Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Isomeric triazines exhibit unique profiles of bioorthogonal reactivity†
David N.
Kamber‡
a,
Sean S.
Nguyen‡
 a,
Fang
Liu
a,
Fang
Liu
 bc,
Jeffrey S.
Briggs
a,
Hui-Wen
Shih
a,
R. David
Row
a,
Zane G.
Long
bc,
Jeffrey S.
Briggs
a,
Hui-Wen
Shih
a,
R. David
Row
a,
Zane G.
Long
 a,
K. N.
Houk
a,
K. N.
Houk
 b,
Yong
Liang
b,
Yong
Liang
 *c and
Jennifer A.
Prescher
*c and
Jennifer A.
Prescher
 *ade
*ade
aDepartment of Chemistry, University of California, Irvine, California 92697, USA. E-mail: jpresche@uci.edu
bDepartment of Chemistry and Biochemistry, University of California, Los Angeles, California 90095, USA
cState Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210023, China. E-mail: yongliang@nju.edu.cn
dDepartment of Molecular Biology & Biochemistry, University of California, Irvine, California 92697, USA
eDepartment of Pharmaceutical Sciences, University of California, Irvine, California 92697, USA
First published on 21st August 2019
Abstract
Expanding the scope of bioorthogonal reactivity requires access to new and mutually compatible reagents. We report here that 1,2,4-triazines can be tuned to exhibit unique reaction profiles with biocompatible strained alkenes and alkynes. Computational analyses were used to identify candidate orthogonal reactions, and the predictions were experimentally verified. Notably, 5-substituted triazines, unlike their 6-substituted counterparts, undergo rapid [4 + 2] cycloadditions with a sterically encumbered strained alkyne. This unique, sterically controlled reactivity was exploited for dual bioorthogonal labeling. Mutually orthogonal triazines and cycloaddition chemistries will enable new multi-component imaging applications.
Bioorthogonal chemistries have been used extensively to tag biomolecules in complex environments.1 The success of these transformations is critically dependent on the stabilities of the reagents in cells and tissues. At the same time, the reagents must be robustly and singularly reactive with complementary probes.2 This chemical paradox has often frustrated efforts to develop reagents that exhibit selective reactivity, let alone multiple reactions that function in concert. In fact, there are only a handful of bioorthogonal reaction pairs that can be used simultaneously.3–16
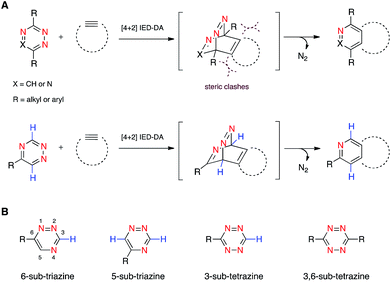
We are developing “privileged” scaffolds that not only meet the requirements for bioorthogonality, but are also compatible with existing reagents to enable tandem application.4,17 Such mutually orthogonal reagents are in demand for chemical tagging of multiple biomolecules.5–14 We recently reported that a 6-substituted 1,2,4-triazine constitutes a new privileged scaffold (Fig. 1A).18 This motif reacts with trans-cyclooctene (TCO) via inverse electron-demand Diels–Alder (IED-DA) cycloaddition. The rate of this cycloaddition can be further improved by scaffold tuning.19 6-Substituted triazines are also remarkably inert to other biological functionality. The enhanced stability of the triazine enabled its direct application in genetic code expansion and recombinant protein production.18 Additionally, while 6-substituted triazines react efficiently with TCO, they do not react with other bioorthogonal alkenes, including norbornene and cyclopropene.18 This result is in sharp contrast to related tetrazine motifs, which react vigorously with a variety of alkenes and alkynes.12,13,20–28
To expand upon the unique features of triazines, we aimed to synthesize and examine the reactivities of alternatively substituted rings. 1,2,4-Triazines react with dienophiles to form new bonds across C3 and C6.18,29 The regioselectivity of this addition could potentially be exploited for orthogonal reaction development: 6-substituted triazines would be less likely to react with bulky dienophiles than their 5-substituted counterparts (Fig. 1B). Thus, different substitution patterns could effectively “tune” triazine reactivity and promote selective cycloaddition. Similar tactics have been used to develop mutually compatible reactions with tetrazines.10 For example, mono-substituted tetrazines react rapidly even with sterically encumbered cyclooctynes.30,31 By contrast, disubstituted tetrazines do not react with similar alkynes due to steric clashes in the transition states.32
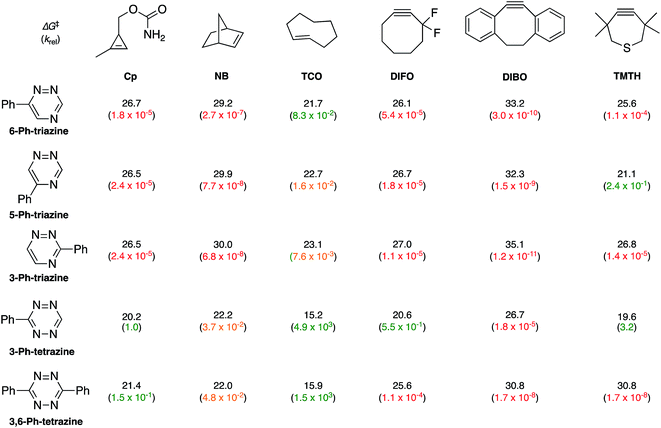
To examine whether differentially substituted triazines could provide mutually orthogonal reactions, we first used density functional theory (DFT) calculations at the M06-2X/6-311+G(d,p)//M06-2X/6-31G(d) level to evaluate the reactivities of model probes.33–36 Isomeric triazines and substituted tetrazines were included in the analyses, along with a panel of bioorthogonal strained dienophiles (Table 1).37–41 The triazine motifs differed in their substitution patterns, with phenyl groups positioned at C3 and C6 (the sites of new bond formation in cycloaddition reactions), or C5. In agreement with our previous work, triazines were predicted to react readily with TCO, although more slowly than their tetrazine counterparts. Minimal or no triazine reactivity was predicted with other strained alkenes, including 1-methylcyclopropene and norbornene. Structurally related tetrazines, by contrast, harbor much lower LUMO+1 energies and were predicted to react robustly with a variety of strained alkenes.42
When evaluating strained alkynes, the reactivity profiles of the triazine isomers diverged. The 5-substituted scaffold was predicted to react with tetramethylthiacycloheptyne (TMTH, 9), a sterically encumbered cycloalkyne developed by the Bertozzi group.43 The 3- and 6-substituted isomers were predicted to exhibit diminished reactivity with TMTH due to steric clashes at the reactive centers. Calculations further suggested that none of the triazine isomers would react efficiently with other strained alkynes, including DIBO and DIFO (molecules with lower HOMO energies), setting the stage for orthogonal reaction development.
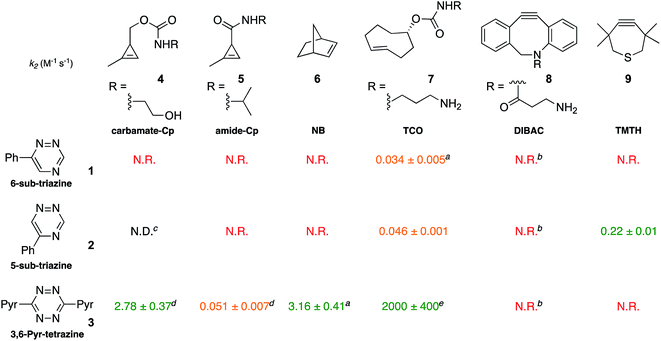
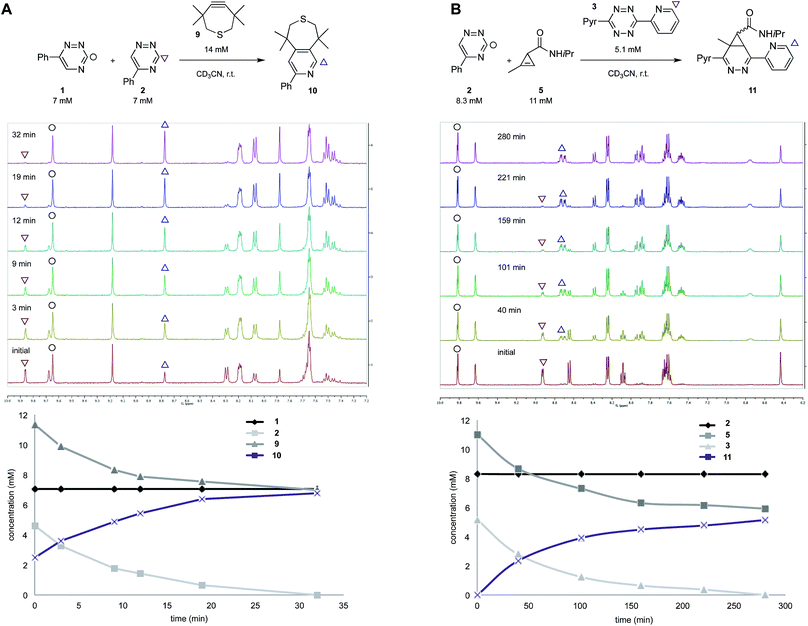
To validate the computational predictions, we synthesized the panel of reagents shown in Table 2. The triazine and tetrazine scaffolds were incubated with a variety of strained alkenes and alkynes, and the reactions were monitored by 1H-NMR spectroscopy (Fig. S1–S10†). The measured bimolecular rate constants closely matched those predicted by DFT calculations (Table 2). The 6-substituted triazine (1) reacted at a reasonable rate with TCO, but no other strained alkene (4–6). The 5-phenyl isomer (2) was also efficiently ligated with TCO, but reacted minimally with cyclopropene 4 (Fig. S3–S5†). Importantly, the most tantalizing prediction—robust reactivity between TMTH (9) and 5-phenyl-1,2,4-triazine (2)—was also validated (Fig. S9–S11†). The reaction proceeded with a rate constant of k2 = 0.22 ± 0.01 M−1 s−1. This rate is on par with many commonly used distortion-accelerated azide–alkyne cycloadditions.2,44–47 Under similar conditions, no reactivity was observed between 6-substituted triazine (1) and TMTH (9) (Fig. S12†). Even with a more reactive triazine (S3), minimal reactivity was observed (Fig. S13†). The selective reactivity of 2 with TMTH (9) was further showcased in a competition experiment (Fig. 2). When the isomeric triazines were combined in equimolar amounts and treated with excess TMTH (9), only the 5-substituted triazine (2) was consumed (Fig. 2A).
The unique reactivity profile of 5-phenyl triazine (2) suggested immediate opportunities for mutually orthogonal reaction development. While triazine 2 reacts quickly with TMTH (9), this isomer is refractory to ligation with bioorthogonal cyclopropenes (scaffolds known to react robustly with tetrazines, Fig. S14 and S15†). Since tetrazines and cyclopropenes are both inert to TMTH and triazines (Fig. S16†), these reagents could be exploited for dual [4 + 2] cycloaddition. To examine this possibility, triazine 2 was first mixed with cyclopropene 5 and tetrazine 3. Over the course of the reaction, the concentration of triazine 2 remained constant, while cyclopropene 5 and tetrazine 3 were consumed (Fig. 2B). When all four model reagents were combined (2.5 mM, 1 equiv.), the two expected cycloadducts were observed (Fig. 3, S17 and S18†). Similar results were obtained using a more reactive cyclopropene in the mixture (Fig. S19†). To our knowledge, these are the first examples of [4 + 2] IED-DA cycloadditions that can be used concurrently without cross-reactivity.3 Bonger and colleagues recently reported a pair of [4 + 2] cycloadditions for dual labeling, but precise reagent stoichiometries were required to prevent off-target reactions.13
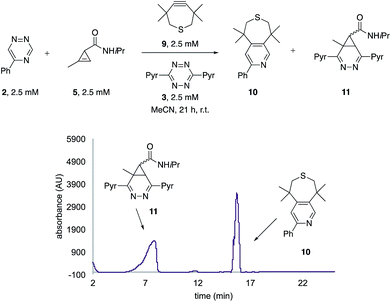 | ||
| Fig. 3 Orthogonal [4 + 2] cycloadditions. All reagents (2.5 mM) were combined and the reactions were monitored by HPLC (210 nm). Two distinct cycloadducts were observed. | ||
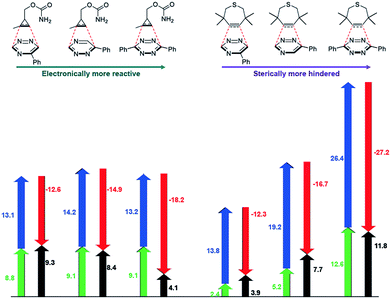
The mutual orthogonality of the reagents from this work arises from the interplay of intrinsic reactivities and steric factors.18,48 Tetrazines are more reactive than triazines with bioorthogonal dienophiles due to the lower LUMO+1 energies of the π-systems.42,49,50 This intrinsic order of reactivity is manifested in the “Distortion/Interaction-Activation Strain Model”51 analysis shown in Fig. 4. The sterically unhindered cyclopropene reacts most slowly with 2, slightly faster with 1, and fastest with the tetrazine analog. These differences in reactivity arise from increased interaction energies that parallel the increasing electrophilicity across the series. The same behavior is observed with other sterically unhindered dienophiles, such as the highly reactive TCO. With a sterically hindered dienophile (e.g., TMTH), though, the opposite trend is observed. The more hindered tetrazine reacts most slowly with TMTH (9), while the less hindered 5-phenyl triazine (2) ligates most rapidly. TMTH reactivity is mainly controlled by distortion energies that are strongly influenced by steric considerations. Balancing electronic interaction energies (that enhance reactivity) with steric effects (that increase distortion energies and thus decrease reactivity) is a general approach for developing mutually orthogonal bioorthogonal reactions.11,36,48
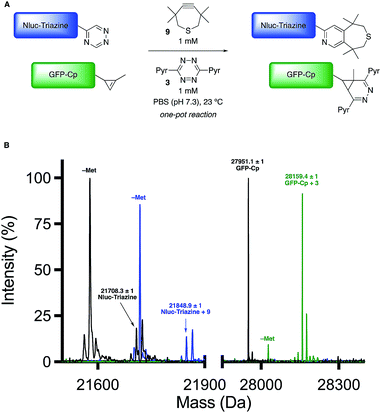
Encouraged by the computational and experimental analyses, we examined whether the orthogonal cycloadditions could be used in more complex environments. Toward this end, we attempted the labeling reactions in concert with two model proteins (GFP and NanoLuciferase, Nluc). We attached a single 5-substituted triazine to Nluc using cysteine-maleimide chemistry (Fig. S20 and Scheme S1†). In this case, Nluc was engineered to harbor a single cysteine at residue 180.52 The resulting conjugate (Nluc-Triazine) was readily ligated with TMTH (9, Fig. S21†). We further prepared a cyclopropene-GFP (GFP-Cp) conjugate via genetic code expansion (Fig. S21†).53 The model proteins were combined 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in PBS (pH 7.3, 2 μM final concentration). The mixture was then treated with TMTH (9) and tetrazine 3. After 3 h, full consumption of the starting proteins was observed via mass spectrometry (Fig. 5, S21 and S22†). No cross-reactivities were observed, suggesting that the cycloadditions can be used to label two biological targets simultaneously. The orthogonal [4 + 2] cycloadditions could also be performed in the presence of lysate, conditions that mimic cellular environments (Fig. S23†). The cycloadditions were also compatible with polar bioorthogonal reactants, enabling simultaneous, triple-component ligations (Fig. S24 and S25†). Collections of three mutually orthogonal reactions are rare.54,55
1 in PBS (pH 7.3, 2 μM final concentration). The mixture was then treated with TMTH (9) and tetrazine 3. After 3 h, full consumption of the starting proteins was observed via mass spectrometry (Fig. 5, S21 and S22†). No cross-reactivities were observed, suggesting that the cycloadditions can be used to label two biological targets simultaneously. The orthogonal [4 + 2] cycloadditions could also be performed in the presence of lysate, conditions that mimic cellular environments (Fig. S23†). The cycloadditions were also compatible with polar bioorthogonal reactants, enabling simultaneous, triple-component ligations (Fig. S24 and S25†). Collections of three mutually orthogonal reactions are rare.54,55
In conclusion, we identified isomeric triazine scaffolds that exhibit unique cycloaddition profiles. Computational and experimental analyses of triazine reactivity were performed. Isomeric triazines were found to react robustly with TCO, but only the least sterically encumbered isomers (5-substituted) reacted with other dienophiles. Notably, 5-substituted 1,2,4-triazines reacted efficiently with TMTH, one of the most sterically encumbered strained alkynes reported to date. The cycloaddition was successfully used in combination with another popular IED-DA reaction, the tetrazine ligation with 1-methylcyclopropene. These mutually compatible reactions can be used in tandem to tag protein targets in biologically relevant environments. Future work will address the need for functional TMTH conjugates. TMTH has been historically difficult to outfit with fluorophores and other reporter groups, although new strategies for derivatization are being pursued. A panel of easily accessible reagents will further enhance multi-component labeling applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the UCI School of Physical Sciences, the Alfred P. Sloan Foundation (J. A. P.) and the National Institutes of Health, National Institute of General Medical Sciences (GM-109078 to K. N. H. and GM126226 to J. A. P.). Y. L. thanks the support from the “Innovation & Entrepreneurship Talents Plan” of Jiangsu Province in China. We thank Rachel Steinhardt for help in preparing TMTH, and members of the Dong, Jarvo, and Overman laboratories for providing reagents. We thank Benjamin Katz for assistance with protein mass spectrometry experiments and Phil Dennison for assistance with NMR experiments. We also thank David Patterson for helpful discussions, along with members of the Prescher and Houk laboratories.References
- M. Grammel and H. C. Hang, Nat. Chem. Biol., 2013, 9, 475–484 CrossRef CAS PubMed.
- D. M. Patterson, L. A. Nazarova and J. A. Prescher, ACS Chem. Biol., 2014, 9, 592–605 CrossRef CAS PubMed.
- D. M. Patterson and J. A. Prescher, Curr. Opin. Chem. Biol., 2015, 28, 141–149 CrossRef CAS PubMed.
- R. D. Row and J. A. Prescher, Acc. Chem. Res., 2018, 51, 1073–1081 CrossRef CAS PubMed.
- A. Sachdeva, K. Wang, T. Elliott and J. W. Chin, J. Am. Chem. Soc., 2014, 136, 7785–7788 CrossRef CAS PubMed.
- J. E. Hudak, R. M. Barfield, G. W. de Hart, P. Grob, E. Nogales, C. R. Bertozzi and D. Rabuka, Angew. Chem., Int. Ed., 2012, 51, 4161–4165 CrossRef CAS PubMed.
- K. A. Andersen, M. R. Aronoff, N. A. McGrath and R. T. Raines, J. Am. Chem. Soc., 2015, 137, 2412–2415 CrossRef CAS PubMed.
- L. I. Willems, N. Li, B. I. Florea, M. Ruben, G. A. van der Marel and H. S. Overkleeft, Angew. Chem., Int. Ed., 2012, 51, 4431–4434 CrossRef CAS PubMed.
- M. R. Karver, R. Weissleder and S. A. Hilderbrand, Angew. Chem., Int. Ed., 2012, 51, 920–922 CrossRef CAS PubMed.
- D. N. Kamber, L. A. Nazarova, Y. Liang, S. A. Lopez, D. M. Patterson, H.-W. Shih, K. N. Houk and J. A. Prescher, J. Am. Chem. Soc., 2013, 135, 13680–13683 CrossRef CAS PubMed.
- M. K. Narayanam, Y. Liang, K. N. Houk and J. M. Murphy, Chem. Sci., 2016, 7, 1257–1261 RSC.
- D. M. Patterson, L. A. Nazarova, B. Xie, D. N. Kamber and J. A. Prescher, J. Am. Chem. Soc., 2012, 134, 18638–18643 CrossRef CAS PubMed.
- S. Eising, B.-T. Xin, F. Kleinpenning, J. J. A. Heming, B. I. Florea, H. S. Overkleeft and K. M. Bonger, ChemBioChem, 2018, 19, 1648–1652 CrossRef CAS PubMed.
- A. R. Sherratt, M. Chigrinova, D. A. MacKenzie, N. K. Rastogi, M. T. M. Ouattara, A. T. Pezacki and J. P. Pezacki, Bioconjugate Chem., 2016, 27, 1222–1226 CrossRef CAS PubMed.
- Z. Shao, W. Liu, H. Tao, F. Liu, R. Zeng, P. A. Champagne, Y. Cao, K. N. Houk and Y. Liang, Chem. Commun., 2018, 54, 14089–14092 RSC.
- U. Reisacher, D. Ploschik, F. Rönicke, G. B. Cserép, P. Kele and H.-A. Wagenknecht, Chem. Sci., 2019, 10, 4032–4037 RSC.
- H.-W. Shih, D. N. Kamber and J. A. Prescher, Curr. Opin. Chem. Biol., 2014, 21, 103–111 CrossRef CAS PubMed.
- D. N. Kamber, Y. Liang, R. J. Blizzard, F. Liu, R. A. Mehl, K. N. Houk and J. A. Prescher, J. Am. Chem. Soc., 2015, 137, 8388–8391 CrossRef CAS PubMed.
- S. J. Siegl, R. Dzijak, A. Vásquez, R. Pohl and M. Vrabel, Chem. Sci., 2017, 8, 3593–3598 RSC.
- M. L. Blackman, M. Royzen and J. M. Fox, J. Am. Chem. Soc., 2008, 130, 13518–13519 CrossRef CAS PubMed.
- K. Lang, L. Davis, S. Wallace, M. Mahesh, D. J. Cox, M. L. Blackman, J. M. Fox and J. W. Chin, J. Am. Chem. Soc., 2012, 134, 10317–10320 CrossRef CAS PubMed.
- K. Lang, L. Davis, J. Torres-Kolbus, C. Chou, A. Deiters and J. W. Chin, Nat. Chem., 2012, 4, 298–304 CrossRef CAS PubMed.
- S. B. Engelsma, L. I. Willems, C. E. van Paaschen, S. I. van Kasteren, G. A. van der Marel, H. S. Overkleeft and D. V. Filippov, Org. Lett., 2014, 16, 2744–2747 CrossRef CAS PubMed.
- H. Wu, B. T. Cisneros, C. M. Cole and N. K. Devaraj, J. Am. Chem. Soc., 2014, 136, 17942–17945 CrossRef CAS PubMed.
- B. L. Oliveira, Z. Guo, O. Boutureira, A. Guerreiro, G. Jiménez-Osés and G. J. L. Bernardes, Angew. Chem., Int. Ed., 2016, 55, 14683–14687 CrossRef CAS PubMed.
- J. Tu, M. Xu, S. Parvez, R. T. Peterson and R. M. Franzini, J. Am. Chem. Soc., 2018, 140, 8410–8414 CrossRef CAS PubMed.
- S. J. Siegl, A. Vázquez, R. Dzijak, M. Dračínský, J. Galeta, R. Rampmaier, B. Klepetářová and M. Vrabel, Chem.–Eur. J., 2018, 24, 2426–2432 CrossRef CAS PubMed.
- B. J. Umlauf, K. A. Mix, V. A. Grosskopf, R. T. Raines and E. V. Shusta, Bioconjugate Chem., 2018, 29, 1605–1613 CrossRef CAS PubMed.
- K. A. Horner, N. M. Valette and M. E. Webb, Chem.–Eur. J., 2015, 21, 14376–14381 CrossRef CAS PubMed.
- G. B. Cserép, O. Demeter, E. Bätzner, M. Kállay, H.-A. Wagenknecht and P. Kele, Synthesis, 2015, 47, 2738–2744 CrossRef.
- I. Nikić, T. Plass, O. Schraidt, J. Szymański, J. A. G. Briggs, C. Schultz and E. A. Lemke, Angew. Chem., Int. Ed., 2014, 53, 2245–2249 CrossRef.
- J. A. Wagner, D. Mercadante, I. Nikić, E. A. Lemke and F. Gräter, Chem.–Eur. J., 2015, 21, 12431–12435 CrossRef CAS PubMed.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 Search PubMed.
- Y. Zhao and D. G. Truhlar, Acc. Chem. Res., 2008, 41, 157–167 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Gaussian Inc., Wallingford, CT, 2013 Search PubMed.
- F. Liu, Y. Liang and K. N. Houk, Acc. Chem. Res., 2017, 50, 2297–2308 CrossRef CAS PubMed.
- M. F. Debets, J. C. M. van Hest and F. P. J. T. Rutjes, Org. Biomol. Chem., 2013, 11, 6439–6455 RSC.
- C. P. Ramil and Q. Lin, Chem. Commun., 2013, 49, 11007–11022 RSC.
- P. Shieh and C. R. Bertozzi, Org. Biomol. Chem., 2014, 12, 9307–9320 RSC.
- K. Lang and J. W. Chin, ACS Chem. Biol., 2014, 9, 16–20 CrossRef CAS PubMed.
- K. Lang and J. W. Chin, Chem. Rev., 2014, 114, 4764–4806 CrossRef CAS PubMed.
- J. Yang, Y. Liang, J. Šečkutė, K. N. Houk and N. K. Devaraj, Chem.–Eur. J., 2014, 20, 3365–3375 CrossRef CAS PubMed.
- G. de Almeida, E. M. Sletten, H. Nakamura, K. K. Palaniappan and C. R. Bertozzi, Angew. Chem., Int. Ed., 2012, 51, 2443–2447 CrossRef CAS PubMed.
- E. M. Sletten, H. Nakamura, J. C. Jewett and C. R. Bertozzi, J. Am. Chem. Soc., 2010, 132, 11799–11805 CrossRef CAS PubMed.
- M. F. Debets, S. S. van Berkel, J. Dommerholt, A. T. J. Dirks, F. P. J. T. Rutjes and F. L. van Delft, Acc. Chem. Res., 2011, 44, 805–815 CrossRef CAS PubMed.
- M. King, R. Baati and A. Wagner, Chem. Commun., 2012, 48, 9308–9309 RSC.
- E. G. Burke, B. Gold, T. T. Hoang, R. T. Raines and J. M. Schomaker, J. Am. Chem. Soc., 2017, 139, 8029–8037 CrossRef CAS PubMed.
- Y. Liang, J. L. Mackey, S. A. Lopez, F. Liu and K. N. Houk, J. Am. Chem. Soc., 2012, 134, 17904–17907 CrossRef CAS PubMed.
- F. Liu, Y. Liang and K. N. Houk, J. Am. Chem. Soc., 2014, 136, 11483–11493 CrossRef CAS PubMed.
- Y.-F. Yang, Y. Liang, F. Liu and K. N. Houk, J. Am. Chem. Soc., 2016, 138, 1660–1667 CrossRef CAS PubMed.
- F. M. Bickelhaupt and K. N. Houk, Angew. Chem., Int. Ed., 2017, 56, 10070–10086 CrossRef CAS PubMed.
- W. Engelen, K. M. van de Wiel, L. H. H. Meijer, B. Saha and M. Merkx, Chem. Commun., 2017, 53, 2862–2865 RSC.
- T. S. Elliott, F. M. Townsley, A. Bianco, R. J. Ernst, A. Sachdeva, S. J. Elsässer, L. Davis, K. Lang, R. Pisa, S. Greiss, K. S. Lilley and J. W. Chin, Nat. Biotechnol., 2014, 32, 465–472 CrossRef CAS PubMed.
- J. S. Italia, P. S. Addy, S. B. Erickson, J. C. Peeler, E. Weerapana and A. Chatterjee, J. Am. Chem. Soc., 2019, 141, 6204–6212 CrossRef CAS PubMed.
- J. Tu, D. Svatunek, S. Parvez, A. C. Liu, B. J. Levandowski, H. J. Eckvahl, R. T. Peterson, K. N. Houk and R. M. Franzini, Angew. Chem., Int. Ed., 2019, 58, 9043–9048 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedures, protein expression procedures, computational details, NMR and mass spectra. See DOI: 10.1039/c9sc01427f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |