Open Access Article
Open Access ArticleDirect conversion of phenols into primary anilines with hydrazine catalyzed by palladium†
Zihang
Qiu
 ,
Leiyang
Lv
,
Leiyang
Lv
 ,
Jianbin
Li
,
Jianbin
Li
 ,
Chen-Chen
Li
,
Chen-Chen
Li
 and
Chao-Jun
Li
and
Chao-Jun
Li
 *
*
Department of Chemistry and FQRNT Centre for Green Chemistry and Catalysis, McGill University, 801 Sherbrooke St. W., Montreal, Quebec H3A 0B8, Canada. E-mail: cj.li@mcgill.ca
First published on 26th March 2019
Abstract
Primary anilines are essential building blocks to synthesize various pharmaceuticals, agrochemicals, pigments, electronic materials, and others. To date, the syntheses of primary anilines mostly rely on the reduction of nitroarenes or the transition-metal-catalyzed Ullmann, Buchwald–Hartwig and Chan–Lam cross-coupling reactions with ammonia, in which non-renewable petroleum-based chemicals are typically used as feedstocks via multiple step syntheses. A long-standing scientific challenge is to synthesize various primary anilines directly from renewable sources. Herein, we report a general method to directly convert a broad range of phenols into the corresponding primary anilines with the cheap and widely available hydrazine as both amine and hydride sources with simple Pd/C as the catalyst.
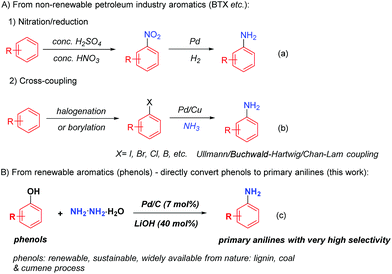
Primary anilines, as the parent molecules of aromatic amines, are vital and well exploited building blocks towards a wide range of pharmaceuticals, agrochemicals, plastics, electronic materials, dyes and resins.1 Due to their importance, various methods have been explored to synthesize primary anilines. The nitro-reduction or the Ullmann, Buchwald–Hartwig and Chan–Lam cross-coupling reactions represent the very successful methods to build primary anilines nowadays. However, in most cases, such methods rely on the starting materials derived from non-renewable petroleum based chemicals (BTX, benzene, toluene, xylenes etc.),2 in which extra pre-functionalization steps of arenes by nitration,3 halogenation4 and borylation5 are required to generate the corresponding aromatic nitro compounds, arylhalides and arylboron compounds in order to form primary anilines (Scheme 1a and b)1c,6. On the other hand, naturally abundant phenols, which constitute some of the main units of lignin bio-mass and are widely obtainable at very low cost from coal and cumene industrial processes, are often considered as the alternative renewable aromatic feedstocks.7 Because of their sustainable feature, it is highly desirable to develop a method to directly convert phenols into primary anilines.
To this end, only gas-phase direct amination of phenol into aniline was successful, however, under very harsh conditions, in which phenol was vaporized to gas and then reacted with a large excess amount of ammonia and hydrogen gases at very high temperature (>250 °C).7d,8 Recently, a liquid phase phenol to aniline transformation was reported, but still requiring high temperature (200 °C) and large excess amounts of ammonia gas and hydrogen gas with a very limited substrate scope (phenol and o/m/p-cresols); and at relatively lower temperature, this transformation suffered from both low conversion of phenol and poor selectivity towards the primary aniline, which can be attributed to the weak nucleophilicity of ammonia and the low stability of the cyclohexylimine intermediate. Such an intermediate is prone to undergo a reduction process to form cyclohexylamine under reductive conditions.9 Therefore, a general and relatively mild phenol to primary aniline transformation is yet to be developed.
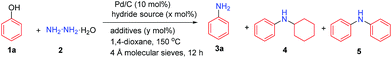
Hydrazine, as the simplest diamine, is an inexpensive, widely available and easy-to-handle amine source.10 It possesses stronger nucleophilicity than ammonia due to the α-effect of the hydrazine nitrogen atom;11 moreover, the corresponding cyclohexanone hydrazone is isolatable and more stable than cyclohexylimine.12 Previously, our group utilized hydrazine as the amine source to couple with phenols to generate various N-cyclohexylanilines, in which the acid protonated the formation of cyclohexanone hydrazone intermediate and facilitated its reduction to the cyclohexylamines.13 With our previous successes to couple phenols with various substituted amines14 and hydrazine to produce N-substituted anilines,13,14 we contemplated the possibility of switching the phenol–hydrazine coupling selectivity towards primary anilines instead. We hypothesized that under less acidic or even basic conditions, the initial reduction of the cyclohexanone hydrazone intermediate might be slowed down. Instead, such a stable hydrazone intermediate could undergo the dehydrogenation process to rearomatize to phenylhydrazine with the catalysis of Pd/C, and then upon the reductive cleavage of the N–N bond of the phenylhydrazine intermediate, the primary aniline could be generated in a highly selective fashion (Scheme 1c).
To examine the feasibility of our hypothesis, we initially tested the reaction using phenol with 1.5 equiv. hydrazine monohydrate, 10 mol% Pd/C as the catalyst, and 50 mol% NaBH4 as the hydride source in 1,4-dioxane (0.2 M) at 150 °C for 12 h (Table 1, entry 1). To our delight, the desired primary aniline 3a could be obtained in 10% yield; however, the selectivity was poor with 46% N-cyclohexylaniline (4) as the main product along with 16% dicyclohexylamine, 1% N-phenylaniline (5), some cyclohexane and benzene. We attributed the poor selectivity to the water molecule in the hydrazine monohydrate reagent, playing the role of a weak Brønsted acid to facilitate the reduction of the cyclohexanone hydrazone intermediate to generate cyclohexylamine, as our group discovered previously.13 To remove the water, various molecular sieves were investigated (Table S1†) and among them, the 4 Å molecular sieves showed the best efficiency, increasing the yield of the primary aniline 3a to 58% (Table 1, entry 2). The solvent had a significant effect on this transformation (Table S2†): for example, switching the solvent from 1,4-dixoane to toluene decreased the primary aniline 3a yield to 43% along with a lower conversion of phenol (Table 1, entry 3). Various catalysts were then examined (Table S3†) and the Pd/C (5 wt%) catalyst showed the best selectivity and highest yield of primary aniline 3a among Pd/C (10 wt%), Pd/Al2O3 (5 wt%) and Ru/C (5 wt%).
| Entry | Hydride source (x mol%) | Additive (y mol%) | Conv. (%) | 3a | Yield (%) 4 | 5 |
|---|---|---|---|---|---|---|
| a Reaction conditions: phenol (0.2 mmol, 1 equiv.), N2H4·H2O (0.3 mmol, 1.5 equiv.), 10 mol% of 5 wt% Pd/C, various hydride sources, and additives with 4 Å molecular sieves (100 mg per 0.3 mmol hydrazine) in 1,4-dioxane (0.2 M) were stirred under argon for 12 h; starting material conversions and NMR yields were given with 1,3,5-trimethoxylbenzene as the internal standard, and yields were calculated based on phenol. b No molecular sieves were added. c Toluene was used as the solvent instead of 1,4-dioxane. d 3.0 equiv. N2H4·H2O was used. e 3.0 equiv. N2H4 in THF solution (1 M) was used. f 2.25 equiv. N2H4 in THF solution (1 M) was used. g Reaction was run at 170 °C. h 7 mol% Pd/C was used. i 0.4 mmol phenol was used. j 4.5 equiv. N2H4·H2O was used. k 6.0 equiv. N2H4·H2O was used. l Isolated yield. For details of optimization (Tables S1–S12), please see the ESI; HOAc = acetic acid. | ||||||
| 1b | NaBH4 (50) | — | 100 | 10 | 46 | 1 |
| 2 | NaBH4 (50) | — | 100 | 58 | 8 | 2 |
| 3c | NaBH4 (50) | — | 81 | 43 | 8 | 1< |
| 4 | NaBH4 (50) | ZnF2 (10) | 100 | 30 | 36 | 11 |
| 5 | NaBH4 (50) | HOAc (10) | 81 | 41 | 12 | 3 |
| 6 | NaBH4 (50) | Na2CO3 (10) | 100 | 55 | 16 | 4 |
| 7 | NaBH4 (50) | NaOtBu (10) | 100 | 59 | 6 | 1 |
| 8 | — | — | 67 | 25 | 4 | 10 |
| 9j | — | — | 78 | 51 | 2 | 2 |
| 10k | — | — | 82 | 49 | 2 | 2 |
| 11e | HCO2Na (1.0) | — | 100 | 64 | 3 | 1< |
| 12e | HCO2Na (1.0) | LiOH (40) | 100 | 68 | 2 | 1< |
| 13f | HCO2Na (1.0) | LiOH (40) | 100 | 69 | 4 | 2 |
| 14f,g,h | HCO2Na (1.0) | LiOH (40) | 100 | 72 | 4 | 2 |
| 15 , , , | — | LiOH (40) | 100 | 72 (71) | <1 | <1 |
To help the tautomerization process of the cyclohexanone hydrazone intermediate,15 catalytic amounts of Brønsted and Lewis acids were tested (Table S4†), however, leading to a decreased yield of primary aniline 3a with an increased yield of N-cyclohexylaniline (4) (Table 1, entries 4 and 5). Conversely, with the basic additives (Table S5†), the yield of primary aniline 3a remained almost unchanged (Table 1, entries 6 and 7). To increase the selectivity of primary aniline 3a over N-cyclohexylaniline (4), a gentler hydride donor might be needed, since a stronger hydride donor would favor the reduction of the cyclohexanone hydrazone intermediate towards the undesired cyclohexylamine intermediate. Hydrazine, on the other hand, might slowly decompose to H2in situ under the Pd/C catalyst, and thus could also serve as a slowly generating mild hydride source.10 To test this postulation, a control experiment without NaBH4 was carried out, in which the primary aniline 3a was generated in 25% yield (Table 1, entry 8). By increasing the amount of hydrazine to 4.5 equiv., the yield of primary aniline 3a could be increased to 51% with slightly increased conversion and better 3a selectivity (Table 1, entry 9 vs. 8); however, no further improvement was observed when increasing the hydrazine amount to 6 equiv. (Table 1, entry 10).
To increase the conversion of phenol, small amounts of various hydride donors were added (Table S8,† entries 5–9) and finally by using the relatively weak hydride donor HCO2Na and with 3 equiv. of hydrazine in THF (1 M), the yield of primary aniline 3a could be slightly increased to 64% (Table 1, entry 11 vs. 2). When LiOH was added to the reaction system, the desired primary aniline 3a yield could be slightly increased to 68% (Table 1, entry 12),10 and decreasing the hydrazine amount from 3.0 equiv. to 2.25 equiv. maintained the same efficiency, generating the primary aniline 3a in 69% yield (Table 1, entry 13). Other metal hydroxides also showed positive effects on the primary aniline 3a formation but were all inferior to LiOH (Table S9,† entries 18–21), while other lithium salts exhibited negative effects (Table S9,† entries 8–14). By elevating the temperature to 170 °C and with the Pd/C catalyst loading decreased to 7 mol%, the primary aniline 3a yield could be increased to 72% (Table 1, entry 14) probably due to the balanced rate of dehydrogenation and hydrogenation processes. Fortunately, by carefully tuning the reaction conditions, we found that with 3.0 equiv. hydrazine monohydrate and 40 mol% LiOH without any extra hydride donor at 170 °C, the primary aniline 3a could be produced in 71% isolated yield with excellent selectivity (Table 1, entry 15 and Table S11†), in which the hydrazine acted as both the amine and hydride sources.
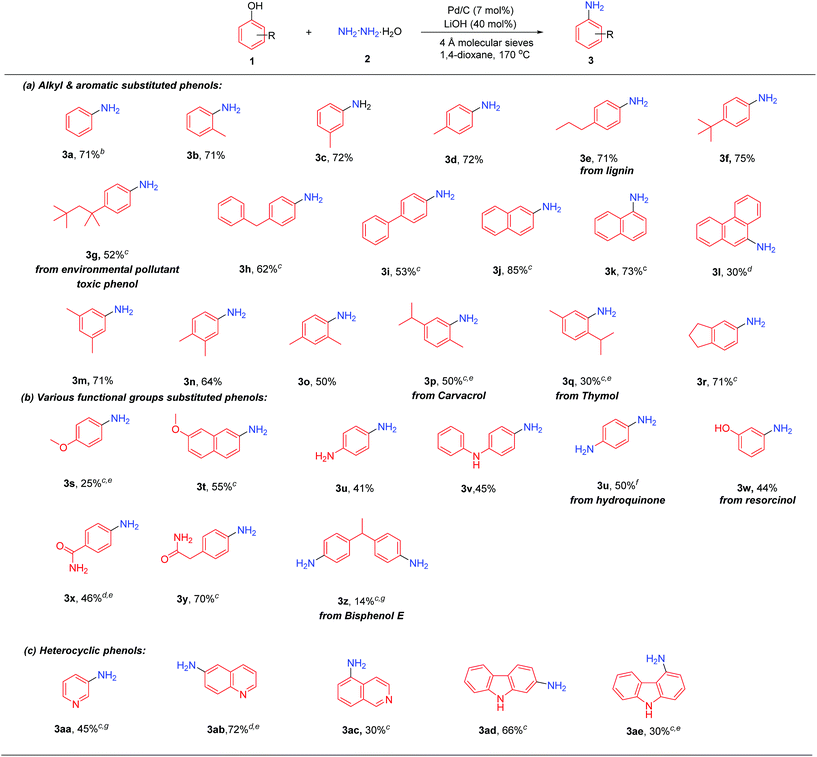
With the optimized conditions in hand, the substrate scope of phenols was investigated. As shown in Table 2, phenols bearing various alkyl and aromatic substituents reacted smoothly to afford the corresponding primary aromatic amines 3b–3r in moderate to excellent yields. 4-Propylphenol, as one of the predominant products derived from lignin,7b could generate the corresponding 4-propylaniline (3e) in a good yield (71%) under the standard conditions. Sterically bulky substituted phenols, such as 4-tert-butylphenol and 4-tert-octylphenol (a potential environmental pollutant exhibiting toxic and estrogenic effects), could afford products 3f and 3g efficiently. 2-Naphthol and 1-naphthol gave the corresponding naphthylamines 3j and 3k in 85% and 73% yield, respectively, while 9-phenanthrol afforded product 3l in relatively lower yield. Disubstituted phenols also worked well in this reaction: 3,5-, 3,4- and 2,4-dimethyl phenols all produced disubstituted anilines 3m–3o in 50–71% yields, while 2,4-dimethylphenol gave relatively lower yield due to the steric hindrance of the ortho methyl group. Carvacrol and thymol, both naturally abundant bioactive monoterpenic phenols, could successfully be converted into the corresponding anilines; the higher yield with carvacrol than thymol is likely due to the higher steric hindrance of the isopropyl group than that of the methyl group (3pvs.3q). 5-Indanol, consisting of a 5-membered fused cyclic ring with phenol, gave a good yield of product 3r.
| a Reaction conditions: phenols (0.4 mmol, 1 equiv.), N2H4·H2O (1.8 mmol, 4.5 equiv.), 7 mol% of 5 wt% Pd/C and 4 Å molecular sieves (100 mg per 0.3 mmol hydrazine) in 1,4-dioxane (0.2 M) were stirred under argon for 16 h; isolated yields were given. b 3 equiv. N2H4·H2O was used and reaction was run for 12 h. c 37.5 mol% NaBH4 was added. d 50 mol% NaBH4 was added. e LiOH was not added. f 5 equiv. N2H4·H2O was used. g 20 mol% of 5 wt% Pd/C was used. |
|---|
|
Next, the scope of phenols with various functional substituents was examined (3s–3z). 4-Methoxylphenol afforded 4-methoxylaniline (3s) in low yield, together with some C–OMe bond cleavage products, while 7-methoxy-2-naphthol gave product 3t in relatively higher yield. 4-Amino phenol reacted smoothly to generate 1,4-diaminophenol (3u) without the need of pre-protection of the amino group. Dihydroxyl-benzene could react well in this transformation, in which both hydroxyl groups of 1,4-dihydroxylbenzene (hydroquinone) would react to generate 1,4-diaminobenzene, whereas for 1,3-dihydroxylbenzene (resorcinol), one hydroxyl group reacted to give 3-aminophenol (3w) selectively. A phenol bearing an amide substituent gave the product 3x in 46% yield, whereas when 2-(4-hydroxyphenyl)acetamide was tested, the aniline product 3y could be obtained in 70% yield. The bisphenol type compound could also undergo this transformation, although in lower yield (3z).
Finally, the scope of heterocyclic phenols was investigated (3aa–3ae). 3-Aminopyridine (3aa) could be directly obtained from 3-hydroxypyridine in moderate yield. Besides, 6-aminoquinoline (3ab) (used as an internal standard in determining serum nicotine)16 was obtained in high yield, while 5-aminoisoquinoline (3ac) was generated in lower yield. Hydroxyl substituted carbazoles were also suitable substrates for this reaction, with 2-hydroxycarbazole giving higher yield than 4-hydroxycarbazole possibly because of the steric hindrance of 5-H in 4-hydroxycarbazole (3advs.3ae). Unfortunately, when the lignin monomers were directly used as starting materials, the desired primary aniline products could not be obtained (for details, please see the ESI†).
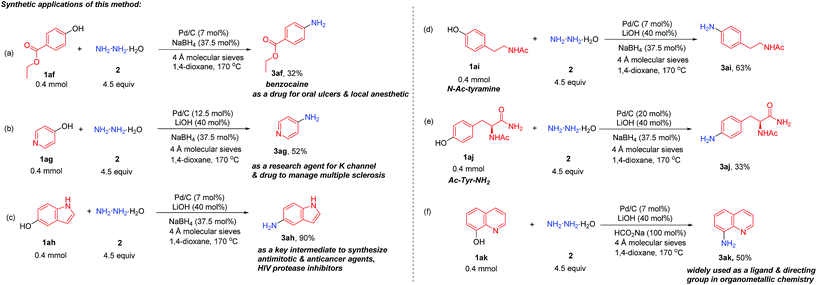
To evaluate the synthetic potential of this direct conversion of phenols to primary anilines, several useful primary anilines applied in pharmaceuticals and organometallic chemistry were directly synthesized from the corresponding phenols (Scheme 2). Benzocaine (3af) (a pharmaceutical drug for oral ulcers and a local anesthetic), 4-aminopyridine (3ag) (used as a research agent to characterize potassium channels and a drug to manage multiple sclerosis17) and 5-aminoindole (3ah) (used as a key intermediate to prepare antimitotic and anticancer agents and HIV protease inhibitors18) could all be obtained in moderate to high yields directly from phenols (Scheme 2a–c). Moreover, protected tyrosine (amino acid) and tyramine (the decarboxylation product from tyrosine) were all suitable substrates for this reaction (3ai and 3aj) to afford the corresponding aniline analogues in one step (Scheme 2d and e). Besides, 8-aminoquinoline (3ak), which is widely used as a ligand and directing group in organometallic chemistry,19 was also produced efficiently in this transformation (Scheme 2f).
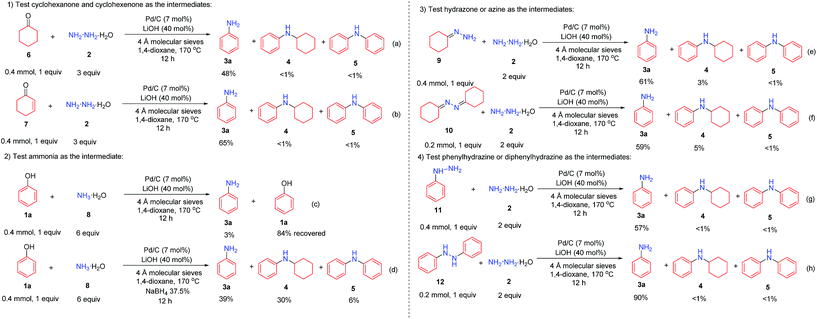
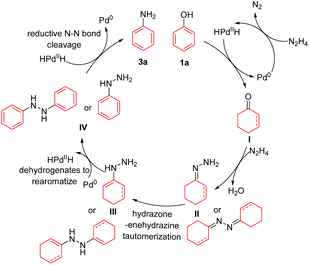
The plausible mechanism was investigated (Scheme 3). First, cyclohexanone (6) and cyclohexenone (7), as the two possible reduced forms of phenol, were reacted with hydrazine under the standard conditions, and both reactions afforded the desired primary aniline product 3a (in 48% and 65% yields, respectively) with excellent selectivity (Scheme 3a and b), indicating the possible involvement of both cyclohexanone (6) and cyclohexenone (7) as the reaction intermediates. Second, since hydrazine might decompose to ammonia under the current Pd/C catalyst system,10 ammonia was used as the amine source to react with phenol (1a) as the control experiments (Scheme 3c and d). Without additional hydride sources, phenol (1a) was mainly recovered in 84% yield (Scheme 3c), whereas in the presence of a small amount of NaBH4, the primary aniline was generated in 39% yield but with poor selectivity (Scheme 3d), showing the unlikely involvement of ammonia as the intermediate. Third, the two possible intermediates hydrazone 9 and azine 10 were tested (Scheme 3e and f). Both of them could generate the primary aniline 3a with good yield and selectivity under standard conditions, demonstrating that they are possible intermediates in this transformation. Finally, the possible intermediates phenylhydrazine 11 and diphenylhydrazine 12 were examined (Scheme 3g and h): in both cases, the desired primary aniline 3a could be obtained in good yield and very high selectivity, indicating that both would be the possible intermediates involved in this transformation.
Based on the above mechanistic studies and literature reports, a tentative mechanism for this direct phenol to primary aniline reaction is proposed in Scheme 4. Initially, HPdIIH (palladium hydride) species can be generated either by the decomposition of N2H4 under the Pd/C catalyst10 or by NaBH4 reacting with Pd/C in the cases that NaBH4 is added.14c,e The palladium hydride then reduces the phenol (1a) to cyclohexanone or cyclohexenone intermediate I.14a,c Then, intermediate I can condense with hydrazine to afford the hydrazone or azine intermediate II, which is followed by the hydrazone–enehydrazine tautomerization to generate intermediate III.15 Then intermediate III can readily undergo dehydrogenation and rearomatize to form the phenylhydrazine type intermediate IV, and meanwhile regenerate the HPdIIH species.14a,c,e Finally, reductive N–N bond cleavage of IV20 produces the desired primary aniline product 3a and regenerates the Pd0 species at the same time.
Conclusions
In conclusion, we have developed a general method to directly convert a wide range of naturally abundant and renewable phenols into the corresponding primary anilines with the cheap and easy-to-handle hydrazine, acting as both amine and hydride sources, under relatively mild conditions with simple Pd/C as the catalyst. Bio-active phenols, such as carvacrol, thymol, hydroquinone, resorcinol, tyrosine and tyramine derivatives, are all appropriate substrates in this transformation and moreover, some useful primary anilines (shown in Scheme 2) could be directly synthesized by this protocol as well. Ongoing studies of other direct phenol amination methods are underway in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the Canada Research Chair Foundation (to C.-J. Li), the CFI, FQRNT Center for Green Chemistry and Catalysis, NSERC, and McGill University for supporting our research.Notes and references
- (a) S. A. Lawrence, Amines: Synthesis, Properties and Applications, Cambridge University Press, 2004 Search PubMed; (b) T. Kahl, K. W. Schröder, F. Lawrence, W. Marshall, H. Höke and R. Jäckh, Aniline, in Ullmann's Encyclopedia of Industrial Chemistry, 2012, ch. 465, vol. 3, pp. 465–477 Search PubMed; (c) J. Schranck and A. Tlili, ACS Catal., 2018, 8, 405–418 CrossRef CAS.
- G. Alfke, W. W. Irion and O. S. Neuwirth, Oil Refining, in Ullmann's Encyclopedia of Industrial Chemistry, 2007, vol. 25, pp. 208–261 Search PubMed.
- J. F. Hartwig, S. Shekhar, Q. Shen and F. Barrios-Landeros, Synthesis of Anilines in the Chemistry of Anilines, 2007, pp. 455–536 Search PubMed.
- M. B. Smith and J. March, Electrophilic Aromatic Substitution, in March's Advanced Organic Chemistry, 2006, ch. 11, pp. 657–751 Search PubMed.
- (a) J. F. Hartwig, Chem. Soc. Rev., 2011, 40, 1992–2002 RSC; (b) T. M. Boller, J. M. Murphy, M. Hapke, T. Ishiyama, N. Miyaura and J. F. Hartwig, J. Am. Chem. Soc., 2005, 127, 14263–14278 CrossRef CAS PubMed.
- (a) N. Xia and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 337–339 CrossRef CAS PubMed; (b) M. Fan, W. Zhou, Y. Jiang and D. Ma, Org. Lett., 2015, 17, 5934–5937 CrossRef CAS PubMed; (c) Q. Shen and J. F. Hartwig, J. Am. Chem. Soc., 2006, 128, 10028–10029 CrossRef CAS PubMed; (d) D. S. Surry and S. L. Buchwald, J. Am. Chem. Soc., 2007, 129, 10354–10355 CrossRef CAS PubMed; (e) H. Rao, H. Fu, Y. Jiang and Y. Zhao, Angew. Chem., Int. Ed., 2009, 48, 1114–1116 CrossRef CAS PubMed; For recent advances in primary aniline synthesis, please see: (f) L. Legnani, G. P. Cerai and B. Morandi, ACS Catal., 2016, 6, 8162–8165 CrossRef CAS; (g) J. Liu, K. Wu, T. Shen, Y. Liang, M. Zou, Y. Zhu, X. Li, X. Li and N. Jiao, Chem.–Eur. J., 2017, 23, 563–567 CrossRef CAS PubMed; (h) X. Jin, Y. Koizumi, K. Yamaguchi, K. Nozaki and N. Mizuno, J. Am. Chem. Soc., 2017, 139, 13821–13829 CrossRef CAS PubMed; (i) J. Liu, X. Qiu, X. Huang, X. Luo, C. Zhang, J. Wei, J. Pan, Y. Liang, Y. Zhu, Q. Qin, S. Song and N. Jiao, Nat. Chem., 2019, 11, 71–77 CrossRef CAS PubMed. For a recent work on conversion of aliphatic OH to NH2, please see: (j) W. Deng, Y. Wang, S. Zhang, K. M. Gupta, M. J. Hulsey, H. Asakura, L. Liu, Y. Han, E. M. Karp, G. T. Beckham, P. J. Dyson, J. Jiang, T. Tanaka, Y. Wang and N. Yan, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 5093–5098 CrossRef CAS PubMed.
- (a) B. M. Upton and A. M. Kasko, Chem. Rev., 2016, 116, 2275–2306 CrossRef CAS PubMed; (b) C. Li, X. Zhao, A. Wang, G. W. Huber and T. Zhang, Chem. Rev., 2015, 115, 11559–11624 CrossRef CAS PubMed; (c) G. W. Huber and A. Corma, Angew. Chem., Int. Ed., 2007, 46, 7184–7201 CrossRef CAS PubMed; (d) M. Weber, M. Weber and M. Kleine-Boymann, Phenol, in Ullmann's Encyclopedia of Industrial Chemistry, 2012, ch. 503, vol. 26, pp. 503–518 Search PubMed.
- H. Zeng, Z. Qiu, A. Domínguez-Huerta, Z. Hearne, Z. Chen and C.-J. Li, ACS Catal., 2016, 7, 510–519 CrossRef.
- T. Cuypers, P. Tomkins and D. E. De Vos, Catal. Sci. Technol., 2018, 8, 2519–2523 RSC.
- (a) J.-P. Schirmann and P. Bourdauducy, Hydrazine, in Ullmann's Encyclopedia of Industrial Chemistry, 2001, vol. 18, pp. 79–96 Search PubMed; (b) L. He, B. Liang, Y. Huang and T. Zhang, Natl. Sci. Rev., 2017, 5, 356–364 CrossRef; (c) A. Furst, R. C. Berlo and S. Hooton, Chem. Rev., 1965, 65, 51–68 CrossRef CAS.
- (a) T. A. Nigst, A. Antipova and H. Mayr, J. Org. Chem., 2012, 77, 8142–8155 CrossRef CAS PubMed; (b) J. O. Edwards and R. G. Pearson, J. Am. Chem. Soc., 1962, 84, 16–24 CrossRef CAS.
- (a) P. Rulliere, G. Benoit, E. M. D. Allouche and A. B. Charette, Angew. Chem., Int. Ed., 2018, 57, 5777–5782 CrossRef CAS PubMed; (b) D. K. Kolmel and E. T. Kool, Chem. Rev., 2017, 117, 10358–10376 CrossRef CAS PubMed.
- J.-S. Li, Z. Qiu and C.-J. Li, Adv. Synth. Catal., 2017, 359, 3648–3653 CrossRef CAS.
- (a) Z. Chen, H. Zeng, S. A. Girard, F. Wang, N. Chen and C.-J. Li, Angew. Chem., Int. Ed., 2015, 54, 14487–14491 CrossRef CAS PubMed; (b) Z. Chen, H. Zeng, H. Gong, H. Wang and C.-J. Li, Chem. Sci., 2015, 6, 4174–4178 RSC; (c) Z. Qiu, J.-S. Li and C.-J. Li, Chem. Sci., 2017, 8, 6954–6958 RSC; (d) A. Dominguez-Huerta, I. Perepichka and C.-J. Li, Communications Chemistry, 2018, 1, 45 CrossRef; (e) H. Zeng, D. Cao, Z. Qiu and C.-J. Li, Angew. Chem., Int. Ed., 2018, 57, 3752–3757 CrossRef CAS PubMed; (f) D. Cao, H. Zeng and C.-J. Li, ACS Catal., 2018, 8, 8873–8878 CrossRef CAS; (g) S. A. Girard, X. Hu, T. Knauber, F. Zhou, M. O. Simon, G.-J. Deng and C.-J. Li, Org. Lett., 2012, 14, 5606–5609 CrossRef CAS PubMed.
- (a) F. Zhan and G. Liang, Angew. Chem., Int. Ed., 2013, 52, 1266–1269 CrossRef CAS PubMed; (b) J. J. Li, Fischer indole synthesis in Name Reactions, Springer International Publishing, Cham, 5th edn, 2014, pp. 253–254 Search PubMed; (c) N. D. Heindel, P. d. Kennewel and M. Pfau, J. Org. Chem., 1970, 35, 80–83 CrossRef CAS.
- P. J. Aragon, A. D. Yapi, F. Pinguet, J. M. Chezal, J. C. Teulade, J. P. Chapat and Y. Blache, Chem. Pharm. Bull., 2004, 52, 659–663 CrossRef CAS PubMed.
- (a) H. B. Jensen, M. Ravnborg, U. Dalgas and E. Stenager, Ther. Adv. Neurol. Disord., 2014, 7, 97–113 CrossRef CAS PubMed; (b) L. S. Chin, C. C. Park, K. M. Zitnay, M. Sinha, A. J. DiPatri Jr., P. Perillan and J. M. Simard, J. Neurosci. Res., 1997, 48, 122–127 CrossRef CAS PubMed.
- (a) V. Gasparotto, I. Castagliuolo, G. Chiarelotto, V. Pezzi, D. Montanaro, P. Brun, G. Palu, G. Viola and M. G. Ferlin, J. Med. Chem., 2006, 49, 1910–1915 CrossRef CAS PubMed; (b) L. D. Via, O. Gia, V. Gasparotto and M. G. Ferlin, Eur. J. Med. Chem., 2008, 43, 429–434 CrossRef PubMed; (c) L. Chiummiento, M. Funicello, P. Lupattelli, F. Tramutola and P. Campaner, Tetrahedron, 2009, 65, 5984–5989 CrossRef CAS.
- (a) M. Corbet and F. De Campo, Angew. Chem., Int. Ed., 2013, 52, 9896–9898 CrossRef CAS PubMed; (b) H. Hudali, J. Kingston and H. Tayim, Inorg. Chem., 1979, 18, 1391–1394 CrossRef CAS.
- (a) A. Gevorgyan, S. Mkrtchyan, T. Grigoryan and V. O. Iaroshenko, Chempluschem, 2018, 83, 375–382 CrossRef CAS; (b) F. Alonso, P. Candela, C. Gómez and M. Yus, Adv. Synth. Catal., 2003, 345, 275–279 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9sc00595a. |
| This journal is © The Royal Society of Chemistry 2019 |