Open Access Article
Open Access ArticleDiketopyrrolopyrrole-based fluorescence probes for the imaging of lysosomal Zn2+ and identification of prostate cancer in human tissue†
Chenchen
Du‡
a,
Shibo
Fu‡
 b,
Xiaohua
Wang
a,
Adam C.
Sedgwick
b,
Xiaohua
Wang
a,
Adam C.
Sedgwick
 c,
Wei
Zhen
a,
Minjie
Li
a,
Xinqiang
Li
d,
Juan
Zhou
*b,
Zhong
Wang
*b,
Hongyu
Wang
c,
Wei
Zhen
a,
Minjie
Li
a,
Xinqiang
Li
d,
Juan
Zhou
*b,
Zhong
Wang
*b,
Hongyu
Wang
 *a and
Jonathan L.
Sessler
*a and
Jonathan L.
Sessler
 *ac
*ac
aDepartment of Chemistry, College of Science, Center for Supramolecular Chemistry & Catalysis, Shanghai University, 99 Shangda Road, Shanghai, 200444, P. R. China. E-mail: wanghy@shu.edu.cn
bDepartment of Urology, Shanghai Ninth People's Hospital, Shanghai Jiaotong University, School of Medicine, Shanghai, 200011, P. R. China
cDepartment of Chemistry, The University of Texas at Austin, 105 E 24th Street A5300, Austin, TX 78712-1224, USA. E-mail: sessler@cm.utexas.edu
dPathology Department, First Affiliated Hospital of Zhengzhou University, 1 Jianshe East Road, Zhengzhou, Henan Province 450052, P. R. China
First published on 1st May 2019
Abstract
A series of diketopyrrolopyrrole-based fluorescent probes (DPP-C2, LysoDPP-C2, LysoDPP-C3, and LysoDPP-C4) have been developed for the detection of low pH and Zn2+ in an AND logic fashion. The chelation of Zn2+ or the protonation of a morpholine moiety within these probes results in a partial increase in the fluorescence intensity, an effect ascribed to suppression of one possible photo-induced electron transfer (PET) pathway. In contrast, a large increase in the observed fluorescence intensity is observed at low pH and in the presence of Zn2+; this is rationalized in terms of both possible PET pathways within the probes being blocked. Job plots, fluorescence titration curves, and isothermal titration calorimetry proved consistent with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Zn2+ complexation stoichiometry. Each probe demonstrated an excellent selectivity towards Zn2+ and the resulting Zn2+ complexes demonstrated pH sensitivity over the 3.5–9 pH range. Fluorescence imaging experiments confirmed that LysoDPP-C4 was capable of imaging lysosomal Zn2+ in live cells. Little evidence of cytotoxicity was seen. LysoDPP-C4 was successfully applied to the bioimaging of nude mice, wherein it was shown capable of imaging the prostate. Histological studies using a human sample revealed that LysoDPP-C4 can discriminate cancerous prostate tissue from healthy prostate tissue.
1 Zn2+ complexation stoichiometry. Each probe demonstrated an excellent selectivity towards Zn2+ and the resulting Zn2+ complexes demonstrated pH sensitivity over the 3.5–9 pH range. Fluorescence imaging experiments confirmed that LysoDPP-C4 was capable of imaging lysosomal Zn2+ in live cells. Little evidence of cytotoxicity was seen. LysoDPP-C4 was successfully applied to the bioimaging of nude mice, wherein it was shown capable of imaging the prostate. Histological studies using a human sample revealed that LysoDPP-C4 can discriminate cancerous prostate tissue from healthy prostate tissue.
Introduction
Zinc (Zn2+) is one of the most common d-block metals found in the human body. It plays an important role in a wide range of biochemical processes and is used to maintain the structural integrity of over 3000 human proteins.1 In addition, so-called labile Zn2+ ions are commonly found in specific areas of the human body, including the prostate.2 In the prostate, Zn2+ is found at concentrations between ∼10 to 100 mM.3,4 This high Zn2+ concentration serves to inhibit m-aconitase activity thus preventing citrate oxidation during the Krebs cycle.2,5,6 This unique function allows increased production and secretion of citrate into the prostatic fluid and is considered vital for the normal function of the prostate. However, in malignant prostate tissue, there is a dramatic decrease in Zn2+ concentration (500 nmol g−1vs. 3000 nmol g−1 – for malignant and healthy tissues, respectively), as well as a downregulation of the zinc transporter, ZIP1.7–10 Indeed, strong correlations between prostatic Zn2+ levels and prostate cancer (PCa) have been reported.5 These correlations are providing an incentive to develop probes for Zn2+ that may be used to image the prostate and differentiate between normal and cancerous tissues in biopsy samples. Here, we report initial efforts to develop such a probe.To date, considerable effort has been devoted to the development of Zn2+ probes.11–20 Fluorescence-based systems have received particular attention in this regard since they provide good sensitivity, selectivity, and spatial and temporal resolution. In 2010, Lippard, et al. reported the fluorescence Zn2+ probe, ZPP1, which was successfully used to identify PCa in a transgenic mouse model.21 Subsequently, they developed a triphenylphosphonium (TPP) functionalized, reaction-based fluorescent probe (DA-ZP1-TPP), which selectively localized at the mitochondria. On the basis of studies with this probe, it was concluded that tumorigenic cancer cells are unable to accumulate Zn2+ within their mitochondria.22 More recently, Liu, et al. reported the Zn2+ sensor 3HC-DPA. This system relies on ESIPT (excited-state intramolecular proton transfer)23 to distinguish between cancerous prostate cells and healthy prostate cells.24 To our knowledge no known Zn2+ sensor has been applied to the problem of prostate tissue differentiation in human samples. Moreover, systems capable of detecting zinc cation concentrations in an organelle-selective way are still limited.22
Lysosomes are acidic organelles with a pH (3.5–6.0) that is distinctly lower than that of the cytoplasm (pH 7.2). Within the acidic lysosomal environment, a diversity of functions is maintained, such as the digestion and degradation of macromolecules by hydrolytic enzymes. These lysosomal functions are closely linked to other cell processes, including plasma membrane repair and metabolism.25 The abnormal function of lysosomes has been linked to the pathogenesis of a number of disorders and diseases.25,26 Probes that provide insight into the functioning of lysosomes may thus have a role to play in the diagnostic and staging of various diseases, including cancer.
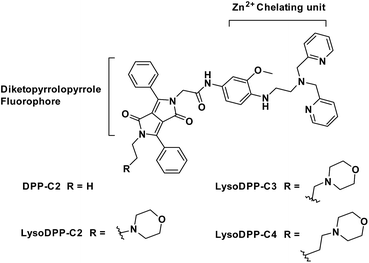
To our knowledge, there are currently no fluorescent probes that may be used to image lysosomal Zn2+ in prostate cancer cells. We believe that a dual detection strategy that monitors both pH and Zn2+ concentrations may allow this goal to be achieved. In recent years several research groups have focused on applying molecular logic to the problem of biological sensing.27–30 So-called AND logic-based fluorescence probes are particularly attractive since they may allow two or more biological analytes to be analysed simultaneously. In this work, we focused on the development of lysosomal targeting AND logic-based fluorescence probes for the detection of both acidic pH and Zn2+ (probes DPP-C2, LysoDPP-C2, LysoDPP-C3, and LysoDPP-C4; Fig. 1). This AND logic approach is designed to overcome the limitations associated with monitoring Zn2+ concentrations in the lysosome by increasing the signal-to-noise ratio between the lysosome (low pH – strong fluorescence) and cytosol (weak fluorescence).31–33
 | ||
| Fig. 1 The development of dual-functionalized diketopyrrolopyrrole (DPP)-based fluorescent probes for the AND logic-based detection of both acidic pH and Zn2+. | ||
Results and discussion
To obtain probes with dual Zn2+ and H+ sensing functions, both a methoxy-based N,N-di-(2-picolyl)ethylenediamine (MeO-DPEN) Zn2+ chelator34 and a lysosome-targeting morpholine moiety were appended synthetically to the lactam units of a 3,6-diphenyl diketopyrrolopyrrole (DPP) fluorophore (Fig. 1). Each of these functionalities was designed to quench the fluorescence via an independent photoinduced electron transfer (PET) pathway. In the presence of Zn2+, MeO-DPEN forms a chelate complex with the Zn2+ cation. This results in a partial increase in the fluorescence emission intensity through inhibition of the PET process associated with this particular subunit. When the resulting Zn2+ probe complex is exposed to an acidic environment (e.g., enters the lysosome), the morpholine unit becomes protonated; this leads to a complete restoration of the fluorescence emission intensity since both PET pathways are now blocked. The basic mechanistic rationale underlying our probe design and expected function is shown in Scheme 1.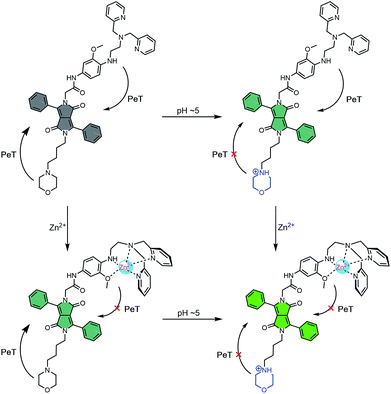 | ||
| Scheme 1 Fluorescence ‘turn on’ mechanism allowing for the concurrent detection of low pH AND Zn2+. Grey – weak fluorescence emission intensity. Green – strong fluorescence emission intensity. | ||
The synthesis of probes DPP-C2, LysoDPP-C2, LysoDPP-C3, and LysoDPP-C4 is detailed in the ESI (Schemes S1–S3†). In brief, dye 1 was asymmetrically acylated under basic conditions with tert-butyl bromoacetate to afford intermediate 2 in 25% yield. Compound 2 was then alkylated with bromoethane or bromo-substituted alkyl morpholine intermediates containing tethers of varying lengths (n = 2 and 3; precursors 3 and 4) using K2CO3 as a base, NaI as a catalyst, and acetone as the solvent to afford compounds 5, 6 and 7 in 56–60% yield. To access the alkyl (n = 4) morpholine functionalized DPP, intermediate 2 was alkylated with 1,4-dibromobutane using similar alkylation conditions. This afforded the bromo-alkyl intermediate 8, which was subsequently reacted with morpholine, giving compound 9 in excellent yield (79%). The tert-butyl esters of intermediates 5, 6, 7, and 9 were hydrolysed using trifluoroacetic acid to give the corresponding free carboxylic acids. Each carboxylic acid was taken onto the next step without purification and reacted with amino functionalized MeO-DPEN to yield the desired AND logic fluorescent probes DPP-C2, LysoDPP-C2, LysoDPP-C3, and LysoDPP-C4.
With each probe in hand, UV-Vis and fluorescence spectroscopic analyses were performed. The polarity of the solvent was shown to have little effect on the UV-Vis absorption or fluorescence emission spectrum of each probe or the corresponding Zn2+/probe complex (Fig. S1–S4, Tables S1 and S2†). Interestingly, the DPP-C2/Zn2+ complex demonstrated poor photostability with the intensity of the UV-Vis absorption and fluorescence maxima being reduced by 58% and 41%, respectively, after one week of exposure to sun light. In contrast, the morpholine-functionalized probes exhibited greater photostability with only a 33%, 26% and 20% reduction in fluorescence emission maxima being seen for LysoDPP-C2/Zn2+, LysoDPP-C3/Zn2+, and LysoDPP-C4/Zn2+, respectively, after a similar week of exposure to sun light (Fig. S5 and S6†).
The Zn2+ affinity of each probe was then assessed. As prepared, each probe proved relatively non-fluorescent (ΦF below 0.8%). However, treatment with Zn2+ (ZnSO4) resulted in an increase in fluorescence intensity. Through quantitative fluorescence titrations with corresponding good fits to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding profile, it was determined that all four probes responded to nanomolar concentrations of Zn2+ (Kd = 2.83, 1.54, 2.57, and 1.91 nm for DPP-C2, LysoDPP-C2, LysoDPP-C3, and LysoDPP-C4, respectively; see Fig. S7–S13†). Job plot analyses provided support for the proposed 1
1 binding profile, it was determined that all four probes responded to nanomolar concentrations of Zn2+ (Kd = 2.83, 1.54, 2.57, and 1.91 nm for DPP-C2, LysoDPP-C2, LysoDPP-C3, and LysoDPP-C4, respectively; see Fig. S7–S13†). Job plot analyses provided support for the proposed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. The ability to interact with Zn2+ was further confirmed through isothermal titration calorimetry (ITC) (Fig. S14 and S15†).
1 stoichiometry. The ability to interact with Zn2+ was further confirmed through isothermal titration calorimetry (ITC) (Fig. S14 and S15†).
Next, we evaluated the ability of each probe to detect Zn2+ at different pH. As shown in Fig. 2, S16 and S17,† the probes proved non-fluorescent in aqueous solution over the 6.5–9 pH range. As expected, the addition of Zn2+ (1 μM) to the non-morpholine functionalized control DPP-C2 probe resulted in the complete restoration of the fluorescence emission intensity. This system also proved insensitive to pH (3.5–9). In contrast, the addition of Zn2+ (1 μM) to the morpholine-functionalized probes (LysoDPP-C2 and LysoDPP-C4) over a pH range of 6.5–9 led to an increase in the fluorescence intensity with the largest fluorescence response being produced by LysoDPP-C4. As the pH gradually became more acidic, the Zn2+ complex of LysoDPP-C2 produced the greatest change in fluorescence intensity. Moreover, the extent of PET fluorescence quenching was found to decrease as the distance between the DPP subunit and the morpholine moiety increased. Per the design expectations, each morpholine-functionalized probe displayed a low fluorescence emission intensity at cytosolic pH and strong fluorescence emission intensity at lysosomal pH and in the presence of Zn2+. These results were considered as important predicates to achieving lysosomal Zn2+ imaging with a good signal to noise ratio.
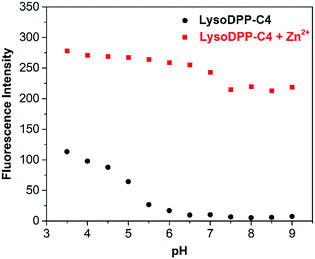 | ||
| Fig. 2 Fluorescence intensity of LysoDPP-C4 (1 μM) and its zinc complex in solutions of varying pH (0.05 M NaCl aqueous solution). λex = 430 nm/λem = 515 nm. | ||
Prior to cellular studies of each probe, metal cation selectivity and competition experiments were performed. As shown in Fig. S18,† no competition involving Zn2+ binding was observed for the commonly found biological metal ions Na+, Ca2+, K+, and Mg2+ in the case of any of the probes. However, the metal ions Mn2+, Fe2+, Fe3+, Co2+, Ni2+, Cu2+, and Cd2+ resulted in partial or total fluorescence quenching. Due to the absolute or labile (free) concentrations of these cations being relatively low in cells, we do not believe they would provide a source of interference in the context of lysosomal Zn2+ analyses.
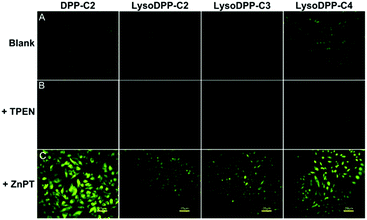
Each probe was then evaluated in vitro. Here, HeLa cells were used as a model system to evaluate cytotoxicity, responsiveness to intracellular Zn2+, and lysosomal targeting. Low cytotoxicity was observed for each probe (Fig. S19†). They were thus used for cellular imaging experiments. As illustrated in Fig. 3, upon incubation the fluorescence produced by both DPP-C2 and LysoDPP-C4 was appreciable, whereas LysoDPP-C2 and LysoDPP-C3 were characterized by low levels of fluorescence emission intensity. Upon the addition of N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN), a membrane permeable zinc chelator,35 a decrease in the fluorescence emission intensity was observed in the case of each probe. In contrast, the addition of Zn2+ in the form of zinc/pyrithione (ZnPT)36 led to a remarkable increase in the fluorescence intensity. On this basis, we concluded that probe LysoDPP-C4, in particular, was worth pursuing further as a dual mode (AND logic) intracellular Zn2+ sensor. On the other hand, probes LysoDPP-C2 and LysoDPP-C3 were not pursued further due to their inability to produce a presumed zinc cation-based background fluorescence response.
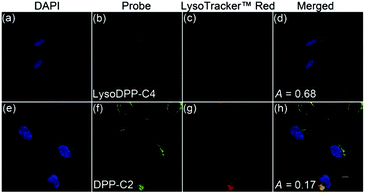
The subcellular distributions of LysoDPP-C4 and the control probe DPP-C2 were then determined through co-staining experiments with LysoTracker™ Red DND-99 (Fig. 4). LysoDPP-C4 was shown to localize at the lysosome through a close overlap with LysoTracker™ Red DND-99 producing a Pearson's coefficient (A) of 0.68 (Fig. 4d). In accord with the design expectation that the morpholine functionality present in LysoDPP-C4 was largely responsible for the observed lysosomal targeting, the morpholine-free control DPP-C2 was shown to have a poor overlap with LysoTracker™ Red DND-99 (Pearson's coefficient of 0.17; Fig. 4h).
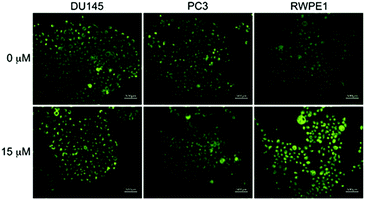
LysoDPP-C4 was then tested for its ability to discriminate between cancerous and normal prostate cells. Toward this end, two prostate cancerous cell lines PC3 and DU145 and one normal human prostate epithelial cell line, RWPE1, were used. LysoDPP-C4 showed negligible cytotoxicity towards these prostate cell lines (Fig. S20†). Addition of Zn2+ to the cancerous cells DU145 and PC3 resulted in no change in the fluorescence intensities. However, the addition of Zn2+ to RWPE1 cells resulted in a significant increase in the observed fluorescence intensity (Fig. 5). This difference is ascribed to the down-regulation of zinc transporters in cancerous PC3 and DU145 cells, which reduces the uptake of extracellular Zn2+.
Support for these findings came from flow cytometry and ICP-MS analyses. These studies revealed that the zinc concentration-dependent fluorescence enhancement could be observed only in RWPE1 cells after incubation with Zn2+ (15 μM), which afforded a 5.9-fold fluorescence increase. An approximate 2.5-fold increase in [Zn2+] in RWPE1 cells was estimated by ICP-MS using Sr2+ as the internal standard (Fig. S21 and Table S3†). On this basis, we conclude that LysoDPP-C4 may be used to discriminate between healthy and cancerous prostate cells.
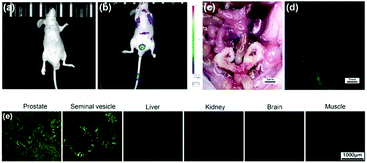
On the basis of these in vitro studies, LysoDPP-C4 was tested in vivo using nude mice models (Fig. 6). As can be seen from an inspection of Fig. 6b, injection of LysoDPP-C4 (500 μg per mouse, 5 mice per set) into male BALB/c nude mice via the tail vein resulted in a bright fluorescence signal in the region of the prostate after 15 minutes. The mice were then sacrificed and dissected. The viscera were observed using a fluoroscope. Both white-light color images and fluorescent images were taken under the same settings (Fig. 6c and d). The prostate and seminal vesicle displayed a strong green fluorescence, which is consistent with both literature reports that a high concentration of Zn2+ are found in these organs and the central hypothesis underlying the present study, namely that LysoDPP-C4 is functioning as an effective Zn2+ probe.
Histological analyses were then carried out and served to confirm the above observations (Fig. 6e). For instance, frozen sections of the prostate and seminal vesicle produced a strong green fluorescence signal, whereas little fluorescence was observed in the case of the liver and kidney sections. Essentially no fluorescence emission was observed in the case of the brain and muscle samples. These results provide support for our contention that LysoDPP-C4 may be used to image the prostate via Zn2+-induced fluorescence signaling.
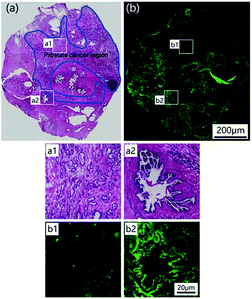
To test the potential of LysoDPP-C4 in prostate cancer biomedical imaging applications, a prostate nodule obtained from a 65 year-old male patient diagnosed with prostate cancer (Gleason's score, 3 + 3) was used. First, hematoxylin and eosin (H & E) staining was used to highlight the cancerous region (area within the blue line in Fig. 7a). Subsequent staining with LysoDPP-C4 allowed a clear visualization of the cancerous regions as shown by regions of relatively low fluorescence emission intensity (Fig. 7b). A significant difference in fluorescence intensity between healthy and cancerous tissue was observed. Therefore, we propose that LysoDPP-C4 may have a role to play in identifying cancerous prostate tissue in human samples.
Experimental
All animal experiments were performed according to the Chinese Regulations for the Administration of Affairs Concerning Experimental Animals and approved by the Subcommittee on Research Animal Care at the Shanghai Ninth People's Hospital. All experimental details are provided in the ESI.†All animal experiments were performed in accord with institutional guidelines and were specifically approved by the Subcommittee on Research Animal Care at the Shanghai Ninth People's Hospital.
Conclusions
In summary, we report here a series of diketopyrrolopyrrole-based AND logic fluorescent probes (DPP-C2, LysoDPP-C2, LysoDPP-C3 and LysoDPP-C4) that were designed to permit the detection of both low pH and Zn2+ in an AND logic-like fashion. In these probes, the aliphatic morpholine moiety serves as both a pH-responsive marker and a lysosome-targeting subunit. Chelation of Zn2+ or the protonation of the morpholine moiety serves to suppress only one PET pathway, resulting in a small increase in the observed fluorescence intensity. However, when both PET pathways are blocked, as occurs in the presence of Zn2+ and H+, the fluorescence is fully recovered. The effectiveness of this strategy in terms of sensing was demonstrated through lysosomal Zn2+ imaging in live cells. The most effective probe in terms of both initial fluorescence intensity and Zn2+-induced increases proved to be LysoDPP-C4. This system demonstrated low cytotoxicity and permitted changes in intracellular Zn2+ concentrations in the lysosome to be monitored. LysoDPP-C4 could be used to discriminate between cancerous and normal prostate cells. In vivo and histological studies demonstrated the effectiveness of LysoDPP-C4 in terms of identifying the prostate and to delineating cancerous regions within a prostate cancer sample obtained from a 65 year old male patient.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (61774099, 81402089) and Program for Outstanding Medical Academic Leader. The work in Austin was supported by the National Institutes of Health (CA68682 to JLS) and the Robert A. Welch Foundation (F-0018 to JLS).Notes and references
- A. Krezel and W. Maret, Arch. Biochem. Biophys., 2016, 611, 3–19 CrossRef CAS PubMed.
- L. C. Costello and R. B. Franklin, Prostate, 1998, 35, 285–296 CrossRef CAS.
- F. Gyorkey, K. W. Min, J. A. Huff and P. Gyorkey, Cancer Res., 1967, 27, 1348–1353 CAS.
- M. V. C. Jordan, S. T. Lo, S. W. Chen, C. Preihs, S. Chirayil, S. R. Zhang, P. Kapur, W. H. Li, L. M. De Leon-Rodriguez, A. J. M. Lubag, N. M. Rofsky and A. D. Sherry, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, E5464–E5471 CrossRef PubMed.
- L. C. Costello, P. Feng, B. Milon, M. Tan and R. B. Franklin, Prostate Cancer Prostatic Dis., 2004, 7, 111–117 CrossRef CAS PubMed.
- L. C. Costello and R. B. Franklin, Arch. Biochem. Biophys., 2016, 611, 100–112 CrossRef CAS PubMed.
- R. B. Franklin, P. Feng, B. Milon, M. M. Desouki, K. K. Singh, A. Kajdacsy-Balla, O. Bagasra and L. C. Costello, Mol. Cancer, 2005, 4, 32 CrossRef PubMed.
- L. C. Costello and R. B. Franklin, J. Biol. Inorg Chem., 2011, 16, 3–8 CrossRef CAS PubMed.
- V. Kolenko, E. Teper, A. Kutikov and R. Uzzo, Nat. Rev. Neurol., 2013, 10, 219–226 CAS.
- L. C. Costello, R. B. Franklin and P. Feng, Mitochondrion, 2005, 5, 143–153 CrossRef CAS PubMed.
- D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Chem. Soc. Rev., 2017, 46, 7105–7123 RSC.
- J. M. Goldberg, F. Wang, C. D. Sessler, N. W. Vogler, D. Y. Zhang, W. H. Loucks, T. Tzounopoulos and S. J. Lippard, J. Am. Chem. Soc., 2018, 140, 2020–2023 CrossRef CAS PubMed.
- M. L. Zastrow, R. J. Radford, W. Chyan, C. T. Anderson, D. Y. Zhang, A. Loas, T. Tzounopoulos and S. J. Lippard, ACS Sens., 2016, 1, 32–39 CrossRef CAS PubMed.
- K. P. Carter, M. C. Carpenter, B. Fiedler, R. Jimenez and A. E. Palmer, Anal. Chem., 2017, 89, 9601–9608 CrossRef CAS PubMed.
- Y. Han, J. M. Goldberg, S. J. Lippard and A. E. Palmer, Sci. Rep., 2018, 8, 15034 CrossRef PubMed.
- K. A. Jolliffe, Acc. Chem. Res., 2017, 50, 2254–2263 CrossRef CAS PubMed.
- L. Fang, G. Trigiante, C. J. Kousseff, R. Crespo-Otero, M. P. Philpott and M. Watkinson, Chem. Commun., 2018, 54, 9619–9622 RSC.
- W. Jiang, Q. Q. Fu, H. Y. Fan and W. Wang, Chem. Commun., 2008, 259–261 RSC.
- H. A. Michaels, C. S. Murphy, R. J. Clark, M. W. Davidson and L. Zhu, Inorg. Chem., 2010, 49, 4278–4287 CrossRef CAS PubMed.
- L. Zhu, Z. Yuan, J. T. Simmons and K. Sreenath, RSC Adv., 2014, 4, 20398–20440 RSC.
- S. K. Ghosh, P. Kim, X. A. Zhang, S. H. Yun, A. Moore, S. J. Lippard and Z. Medarova, Cancer Res., 2010, 70, 6119–6127 CrossRef CAS PubMed.
- W. Chyan, D. Y. Zhang, S. J. Lippard and R. J. Radford, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 143–148 CrossRef CAS PubMed.
- A. C. Sedgwick, L. L. Wu, H. H. Han, S. D. Bull, X. P. He, T. D. James, J. L. Sessler, B. Z. Tang, H. Tian and J. Yoon, Chem. Soc. Rev., 2018, 47, 8842–8880 RSC.
- X. Li, J. Li, X. W. Dong, X. Gao, D. Zhang and C. L. Liu, Sens. Actuators, B, 2017, 245, 129–136 CrossRef CAS.
- J. P. Luzio, P. R. Pryor and N. Bright, Nat. Rev. Mol. Cell Biol., 2007, 8, 622–632 CrossRef CAS PubMed.
- F. M. Platt, B. Boland and A. C. van der Spoel, J. Cell Biol., 2012, 199, 723–734 CrossRef CAS PubMed.
- S. Erbas-Cakmak, S. Kolemen, A. C. Sedgwick, T. Gunnlaugsson, T. D. James, J. Yoon and E. U. Akkaya, Chem. Soc. Rev., 2018, 47, 2228–2248 RSC.
- J. L. Kolanowski, F. Liu and E. J. New, Chem. Soc. Rev., 2018, 47, 195–208 RSC.
- A. Romieu, Org. Biomol. Chem., 2015, 13, 1294–1306 RSC.
- H. M. Kim, M. S. Seo, M. J. An, J. H. Hong, Y. S. Tian, J. H. Choi, O. Kwon, K. J. Lee and B. R. Cho, Angew. Chem., Int. Ed., 2008, 47, 5167–5170 CrossRef CAS PubMed.
- H. Zhu, J. L. Fan, S. L. Zhang, J. F. Cao, K. D. Song, D. Ge, H. J. Dong, J. Y. Wang and X. J. Peng, Biomater. Sci., 2014, 2, 89–97 RSC.
- C. C. Du, S. B. Fu, X. L. Ren, X. H. Wang, Z. Wang, J. Zhou and H. Y. Wang, New J. Chem., 2018, 42, 3493–3502 RSC.
- H. J. Lee, C. W. Cho, H. Seo, S. Singha, Y. W. Jun, K. H. Lee, Y. Jung, K. T. Kim, S. Park, S. C. Bae and K. H. Ahn, Chem. Commun., 2016, 52, 124–127 RSC.
- Z. C. Xu, J. Yoon and D. R. Spring, Chem. Soc. Rev., 2010, 39, 1996–2006 RSC.
- S. L. Sensi, D. Ton-That, P. G. Sullivan, E. A. Jonas, K. R. Gee, L. K. Kaczmarek and J. H. Weiss, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 6157–6162 CrossRef CAS PubMed.
- B. L. Barnett, H. C. Kretschmar and F. A. Hartman, Inorg. Chem., 1977, 16, 1834–1838 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedures, 1H, 13C NMR, and mass spectra, UV-Vis and fluorescence spectroscopic analyses, and other materials. See DOI: 10.1039/c9sc01153f |
| ‡ Both authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |