Open Access Article
Open Access ArticleSynthesis of aminyl biradicals by base-induced Csp3–Csp3 coupling of cationic azo dyes†
Yizhu
Liu
a,
Paul
Varava
a,
Alberto
Fabrizio
a,
Léonard Y. M.
Eymann
a,
Alexander G.
Tskhovrebov
a,
Ophélie Marie
Planes
a,
Euro
Solari
a,
Farzaneh
Fadaei-Tirani
 a,
Rosario
Scopelliti
a,
Andrzej
Sienkiewicz
a,
Rosario
Scopelliti
a,
Andrzej
Sienkiewicz
 b,
Clémence
Corminboeuf
b,
Clémence
Corminboeuf
 a and
Kay
Severin
a and
Kay
Severin
 *a
*a
aInstitut des Sciences et Ingénierie Chimiques, Ecole Polytechnique Fédérale de Lausanne (EPFL), 1015 Lausanne, Switzerland. E-mail: kay.severin@epfl.ch
bInstitute of Physics, EPFL, Switzerland
First published on 9th May 2019
Abstract
The synthesis of the industrially important polymer parylene is achieved by polymerization of p-quinodimethane (p-QDM). The polymerization is thought to proceed via a biradical p-QDM dimer, but isolation or characterization of such a biradical has remained elusive. Here, we describe the synthesis of an aza-analogue of this p-QDM dimer. The biradical is formed by base-induced dimerization of an azoimidazolium dye. Due to the presence of sterically shielded aminyl radicals instead of terminal H2Ċ groups, the stability of this dimer is sufficient for analyses by ESR spectroscopy and X-ray crystallography. A similar Csp3–Csp3 coupling was observed for an azotriazolium dye, suggesting that base-induced C–C coupling reactions can be realized for different types of azo dyes.
Introduction
p-Quinodimethane (p-QDM, or p-xylylene) is an important hydrocarbon, from both a fundamental and application point of view.1p-QDM was first obtained through pyrolysis of p-xylene.2 Later, it was found that [2.2]-p-cyclophane is a more convenient starting material.3,4p-QDM readily polymerizes in the condensed phase generating poly(p-xylylene), also known as parylene (Scheme 1a).5 This polymer has interesting materials properties, and it is used for numerous applications.4,5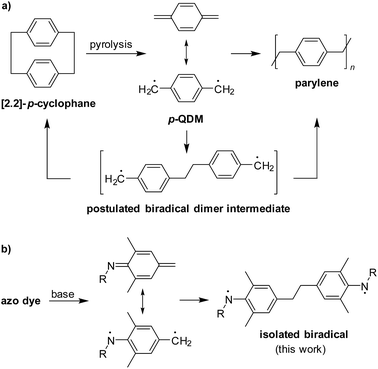 | ||
| Scheme 1 The chemistry of p-QDM (a), and synthesis of an aminyl biradical by base-induced dimerization of an azo dye (b). | ||
Mechanistically, the polymerization of p-QDM is intriguing. Despite its radical-type reactivity,6p-QDM is characterized by a closed-shell quinoidal electronic ground state.7 Recent theoretical investigations suggest that the triplet open-shell electronic state lies as high as 38 kcal mol−1 above the singlet closed-shell ground state.8 However, the initial C–C coupling step involving a singlet-to-triplet spin transition requires a lower activation energy,9 and it is assumed that the polymerization of p-QDM starts with the formation of a dimeric biradical (Scheme 1a).5c,9 This proposition is supported by the fact that solutions of freshly prepared p-QDM give [2.2]-p-cyclophane and larger macrocycles, along with oligomers.10 Further evidence for a stepwise oligomerization mechanism involving biradicals was provided by studying structurally related compounds.11
The postulated biradical dimer of p-QDM is highly reactive,9 and its isolation or direct characterization has remained elusive. Here we describe the synthesis of a structural analogue of the p-QDM dimer, in which the terminal H2Ċ groups are replaced by aminyl radicals (Scheme 1b). The stability of this aminyl biradical12 is sufficient for analyses by ESR spectroscopy and X-ray crystallography.
Results and discussion
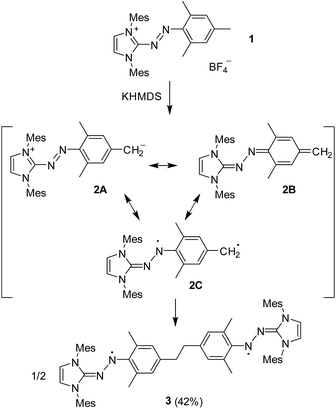
Azoimidazolium salts are industrially important dyes, which are used for the dying of synthetic and natural fibres.13 We have recently reported a new route for preparing these dyes.14 Importantly, this methodology can be used for the synthesis of dyes with aryl substituents attached to the heterocycle. Subsequent studies have shown that azoimidazolium dyes with N-aryl groups can be reduced to give stable aminyl radicals (Scheme 2a).15 Deprotonation of these dyes with potassium bis(trimethylsilyl)amide (KHMDS) results in the formation of mesoionic carbenes (‘Azo-MICs’), which can be captured with metal complexes (Scheme 2b).16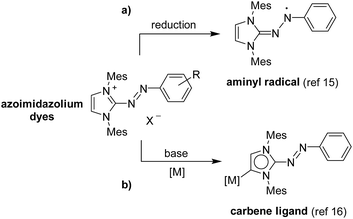 | ||
| Scheme 2 Azoimidazolium dyes can be used as precursors for stable aminyl radicals (a), and they can be converted into mesoionic carbene ligands (b). | ||
While studying the formation of mesoionic carbenes, we made an unexpected observation: when one equivalent of KHMDS was added to a solution of dye 1 (Scheme 3) in THF-d8, the color of the solution changed immediately from bright orange to dark rose-brown, and the 1H NMR signals vanished except for the solvent and HMDS peaks (Fig. S1, ESI†).
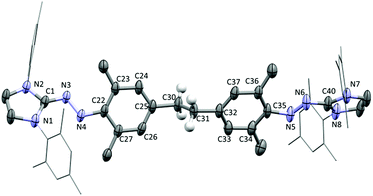
When the reaction was performed with temperature control −78 °C → RT on a preparative scale, black crystals of 3 were isolated in 42% yield (Scheme 3). The molecular structure of 3 was unambiguously established by single crystal X-ray crystallography (Fig. 1). Compound 3 can be regarded as a dimer of the hypothetical intermediate 2, which is formed upon deprotonation of 1. The two parts are linked via an ethylene bridge. The average length of this C–C bond is 1.484 Å, which is comparable to what has been reported for para-N-containing 1,2-diphenylethanes.17 Given that compound 3 has an overall charge of zero, each side of the molecule is expected to feature one unpaired electron. In fact, compound 3 is structurally similar to our previously reported aminyl radicals (Scheme 2a).15 For example, the bond length alternation (BLA)18 of 3 as a measure of the aromaticity is 0.014 Å, and comparable values were observed for the reduced dyes.15 The N–N–Cimidazole moieties are coplanar with the heterocyclic rings, but dihedral angles of ca. 27° are observed between the N2C groups and the arene rings. Moreover, like the aminyl radicals reported earlier,153 is characterized with short N–Cimidazole (avg. 1.317 Å) bonds, long N–Carene (avg. 1.386 Å) bonds, and moderate N–N (avg. 1.350 Å) bonds. These structural data suggest that 3 can be described as an aminyl biradical, as depicted in Scheme 3.
The biradical nature of 3 is further supported by ESR spectroscopy. Compound 3 displays limited stability in solution, and the ESR spectrum was thus recorded in the solid state (for details, see ESI†). The spectrum pointed to a spin doublet with the g-factor of 2.0037 (ESI, Fig. S18†). This value is again very similar to what was found for monomeric aminyl radicals prepared by reduction of azoimidazolium dyes.15
The generation of the biradical 3 by reaction of 1 with base is an unexpected finding, considering that other azoimidazolium dyes are deprotonated at the heterocycle to give mesoionic carbenes (Scheme 2b).16 The apparent Csp3–Csp3 coupling reaction and the generation of a biradical suggests a radical pathway, notwithstanding the fact that both the starting material 1 and the base are closed-shell compounds. Therefore, we propose that the observed reactivity resembles that of p-QDM. Deprotonation of 1 at the benzylic position gives zwitterionic 2A, which is in resonance with the quinoidal form 2B (Scheme 3). Furthermore, a diradical12 structure is conceivable (2C). According to density functional theory (DFT) computations at (U)TPSS-D3/cc-pVDZ level, the open-shell triplet form of 2C is energetically disfavored by 25 kcal mol−1 compared to the closed-shell quinoidal structure 2B (see ESI†). As discussed in the introduction, a similar situation is found for p-QDM.8 Moreover, the optimized structure of 2 shows very similar structural parameters for the central benzene ring and the terminal methylene group as p-QDM (ESI, Table S1†).7a All these results suggest that the electronic structures of 2 and p-QDM are similar. The formation of the biradical 3 can thus be regarded as an aza-version of the p-QDM dimerization. Further oligomerization is prohibited by steric hindrance of the aminyl radical.
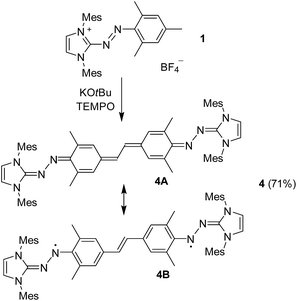
The biradical 3 is labile in solution and spontaneously converts into compound 4 at a slow rate. In fact, crystallization of 3 was only achievable at low temperature (−40 °C), whereas attempts performed at RT gave crystals of 4. The conversion of 1 into 4 can be facilitated with an external hydrogen atom abstractor such as TEMPO, and the best yield was obtained when using KOtBu as base (Scheme 4). In comparison to the dark rose brown parental 3, 4 displays a turquoise color in solution.
Two resonance structures have to be considered for 4: a closed-shell, quinoidal form 4A, and an open-shell diradical12 form 4B, which would benefit from the formation of two aromatic rings.20 Experimental (X-ray, NMR) and computational results suggest that 4 is best described as a closed shell system with low diradicaloid character.20
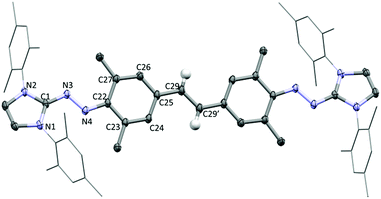
The molecular structure of compound 4 in the crystal is depicted in Fig. 2. Comparison with 3 in some structural parameters is given in Table S3 (ESI†). In contrast to 3, the central hydrocarbon fragment of 4 is essentially co-planar, consistent with a more conjugated framework. Significant changes for the bridging moieties are observed, including an increased “zigzag” angle (avg. 112.8° in 3vs. 124.6° in 4), and a shortened distance of the central C–C bond (1.484 Å in 3vs. 1.408 Å in 4). The bridging hydrocarbon fragment undergoes considerable quinoidization, with C25–C29, C23–C24 (and C26–C27), and C22–41 bonds shortened substantially, and the BLA increased to 0.07 Å (compared to 0.014 Å for 3). It is worth noting that the BLA of 4 is larger than that of ‘Tschitschibabin's hydrocarbon’ (Ph2C(C6H4)2CPh2, BLA = 0.052 Å, see Table S3, ESI,† for more details).21 This compound has been studied extensively, and it was concluded to have a closed-shell quinoidal structure in the ground state.20,21
DFT computations ((U)TPSS-D3/cc-pVDZ) suggest that 4 is characterized by a closed-shell ground state, stabilized relative to the open-shell triplet and singlet states by 8.6 and 38.8 kcal mol−1, respectively.
In line with these results, a solution of 4 in THF-d8 gave rise to a well-resolved 1H NMR spectrum. At room temperature, only one set of signals was observed for the four mesityl groups. Apparently, rotation around the Cimidazole–N bond is fast on the NMR time scale. At lower temperatures, however, the 1H NMR spectra are more complex, indicating a reduced rotational freedom.
The ESR characterization of 4 was also performed. Surprisingly, the ESR spectrum suggested a spin doublet. However, the intensity of the signal was weak, and its percentage varied from batch to batch. We assume that the signal is due to small impurities of a radical cation, which is formed from 4 by oxidation. In fact, compound 4 is a strong reducing agent with a reversible redox transition at −0.76 V vs. ferrocenium/ferrocene (Fc+/Fc) as revealed by cyclic voltammetry (for details see ESI†). It should be noted that ESR-active impurities have been observed for structurally related compounds such as ‘Tschitschibabin's hydrocarbon’.21
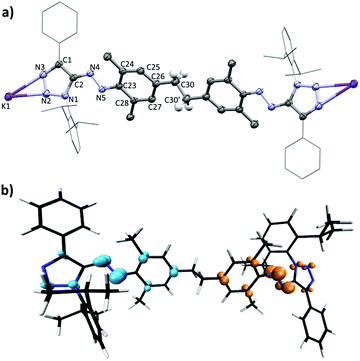
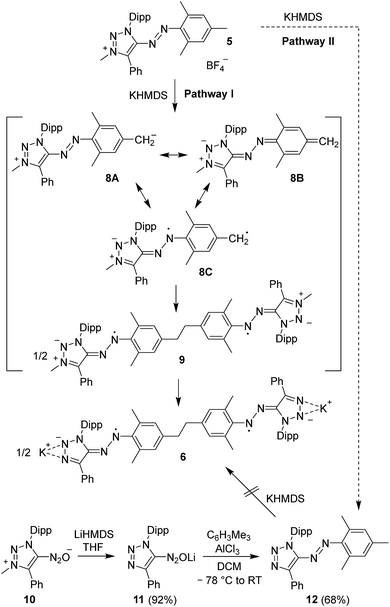
In order to examine if similar Csp3–Csp3 coupling reactions could be observed for other cationic azo dyes, we have investigated the reaction of the azotriazolium dye 5 (ref. 14a) with KHMDS. When base was added to a solution of 5 in THF-d8, vanishing of the 1H NMR signals was observed, as in the case of 1 (ESI, Fig. S8†). Attempts to isolate a defined product from reactions on preparative scale were first not successful. However, when two equivalents of KHMDS were employed, we were able to isolate the N-demethylated dimer 6 in 36% yield (Scheme 5). Dark brown-green crystals, suitable for X-ray crystallography, were obtained by slow diffusion of pentane into a THF solution of 6 at −40 °C.
Fig. 3a shows the structure of 6 in the crystal. As observed for 1, the reaction with KHMDS had resulted in a C–C coupling reaction involving the p-CH3 groups of the mesityl substituent. The dimer has an overall charge of minus two, and charge compensation is achieved by two potassium ions. The latter are coordinated in a κ2-type fashion to the heterocycle, with the remaining coordination sites being occupied by THF molecules (not shown). The shorter K1–N2 distance (2.810(4) Å) compared to the K1–N3 distance (2.969(5) Å) suggests a higher charge density at the N2 atom. Both N–K distances fall in the range reported for compounds with a side-on κ2-K(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)− motif.22 Compared to the structure of the starting material 5,14a a comprehensive expansion of the triazole rings is observed, with a lengthening of all five bonds. This change is consistent with the conversion of a cationic into an anionic ring system.
N)− motif.22 Compared to the structure of the starting material 5,14a a comprehensive expansion of the triazole rings is observed, with a lengthening of all five bonds. This change is consistent with the conversion of a cationic into an anionic ring system.
Like 3, compound 6 was expected to feature two unpaired electrons. This assumption is in line with the observed vanishing of the 1H NMR signals of 5 upon addition of base. Further evidence of its biradical nature was obtained through ESR measurements. The ESR spectrum acquired at room temperature for 6 in the solid state pointed to a spin doublet with the g-factor of 2.0036 (ESI, Fig. S19†). The electronic structure computations ((U)TPSS-D3/cc-pVDZ) shows that dimer 6 favors an open-shell singlet ground state, with the triplet state almost isoenergetic. The largest spin density is found on the azo bridge, with the nitrogen atoms accounting symmetrically and in pairs for 21% (N5 and N10) and for 12% (N4 and N9) of the α- and β-spin densities (Fig. 3b).
Crystallization of a THF solution of 6 under air gave bright red crystals of the neutral dimer 7, the structure of which was analyzed by X-ray crystallography (ESI, Fig. S20†). The ethylene bridge is well preserved, while the remaining parts of the molecule restore complete aromaticity, with small BLA values (0.019 Å) for the benzene rings, well-defined azo (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) bonds, and characteristic neutral triazole rings.
N) bonds, and characteristic neutral triazole rings.
The generation of the biradical 6 from the cationic monomer 5 requires two chemical transformations, namely N-demethylation of the heterocycle,23 and base-induced C–C coupling. A priori, two reaction pathways can be envisaged, depending on the sequence of these two transformations (Scheme 6). In order to address this point, we have synthesized the neutral azo dye 12, which represents the expected intermediate if N-demethylation proceeds before C–C coupling (Pathway II). The synthesis of 12 was accomplished by demethylation of the known diazotate 10 (ref. 14a) with LiHMDS, followed by azo coupling with mesitylene (Scheme 6, for a crystallographic characterization of the intermediate lithium salt 11, see ESI†). Compound 12 turned out to be inert towards KHMDS. Consequently, we propose that the formation of 6 proceeds via Pathway I, involving the initial formation of 8, which displays a p-QDM-like reactivity. Dimerization gives the biradical 9, which undergoes a two-fold N-demethylation to afford 6.
Conclusions
In summary, we have shown that cationic azo dyes with diazenylmesityl groups can undergo a base-induced Csp3–Csp3 coupling reaction to give biradicals. The presence of a CH3 group in the para position to the azo group is essential for this coupling reaction, because dyes with CH3 groups in ortho or meta position are deprotonated at imidazolium heterocycle.16 The dimerization of the azo dyes is proposed to involve the formation of the neutral monomers 2 and 8, which display p-QDM-like structures and reactivity.The polymerization of p-QDM is an intriguing reaction, because it appears to proceed via a radical mechanism, even though p-QDM has a closed-shell structure. It is generally assumed that the polymerization is initiated by the formation of a biradical p-QDM dimer, but the characterization of this highly reactive intermediate has remained elusive. Our results show that it is possible to intercept dimers with a biradical structure if the terminal radical sites are stabilized. In the case of 3 and 6, this stabilization is achieved by replacing the H2Ċ groups with sterically shielded aminyl radicals.
The dimerization reactions described in this contribution are also interesting from a synthetic chemistry point of view, because the homo-coupling of azo dyes represents a new way to synthesize more complex dye structures. Polyconjugated compounds such as 4, for example, could also be of interest for optoelectronic applications.19a,20a
Conflicts of interest
There are no conflicts to declare.Author contributions
Y. L., P. V., L. Y. M. E. and A. G. T. synthesized and analyzed the compounds, A. F. and C. C. performed the computational study, O. M. P. performed the CV experiments, E. S., F. F.-T., and R. S. carried out the crystallographic analyses, A. S. performed the ESR analyses, K. S. initiated and coordinated the study, and all authors except E. S. and A. G. T. contributed to writing of the manuscript.Acknowledgements
Y. L. was an EPFL Fellow co-funded by the Marie Skłodowska-Curie program (grant No. 665667). The work was supported by the Swiss National Science Foundation, and by the Ecole Polytechnique Fédérale de Lausanne (EPFL). We thank Abdusalom Suleymanov for help with the ESR measurements, and Emilie Baudat for NMR measurements.References
- (a) A. Konishi and T. Kubo, Top. Curr. Chem., 2017, 375, 83 CrossRef PubMed; (b) J. Casado, Top. Curr. Chem., 2017, 375, 73 CrossRef PubMed.
- (a) L. A. Errede and M. Szwarc, Q. Rev., Chem. Soc., 1958, 12, 301 RSC; (b) M. Szwarc, J. Chem. Phys., 1948, 16, 128 CrossRef CAS; (c) M. Szwarc, Nature, 1947, 160, 403 CrossRef CAS; (d) M. Szwarc, Discuss. Faraday Soc., 1947, 2, 46–49 RSC.
- W. F. Gorham, J. Polym. Sci., Part A-1: Polym. Chem., 1966, 4, 3027–3039 CrossRef CAS.
- H. Hopf, Angew. Chem., Int. Ed., 2008, 47, 9808 CrossRef CAS PubMed.
- (a) C. P. Tan and H. C. Craighead, Materials, 2010, 3, 1803 CrossRef CAS; (b) J. B. Fortin and T.-M. Lu, Chemical vapor deposition polymerization: the growth and properties of parylene thin films, Kluwer, Boston, 2004 CrossRef; (c) M. Szwarc, Polym. Eng. Sci., 1976, 16, 473 CrossRef CAS.
- L. A. Errede and J. M. Hoyt, J. Am. Chem. Soc., 1960, 82, 436 CrossRef CAS.
- For the spectroscopic characterization of p-DQM, see: (a) P. G. Mahaffy, J. D. Wieser and L. K. Montgomery, J. Am. Chem. Soc. , 1977, 99, 4514 CrossRef CAS; (b) T. Koenig, R. Wielesek, W. Snell and T. Balle, J. Am. Chem. Soc. , 1975, 97, 3225 CrossRef CAS; (c) Y. Yamakita, Y. Furukawa and M. Tasumi, Chem. Lett. , 1993, 22, 311 CrossRef; (d) J. M. Pearson, H. A. Six, D. J. Williams and M. Levy, J. Am. Chem. Soc. , 1971, 93, 5034 CrossRef CAS; (e) D. J. Williams, J. M. Pearson and M. Levy, J. Am. Chem. Soc. , 1970, 92, 1436 CrossRef CAS.
- M. Bobrowski, P. Skurski and S. Freza, Chem. Phys., 2011, 382, 20 CrossRef CAS.
- K. Smalara, A. Giełdoń, M. Bobrowski, J. Rybicki and C. Czaplewski, J. Phys. Chem. A, 2010, 114, 4296 CrossRef CAS PubMed.
- (a) W. S. Trahanovsky and S. P. Lorimor, J. Org. Chem., 2006, 71, 1784 CrossRef CAS PubMed; (b) L. A. Errede, R. S. Gregorian and J. M. Hoyt, J. Am. Chem. Soc., 1960, 82, 5218 CrossRef CAS.
- (a) D. A. Klumpp, T. M. Gilbert and W. S. Trahanovsky, Eur. J. Org. Chem., 2016, 5559 CrossRef CAS; (b) W. S. Trahanovsky and D. A. Klumpp, Tetrahedron Lett., 2016, 57, 2386 CrossRef CAS; (c) X. Fu and D. Zhao, Org. Lett., 2015, 17, 5694 CrossRef CAS PubMed; (d) W. S. Trahanovsky, D. L. Miller and Y. Wang, J. Org. Chem., 1997, 62, 8980 CrossRef CAS.
- The term ‘biradical’ is used for molecules in which the two unpaired electrons are largely independent. Electronically coupled systems are referred to as ‘diradicals’ or ‘diradicaloids’. For a more detailed discussion see: M. Abe, Chem. Rev., 2013, 113, 7011 CrossRef CAS PubMed.
- R. Raue, Methine Dyes and Pigments. Ullmann’s Encyclopedia of Industrial Chemistry, 2000, pp. 1−55 Search PubMed.
- (a) L. Y. M. Eymann, R. Scopelliti, F. Fadaei Tirani and K. Severin, Chem.–Eur. J., 2018, 24, 7957 CrossRef CAS PubMed; (b) A. G. Tskhovrebov, L. C. E. Naested, E. Solari, R. Scopelliti and K. Severin, Angew. Chem., Int. Ed., 2015, 54, 1289 CrossRef CAS PubMed.
- L. Y. M. Eymann, A. G. Tskhovrebov, A. Sienkiewicz, J. L. Bila, I. Živković, H. M. Rønnow, M. D. Wodrich, L. Vannay, C. Corminboeuf, P. Pattison, E. Solari, R. Scopelliti and K. Severin, J. Am. Chem. Soc., 2016, 138, 15126 CrossRef CAS PubMed.
- F. M. Chadwick, B. F. E. Curchod, R. Scopelliti, F. Fadaei Tirani, E. Solari and K. Severin, Angew. Chem., Int. Ed., 2019, 58, 1764 CrossRef CAS PubMed.
- For selected examples, see: (a) B. Manna, B. Joarder, A. V. Desai, A. Karmakar and S. K. Ghosh, Chem.–Eur. J., 2014, 20, 12399 CrossRef CAS PubMed; (b) N. Wang, J.-G. Ma, W. Shi and P. Cheng, CrystEngComm, 2012, 14, 5634 RSC; (c) B. Manna, A. K. Chaudhari, B. Joarder, A. Karmakar and S. K. Ghosh, Angew. Chem., Int. Ed., 2012, 52, 998 CrossRef PubMed; (d) A. Ghames, T. Douadi, D. Haffar, S. Chafaa, M. Allain, M. A. Khan and G. M. Bouet, Polyhedron, 2006, 25, 3201 CrossRef CAS; (e) M. Albrecht, I. Janser, H. Houjou and R. Fröhlich, Chem.–Eur. J., 2004, 10, 2839 CrossRef CAS PubMed; (f) Z. Feng, A. Fan, S. Valiyaveettil and J. J. Vittal, Cryst. Growth Des., 2003, 3, 555 CrossRef CAS; (g) P. Mandal, S. Paul, K. Goubitz and H. Schenik, Mol. Cryst. Liq. Cryst. Sci. Technol., Sect. A, 1995, 258, 209 CrossRef CAS.
- M. Kertesz, C. H. Choi and S. Yang, Chem. Rev., 2005, 105, 3448 CrossRef CAS PubMed.
- (a) J. Messelberger, A. Grünwald, P. Pinter, M. M. Mansmann and D. Munz, Chem. Sci., 2018, 9, 6107 RSC; (b) S. Grimme and A. Hansen, Angew. Chem., Int. Ed., 2015, 54, 12308 CrossRef CAS PubMed.
- For selected review articles about diradicaloids, see: (a) X. Hu, W. Wang, D. Wang and Y. Zheng, J. Mater. Chem. C, 2018, 6, 11232 RSC; (b) Y. Huang and E. Egap, Polym. J., 2018, 50, 603–614 CrossRef CAS; (c) T. Y. Gopalakrishna, W. Zeng, X. Lu and J. Wu, Chem. Commun., 2018, 54, 2186 RSC; (d) G. Tan and X. Wang, Acc. Chem. Res., 2017, 50, 1997–2006 CrossRef CAS PubMed; (e) Y. Ni and J. Wu, Tetrahedron Lett., 2016, 557, 5426 CrossRef; (f) G. Gryn’ova, M. L. Coote and C. Corminboeuf, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2015, 5, 440 Search PubMed; (g) Z. Zeng, X. Shi, C. Chi, J. T. Navarette, J. Casado and J. Wu, Chem. Soc. Rev., 2015, 44, 6578 RSC.
- L. K. Montgomery, J. C. Huffman, E. A. Jurczak and M. P. Grendze, J. Am. Chem. Soc., 1986, 108, 6004 CrossRef CAS PubMed.
- (a) C. Pi, Y. Wang, W. Zheng, L. Wan, H. Wu, L. Weng, L. Wu, Q. Li and P. von Ragué Schleyer, Angew. Chem., Int. Ed., 2010, 49, 1842 CrossRef CAS PubMed; (b) K. Kunz, H.-W. Lerner and M. Bolte, Acta Crystallogr., 2009, E65, m171 Search PubMed; (c) M.-Á. Velázquez-Carmona, A.-J. Metta-Magaña, S.-A. Cortés-Llamas, V. Montiel-Palma and M.-Á. Muñoz-Hernández, Polyhedron, 2009, 28, 205 CrossRef; (d) L. Wan, C. Pi, L. Zhang, W. Zheng, L. Weng, Z. Chen and Y. Zhang, Chem. Commun., 2008, 2266 RSC; (e) T. J. Hebden, W. W. Brennessel, C. J. Flaschenriem and P. L. Holland, Dalton Trans., 2006, 3855 RSC; (f) S.-Á. Cortés-Llamas, R. Hernández-Lamoneda, M.-Á. Velázquez-Carmona, M.-A. Muñoz-Hernández and R. A. Toscano, Inorg. Chem., 2006, 45, 286 CrossRef PubMed; (g) X.-R. Hu, Y.-W. Wang and J.-M. Gu, Acta Crystallogr., 2005, E61, m1686 Search PubMed.
- For examples of N-dealkylation of 1,2,3-triazolium salts see: (a) Z. Monasterio, A. Irastorza, J. I. Miranda and J. M. Aizpurua, Org. Lett., 2016, 18, 2511 CrossRef CAS PubMed; (b) R. Maity, T. Tichter, M. van der Meer and B. Sarkar, Dalton Trans., 2015, 44, 18311 RSC; (c) S. Koguchi and K. Izawa, Synthesis, 2012, 44, 3603 CrossRef CAS; (d) J. Bouffard, B. K. Keitz, R. Tonner, G. G. Barrios, G. Frenking, R. H. Grubbs and G. Bertrand, Organometallics, 2011, 30, 2617 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1903893–1903897. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc01502g |
| This journal is © The Royal Society of Chemistry 2019 |