–acceptor iron(II) carbene intermediate.1c,6 Based on the obtained experimental results and mechanism study, we propose that the donor–acceptor diazo compound was decomposed by the bis(arylimino)pyridine iron(II) catalyst to generate an iron(II) carbene intermediate, which readily undergoes cyclopropanation of olefins to afford the cyclopropane product (Scheme 4b).
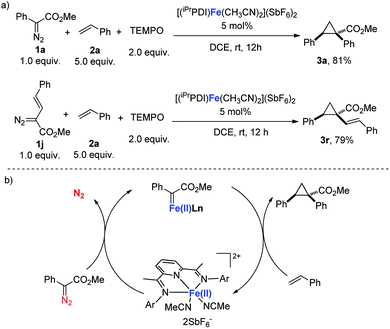 | ||
| Scheme 4 (a) Mechanism study. (b) Proposed mechanism of bis(arylimino)pyridine iron(II)-catalyzed cyclopropanation. | ||
Conclusions
In summary, the effective catalytic activity of bis(arylimino)pyridine iron(II) complexes for carbene transfer reactions of donor–acceptor diazo compounds has been demonstrated by a range of metal carbene transformations from cyclopropanation and insertions to ylide formation. Notably, the asymmetric cyclopropanation of methyl phenyldiazoacetate and styrene has been achieved by a new chiral iron catalyst based on the bis(imino)pyridine ligand derivatized from L-valine methyl ester. Future studies will be aimed at developing new asymmetric bis(imino)pyridine iron catalysts for highly enantioselective metal carbene reactions, as well as elucidating the mechanism of such process and the nature of the iron carbene intermediate.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Support for this research from Western Kentucky University and the National Science Foundation under Cooperative Agreement No. 1355438 (OIA-1355438-3200000271-19-136) is gratefully acknowledged. Dedicated to Professor Michael P. Doyle on the occasion of the 50th anniversary of his academic career.Notes and references
- For selected books and reviews, see: (a) M. P. Doyle, M. A. McKervey and T. Ye, Modern Catalytic Methods for Organic Synthesis with Diazo Compounds: From Cyclopropanes to Ylides, Wiley, New York, 1998 Search PubMed; (b) M. Xiaochu, M. C. Stefan, Y. Fan, H. Wenhao and O. S. Herman, Curr. Org. Chem., 2016, 20, 82 Search PubMed; (c) H. M. L. Davies and J. R. Denton, Chem. Soc. Rev., 2009, 38, 3061 RSC; (d) H. M. L. Davies and J. R. Manning, Nature, 2008, 451, 417 CrossRef CAS.
- For selected reviews of diazo compounds: (a) K. A. Mix, M. R. Aronoff and R. T. Raines, ACS Chem. Biol., 2016, 11, 3233 CrossRef CAS; (b) A. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire and M. A. McKervey, Chem. Rev., 2015, 115, 9981 CrossRef CAS; (c) G. Maas, Angew. Chem., Int. Ed., 2009, 48, 8186 ( Angew. Chem. , 2009 , 121 , 8332 ) CrossRef CAS.
- For selected reviews of insertions: (a) H. M. L. Davies and D. Morton, J. Org. Chem., 2016, 81, 343 CrossRef CAS; (b) D. Gillingham and N. Fei, Chem. Soc. Rev., 2013, 42, 4918 RSC; (c) H. M. L. Davies and D. Morton, Chem. Soc. Rev., 2011, 40, 1857 RSC; (d) M. P. Doyle, R. Duffy, M. Ratnikov and L. Zhou, Chem. Rev., 2010, 110, 704 CrossRef CAS; (e) H. M. L. Davies and R. E. J. Beckwith, Chem. Rev., 2003, 103, 2861 CrossRef CAS. For selected reviews of cycloadditions: (f) Q.-Q. Cheng, Y. Deng, M. Lankelma and M. P. Doyle, Chem. Soc. Rev., 2017, 46, 5425 RSC; (g) H. M. L. Davies and Y. Lian, Acc. Chem. Res., 2012, 45, 923 CrossRef CAS; (h) H.-S. Yeom and S. Shin, Acc. Chem. Res., 2014, 47, 966 CrossRef CAS; (i) H. M. L. Davies and E. G. Antoulinakis, Org. React., 2001, 57, 1 CAS. For selected reviews of ylide formation: (j) X. Guo and W. Hu, Acc. Chem. Res., 2013, 46, 2427 CrossRef CAS; (k) A.-H. Li, L.-X. Dai and V. K. Aggarwal, Chem. Rev., 1997, 97, 2341 CrossRef CAS.
- For selected reviews of dirhodium catalyzed metal carbene reactions: (a) Y. Deng, H. Qiu, H. D. Srinivas and M. P. Doyle, Curr. Org. Chem., 2015, 20, 61 CrossRef; (b) J. Hansen and H. M. L. Davies, Coord. Chem. Rev., 2008, 252, 545 CrossRef CAS; (c) M. P. Doyle, J. Org. Chem., 2006, 71, 9253 CrossRef CAS.
- For selected reviews: (a) L. Liu and J. Zhang, Chem. Soc. Rev., 2016, 45, 506 RSC; (b) M. R. Fructos, M. M. Díaz-Requejo and P. J. Pérez, Chem. Commun., 2016, 52, 7326 RSC; (c) X. Zhao, Y. Zhang and J. Wang, Chem. Commun., 2012, 48, 10162 RSC; (d) Y. Zhang and J. Wang, Eur. J. Org. Chem., 2011, 2011, 1015 CrossRef; (e) C.-Y. Zhou, J.-S. Huang and C.-M. Che, Synlett, 2010, 2010, 2681 CrossRef; (f) H. M. L. Davies and S. J. Hedley, Chem. Soc. Rev., 2007, 36, 1109 RSC.
- S.-F. Zhu and Q.-L. Zhou, Natl. Sci. Rev., 2014, 1, 580 CrossRef CAS.
- J. R. Wolf, C. G. Hamaker, J.-P. Djukic, T. Kodadek and L. K. Woo, J. Am. Chem. Soc., 1995, 117, 9194 CrossRef CAS.
- For selected recent examples: (a) S. B. J. Kan, R. D. Lewis, K. Chen and F. H. Arnold, Science, 2016, 354, 1048 CrossRef CAS; (b) J. Day, B. McKeever-Abbas and J. Dowden, Angew. Chem., Int. Ed., 2016, 55, 5809 ( Angew. Chem. , 2016 , 128 , 5903 ) CrossRef CAS; (c) P. S. Coelho, E. M. Brustad, A. Kannan and F. H. Arnold, Science, 2013, 339, 307 CrossRef CAS; (d) B. Morandi and E. M. Carreira, Science, 2012, 335, 1471 CrossRef CAS; (e) H. M. Mbuvi, E. R. Klobukowski, G. M. Roberts and L. K. Woo, J. Porphyrins Phthalocyanines, 2010, 14, 284 CrossRef CAS; (f) L. K. Baumann, H. M. Mbuvi, G. Du and L. K. Woo, Organometallics, 2007, 26, 3995 CrossRef CAS; (g) G. A. Mirafzal, G. Cheng and L. K. Woo, J. Am. Chem. Soc., 2002, 124, 176 CrossRef CAS; (h) J. R. Griffin, C. I. Wendell, J. A. Garwin and M. C. White, J. Am. Chem. Soc., 2017, 139, 13624 CrossRef CAS.
- For review: (a) S.-F. Zhu and Q.-L. Zhou, Acc. Chem. Res., 2012, 45, 1365 CrossRef CAS. For selected recent examples: (b) H. Xu, Y.-P. Li, Y. Cai, G.-P. Wang, S.-F. Zhu and Q.-L. Zhou, J. Am. Chem. Soc., 2017, 139, 7697 CrossRef CAS; (c) J.-J. Shen, S.-F. Zhu, Y. Cai, H. Xu, X.-L. Xie and Q.-L. Zhou, Angew. Chem., Int. Ed., 2014, 53, 13188 ( Angew. Chem. , 2014 , 126 , 13404 ) CrossRef CAS PubMed; (d) Y. Cai, S.-F. Zhu, G.-P. Wang and Q.-L. Zhou, Adv. Synth. Catal., 2011, 353, 2939 CrossRef CAS; (e) S.-F. Zhu, Y. Cai, H.-X. Mao, J.-H. Xie and Q.-L. Zhou, Nat. Chem., 2010, 2, 546 CrossRef CAS.
- For selected reviews: (a) P. J. Chirik, Acc. Chem. Res., 2015, 48, 1687 CrossRef CAS; (b) V. C. Gibson, C. Redshaw and G. A. Solan, Chem. Rev., 2007, 107, 1745 CrossRef CAS. For selected recent examples: (c) J. M. Hoyt, V. A. Schmidt, A. M. Tondreau and P. J. Chirik, Science, 2015, 349, 960 CrossRef CAS; (d) M. Darmon, R. P. Yu, S. P. Semproni, Z. R. Turner, S. C. E. Stieber, S. DeBeer and P. J. Chirik, Organometallics, 2014, 33, 5423 CrossRef PubMed; (e) J. M. Hoyt, K. T. Sylvester, S. P. Semproni and P. J. Chirik, J. Am. Chem. Soc., 2013, 135, 4862 CrossRef CAS; (f) S. C. E. Stieber, C. Milsmann, J. M. Hoyt, Z. R. Turner, K. D. Finkelstein, K. Wieghardt, S. DeBeer and P. J. Chirik, Inorg. Chem., 2012, 51, 3770 CrossRef CAS.
- S. K. Russell, J. M. Hoyt, S. C. Bart, C. Milsmann, S. C. E. Stieber, S. P. Semproni, S. DeBeer and P. J. Chirik, Chem. Sci., 2014, 5, 1168 RSC.
- (a) S. C. Bart, E. Lobkovsky, E. Bill and P. J. Chirik, J. Am. Chem. Soc., 2006, 128, 5302 CrossRef CAS; (b) S. K. Russell, E. Lobkovsky and P. J. Chirik, J. Am. Chem. Soc., 2009, 131, 36 CrossRef CAS.
- A. Varela-Álvarez and D. G. Musaev, Chem. Sci., 2013, 4, 3758 RSC.
- [(iPrPDI)Fe(CH3CN)2](SbF6)2 was prepared according to the reported procedure: G. J. P. Britovsek, J. England, S. K. Spitzmesser and A. J. P. White, Dalton Trans., 2005, 945 RSC.
- C. Bianchini, G. Mantovani, A. Meli, F. Migliacci, F. Zanobini, F. Laschi and A. Sommazzi, Eur. J. Inorg. Chem., 2003, 2003, 1620 CrossRef.
- For further condition screening of the asymmetric cyclopropanation, see: Table S2†.
- (a) P. J. Chirik and K. Wieghardt, Science, 2010, 327, 794 CrossRef CAS; (b) S. C. Bart, K. Chłopek, E. Bill, M. W. Bouwkamp, E. Lobkovsky, F. Neese, K. Wieghardt and P. J. Chirik, J. Am. Chem. Soc., 2006, 128, 13901 CrossRef CAS.
- For the use of TEMPO to study metal carbene radical: (a) N. D. Paul, S. Mandal, M. Otte, X. Cui, X. P. Zhang and B. de Bruin, J. Am. Chem. Soc., 2014, 136, 1090 CrossRef CAS; (b) W. I. Dzik, X. Xu, X. P. Zhang, J. N. H. Reek and B. de Bruin, J. Am. Chem. Soc., 2010, 132, 10891 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and copies of NMR spectra. See DOI: 10.1039/c9sc02189b |
| This journal is © The Royal Society of Chemistry 2019 |