Open Access Article
Open Access ArticleAu-catalyzed skeletal rearrangement of O-propargylic oximes via N–O bond cleavage with the aid of a Brønsted base cocatalyst†
Keigo
Shiga
b,
Ilya D.
Gridnev
b,
Masahiro
Terada
 b and
Itaru
Nakamura
b and
Itaru
Nakamura
 *a
*a
aResearch and Analytical Center for Giant Molecules, Graduate School of Science, Tohoku University, Sendai, 980-8578, Japan. E-mail: itaru-n@tohoku.ac.jp
bDepartment of Chemistry, Graduate School of Science, Tohoku University, Sendai, 980-8578, Japan
First published on 18th April 2019
Abstract
O-Propargylic oximes that possess an electron-withdrawing aryl group on the oxime moiety undergo Au-catalyzed skeletal rearrangements via N–O bond cleavage to afford the corresponding 2H-1,3-oxazine derivatives. Our studies show that the inclusion of a Brønsted base cocatalyst not only accelerates the reaction but also switches pathways of the skeletal rearrangement reaction, realizing divergent synthesis of heterocyclic compounds. Computational studies indicate that the elimination of propargylic proton in the cyclized vinylgold intermediate is rate-determining and both electron-withdrawing substituents at the oxime moiety and base cocatalyst facilitate the proton elimination. Moreover, the protodeauration process proceeds stepwise involving N–O bond cleavage followed by recyclization to construct the oxazine core.
Introduction
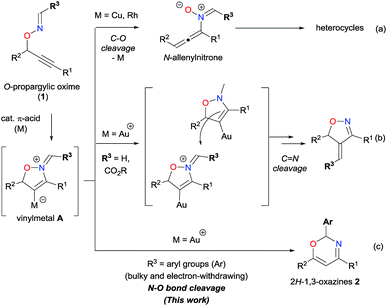
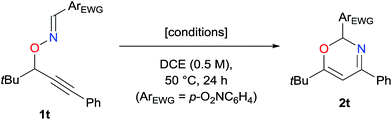
π-Acidic metal catalysis is a powerful tool to synthesize a wide variety of heterocyclic compounds in an efficient and environmentally benign manner. In particular, π-acidic metal-catalyzed skeletal rearrangements allow for the rapid construction of highly elaborate organic molecules, inaccessible by conventional methods, through the cleavage of skeletal σ bonds under mild reaction conditions with high functional group compatibility.1,2 Notably, reaction pathways of catalytic skeletal rearrangements are often changed by metal catalysts as well as functional groups introduced on molecular platforms, such as enynes1 or propargylic esters,2 thus leading to functionalized heterocyclic compounds. Therefore, it is beneficial for pharmaceutical science and material science to provide diverse heterocycles by further exploring catalytic skeletal rearrangement reactions with suitable design of starting materials and appropriate choice of π-acidic metal catalysts.We have recently reported that O-propargylic oximes 1 can serve as a unique platform for the skeletal rearrangement reactions to efficiently construct a variety of heterocycles (Scheme 1).3,4 For example, Cu- and Rh-catalyzed reactions proceeded via C–O bond cleavage resulting in N-allenylnitrone intermediates that subsequently transformed into azaheterocycles of various ring sizes (Scheme 1a).3 We further reported on Au-catalyzed reactions of formaldoxime (R3 = H)4a and glyoxylate oxime (R3 = CO2R)4c that afforded the corresponding 4-methyleneisoxazolines (Scheme 1b), via C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond cleavage.5 Our experimental results imply that these Au-catalyzed reactions proceed via an intermolecular nucleophilic attack on the iminium moiety of the long-lived vinylgold intermediate A (M = Au), because the C–Au bond is much stronger than the C–Cu due to the relativistic nature of the Au catalyst,6 resulting in deceleration of the C–O bond cleavage with the elimination of the metal catalyst. Accordingly, we envisaged that oximes that possess a bulky R3 would decelerate the intermolecular nucleophilic attack and thus favor alternate reactivities of vinylgold intermediate A. Herein, we report on the Au-catalyzed reactions of O-propargylic oximes 1 having an aryl group at the oxime moiety, which proceed via N–O bond cleavage, in affording the corresponding 2H-1,3-oxazine derivatives 2 in good to high yields (Scheme 1c).‡,7
N bond cleavage.5 Our experimental results imply that these Au-catalyzed reactions proceed via an intermolecular nucleophilic attack on the iminium moiety of the long-lived vinylgold intermediate A (M = Au), because the C–Au bond is much stronger than the C–Cu due to the relativistic nature of the Au catalyst,6 resulting in deceleration of the C–O bond cleavage with the elimination of the metal catalyst. Accordingly, we envisaged that oximes that possess a bulky R3 would decelerate the intermolecular nucleophilic attack and thus favor alternate reactivities of vinylgold intermediate A. Herein, we report on the Au-catalyzed reactions of O-propargylic oximes 1 having an aryl group at the oxime moiety, which proceed via N–O bond cleavage, in affording the corresponding 2H-1,3-oxazine derivatives 2 in good to high yields (Scheme 1c).‡,7
Results and discussion
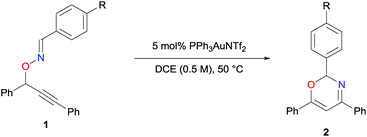
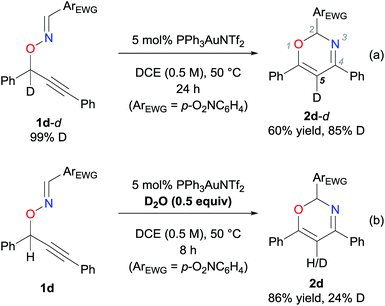
Initially, the reaction of oxime 1a, which possesses a phenyl group at the oxime moiety, was carried out in the presence of PPh3AuNTf2 (5 mol%) in 1,2-dichloroethane (DCE) at 50 °C for 24 h, to afford oxazine 2a in 16% yield (Table 1, entry 1). The efficiency of the present reaction was significantly improved by an electron-deficient aryl group at the oxime moiety (entries 2–4). Particularly, the Au-catalyzed reaction of 1d having a p-nitrophenyl group was complete within 8 h, affording desired product 2d in an excellent yield (93%, entry 4).To gain insight into the reaction mechanism, specifically to track the movement of the propargylic hydrogen, experiments were carried out using deuterated substrates (Scheme 2). As shown in Scheme 2a, the Au-catalyzed reaction of 1d-d, which was deuterated (>99%) at the propargylic position, afforded 2d-d with a decreased deuterium content (85% at the 5 position of the oxazine ring) in a 60% yield.§ It is important to note that the reaction of deuterated substrate 1d-d was slower than that of non-deuterated 1d, and required 24 h for the complete consumption of the deuterated substrate. In contrast, the reaction of the non-deuterated 1d was carried out in the presence of D2O (0.5 equivalent) to afford 2d-d, with a deuterium content of 24% (Scheme 2b). These results strongly suggest that the hydrogen was transferred as a proton interconvertible with external water over the course of the reaction. In other words, direct [1,2]-hydrogen shift is unlikely.
Based on these investigations and our previous studies,4 a reaction mechanism for the skeletal rearrangement can be outlined as illustrated in Scheme 3. First, the π-acidic Au catalyst is coordinated by the alkyne moiety of 1 to form π-complex 3. Consequently, the electrophilically activated alkyne moiety undergoes a nucleophilic attack by the oxime nitrogen, leading to the cyclized vinylgold 4. Next, the propargylic proton is eliminated to form aromatized intermediate 5, which would further undergo protodeauration, N–O bond cleavage, and re-cyclization, although the order of these processes were unidentifiable at this stage. A strong isotope effect on the reaction rate (Scheme 2aversusTable 1, entry 4) would suggest that the rate-determining step is the elimination of the proton from 4 to 5. Accordingly, a p-nitrophenyl group would probably enhance the acidity of the eliminating proton, and facilitate the entire process.
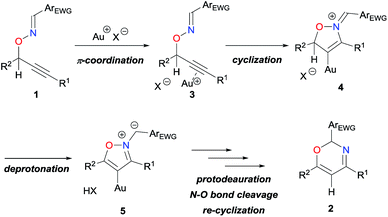 | ||
| Scheme 3 Outline of the reaction mechanism for Au-catalyzed reaction of O-propargylic oximes 1via N–O bond cleavage. | ||
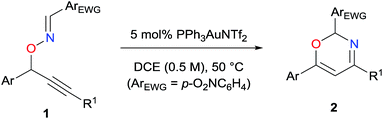
The present transformation was applied toward substrates 1e–s with various aryl groups at the propargylic position, as listed in Table 2. Substrates 1e–g, which possess an aryl group at the alkyne terminus, were efficiently converted to the corresponding oxazines 2e–g in good to excellent yields, regardless of the electronic nature of the aryl group (entries 1–3). 1-Cyclohexenyl group was tolerated as an alkyne substituent (entry 4). Substrates 1i–k, which possess a secondary alkyl group at the alkyne terminus, were transformed to the corresponding desired products 2i–k in good to acceptable yields (entries 5–7). In contrast, 1l–n, which possess a primary alkyl group, a t-butyl group, and a terminal alkyne, respectively, did not afford the corresponding desired products (entries 8–10). As expected, substrates with electron-deficient aryl group substituent at the propargylic position were more effective than that with an electron-rich anisyl substituent, presumably due to the enhanced acidity of the propargyl proton (entries 11–14).
| 1 | R1 | Ar | Time (h) | 2 | Yieldb (%) | |
|---|---|---|---|---|---|---|
| a Reaction of 1 (0.2 mmol) were conducted in the presence of PPh3AuNTf2 (0.01 mmol) in DCE (0.4 mL) at 50 °C. b Isolated yield. c 76% of 1m was recovered. d 28% of 1n was recovered. | ||||||
| 1 | 1e | p-MeOC6H4 | Ph | 8 | 2e | 92 |
| 2 | 1f | p-ClC6H4 | Ph | 8 | 2f | 94 |
| 3 | 1g | p-F3CC6H4 | Ph | 20 | 2g | 86 |
| 4 | 1h | 1-Cyclohexenyl | Ph | 8 | 2h | 51 |
| 5 | 1i | Cy | Ph | 12 | 2i | 81 |
| 6 | 1j | iPr | Ph | 15 | 2j | 53 |
| 7 | 1k | 1-Phenylpentyl | Ph | 8 | 2k | 61 |
| 8 | 1l | nPr | Ph | 24 | — | <1 |
| 9 | 1m | tBu | Ph | 6 | — | <1c |
| 10 | 1n | H | Ph | 24 | — | <1d |
| 11 | 1o | Ph | p-MeOC6H4 | 8 | 2o | 65 |
| 12 | 1p | Ph | p-ClC6H4 | 8 | 2p | 92 |
| 13 | 1q | Ph | p-F3CC6H4 | 8 | 2q | 91 |
| 14 | 1r | 4-MeOC6H4 | p-F3CC6H4 | 12 | 2r | 93 |
| 15 | 1s | Ph | 1-Naphthyl | 8 | 2s | 93 |
Despite using optimized reaction conditions, the Au-catalyzed reactions of the substrates that possess an alkyl group at the propargylic position did not afford the oxazine; considerable amounts of starting materials were recovered (Table 3, entry 1, see also ESI†). In accordance with our proposed mechanism, the alkyl substituent decreases the acidity of the eliminating proton of the cyclized vinylgold intermediate 4 (Scheme 3). Consequently, we decided to explore the use of other counteranions, which could assist the proton elimination (Table 3, entries 1–3).8 To our delight, the use of tosylate, instead of triflic imidate in the reaction of 1t having a t-butyl group resulted in efficient formation of 2t (entry 3), whereas triflate was ineffective (entry 2). More interestingly, we disclosed that pyridine served as an efficient cocatalyst without poisoning the catalytic activities of the π-acidic Au catalyst (entry 4).9 The reaction using bulkier 2,6-lutidine than pyridine was sluggish, and that using trimethylamine resulted in formation of unidentified byproducts, while KOtBu totally diminished the activity of the gold catalyst (entries 5–7). We selected a combination of PPh3AuNTf2 and pyridine (entry 4) as the reaction conditions for the alkyl-substituted substrate in order to further know the applicability of the intriguing cocatalyst system.
| Entry | Catalyst (mol%) | Additive (mol%) | Yielda (%) | Recoverya (%) |
|---|---|---|---|---|
| a The yields were determined by 1H NMR using dibromomethane as an internal standard. Isolated yield in parentheses. b 48 h. c 72 h. | ||||
| 1 | Ph3PAuNTf2 (5) | — | <1 | 90 |
| 2 | PPh3AuCl (5), AgOTf (5) | — | <1 | 91 |
| 3 | PPh3AuCl (5), AgOTs (5) | — | 90b | <1 |
| 4 | PPh3AuNTf2 (5) | Pyridine (10) | (90)c | <1 |
| 5 | PPh3AuNTf2 (5) | 2,6-Lutidine (10) | 10 | 64 |
| 6 | PPh3AuNTf2 (5) | Et3N (10) | 6 | 33 |
| 7 | PPh3AuNTf2 (5) | KOtBu (10) | <1 | 75 |
Substrates 1u–w having a secondary alkyl group were converted to the corresponding products in good to moderate yields by using the gold-pyridine cocatalyst system (Table 4, entries 1–3). In contrast, the reaction of 1x having a primary alkyl group resulted in the decomposition of starting material 1x, presumably due to the instability of the corresponding oxazine (entry 4). It should be noted that the reaction of 1y having a bulky cyclohexyl group at the alkyne terminus was efficiently promoted by the gold-pyridine cocatalyst system (entry 5), whereas that using PPh3AuCl and AgOTs was sluggish (entry 6). Moreover, the substrate 1m, which has tert-butyl and phenyl groups at the alkyne terminus and propargylic position (R2), respectively, was converted to the desired product 2m by using the gold-pyridine cocatalyst system (entry 7 versusTable 2, entry 9).¶ These results indicate that gold-pyridine cooperative catalyst system is effective for not only the proton elimination process but also the cyclization process when the alkyne substituent is bulky (3 to 4 in Scheme 3). Probably, pyridine cocatalyst can maintain the π-acidity of the gold catalyst and even enhance the nucleophilicity of the oxime moiety. In addition, chemical yield of the reaction of 1a, which have a less electron-withdrawing phenyl group at the oxime moiety, was slightly improved from 16% to 28% by the use of the gold-pyridine cocatalyst system (entry 8 versusTable 1, entry 1).
| 1 | R1 | R2 | R3 | Time (h) | 2 | Yieldb (%) | |
|---|---|---|---|---|---|---|---|
| a Reaction of 1 (0.2 mmol) were conducted in the presence of PPh3AuNTf2 (0.01 mmol) and pyridine (0.02 mmol) in DCE (0.4 mL) at 50 °C. b Isolated yield. c PPh3PAuNTf2 (0.02 mmol) and pyridine (0.02 mmol) were used. d PPh3AuCl (0.02 mmol) and AgOTs (0.02 mol) were used, instead of PPh3AuNTf2 and pyridine. e Determined by 1H NMR using dibromomethane as an internal standard. f 21% of 1m was recovered. g 44% of 1a was recovered. | |||||||
| 1 | 1u | Ph | iPr | p-O2NC6H4 | 21 | 2u | 40 |
| 2 | 1v | Ph | Cy | p-O2NC6H4 | 20 | 2v | 65 |
| 3 | 1w | Ph | Cyclopropyl | p-O2NC6H4 | 24 | 2w | 60 |
| 4 | 1x | Ph | nPr | p-O2NC6H4 | 24 | — | <1 |
| 5c | 1y | Cy | tBu | p-O2NC6H4 | 36 | 2y | 72 |
| 6d | 1y | Cy | tBu | p-O2NC6H4 | 48 | 2y | 18e |
| 7 | 1m | tBu | Ph | p-O2NC6H4 | 30 | 2m | 75f |
| 8 | 1a | Ph | Ph | Ph | 24 | 2a | 28g |
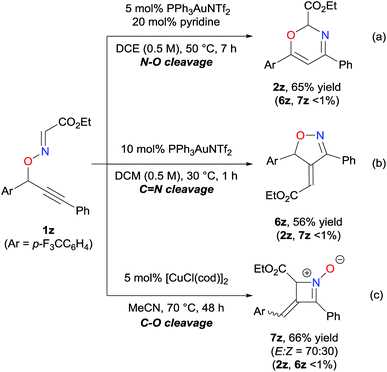
The uniqueness of the Au-pyridine cooperative catalytic system can be demonstrated by the different pathways for the skeletal rearrangement reactions. In the absence of any cocatalysts, the Au-catalyzed reaction of O-propargylic oxime 1z, which possesses an electron-withdrawing and less bulky ethoxycarbonyl group at the oxime moiety, afforded isoxazoline derivative 6z through a sequence of cyclization followed by intermolecular methylene transfer (Scheme 4b).4c In sharp contrast, in the presence of the cooperative Au-pyridine catalyst system, the reaction of oxime 1z selectively afforded oxazine 2z in good yield (Scheme 4a). It is noteworthy that, although a Brønsted base cocatalyst has been typically functioned as an activator of nucleophiles in Au-catalyzed reactions,10 the base cocatalyst serves as a switch of the reaction pathway in the present cascade reaction. Moreover, the reaction of 1z using the Cu catalyst afforded the azete-N-oxide 7zvia C–O bond cleavage (Scheme 4c). Accordingly, our studies reveal that by including or excluding a cocatalyst, as well as choosing appropriate metal catalysts, the skeletal rearrangement reaction can allow for the synthesis of diverse heterocycles from a common starting material through switching among reaction pathways, and can be highly beneficial within the field of drug discovery.
Experimental results indicated that elimination of proton from the propargylic position was rate-determining (Scheme 2). Thus we conducted DFT calculations to further elucidate reaction mechanism, specifically to identify the order of protodeauration, N–O bond cleavage, and re-cyclization processes, which would occur after the deprotonation. The profile for the present skeletal rearrangement reaction was calculated by using 1d as a model substrate (Fig. 1). All calculations were performed with the Gaussian 16 package (Revision A.03) at the level of ωB97XD/SDD for Au and 6-31G(d,p) for other elements in the solution phase according to the SMD solvation model (dichloroethane). The computations indicated that elimination of a proton by the imidate anion from the cyclized vinylgold intermediate 4d to form the isoxazolium species 5d was rate-determining; the free activation energy for this process (4d to TS2d, Fig. 2) was computed to be 24.4 kcal mol−1. In contrast, an energy barrier for direct [1,2]-hydrogen shift for the vinylgold species 4d was computed to be much higher than the proton elimination (36.0 kcal mol−1). Subsequent transfer of a proton from Tf2NH to the gold-bound carbon induced simultaneous ring opening with cleavage of the N–O bond. It is noteworthy that the process was computed to be highly exergonic (56.1 kcal mol−1), which serves as a driving force of the present skeletal rearrangement reaction. While recent theoretical studies have indicated that protodeauration of vinylgold specie proceeds in a concerted manner,11 our computational studies suggest that protodeauration can proceed stepwise when the approach of a proton to the gold-bound carbon atom induces cleavage of a weak covalent bond, such as N–O. Free activation energies for re-cyclization (8d to 9d) and elimination of the gold catalyst were computed to be very low (4.2 and 2.2 kcal mol−1, respectively).|| Elimination of proton from 9d to the vinylgold species 10d was computed to be much slower than elimination of the gold catalyst (23.3 versus 2.2 kcal mol−1). This result implies that decrease of the deuterium content in the Au-catalyzed reaction of deuterated substrate 1d-d (Scheme 2a) is primarily due to proton exchange between the eliminated Tf2ND and external water in the reaction media prior to protonation of 5d.
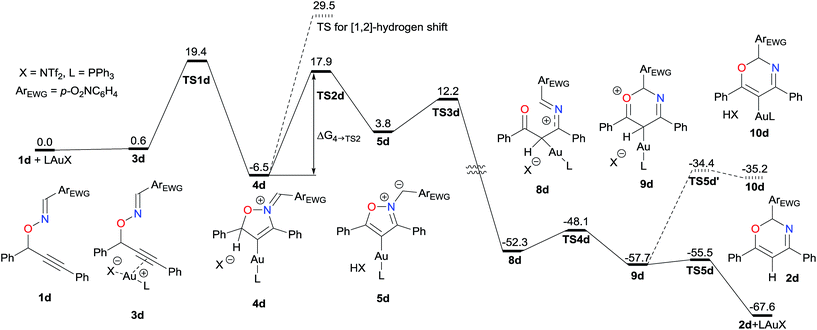 | ||
| Fig. 1 Energy profile of the Au-catalyzed skeletal rearrangement reaction of 1d at the level of ωB97XD/SDD for Au and 6-31G(d,p) for other elements. | ||
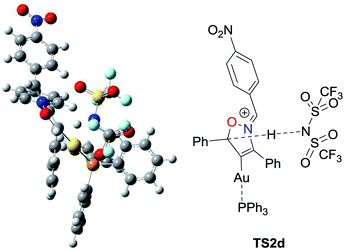 | ||
| Fig. 2 Transition state TS2d of the proton eliminating process in the Au-catalyzed reaction of 1d at the level of ωB97XD/SDD for Au and 6-31G(d,p) for other elements. | ||
Based on the theoretically obtained reaction profile, the activation energy ΔG4→TS2 of the proton elimination process (4 to TS2) was calculated in order to clarify the substituent effect at the oxime moiety as well as the role of base cocatalyst on this process (Table 5). The activation energy ΔG4→TS2 for the reaction of 1a having a phenyl group at the oxime moiety was computed to be much higher than that of 1d (31.2 kcal mol−1 for 1aversus 24.4 kcal mol−1 for 1d), which are in good agreement with our experimental results that the reaction of 1a required a longer reaction time (24 h versus 8 h) to obtain 2a in a much lower chemical yield (16% versus 93%). Thus, the computation well supports our qualitative understanding of the role of electron-withdrawing substituents at the oxime carbon facilitating elimination of the proton by increasing its acidity. In addition, DFT calculations indicated that pyridine approached to a proton on the isoxazoline ring from the opposite side of the imidate anion in the reaction of 1t, which has an alkyl group at the propargylic position, in the presence of pyridine (Fig. 3). This cooperation of the base cocatalyst significantly reduced the activation energy ΔG4→TS2 (from 31.9 to 16.3 kcal mol−1, entries 3 and 4). Further elucidation to understand the role of the base cocatalyst in the present transformation is underway in our laboratory.
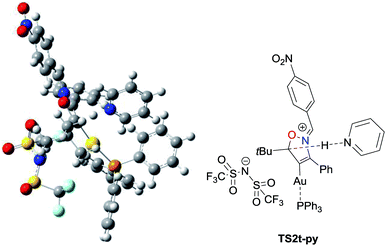 | ||
| Fig. 3 Transition state TS2t-py of the proton elimination process in the Au-catalyzed reaction of 1t in the presence of pyridine at the level of ωB97XD/SDD for Au and 6-31G(d,p) for other elements. | ||
Conclusions
We have successfully developed an novel approach for the synthesis of 2H-1,3-oxazine derivatives via Au-catalyzed skeletal rearrangement. Although oxazine derivatives exist within biologically active compounds,12 and serve as synthetic intermediates for nitrogenous compounds,13 syntheses of such compounds have remained challenging due to the instability of oxazines under thermal and acidic conditions.13a,13c,14 From a synthetic viewpoint, our methodology offers the potential toward the syntheses of various oxazines under mild reaction conditions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP16H00996 in Precisely Designed Catalysts with Customized Scaffolding and a Grant-in-Aid for Scientific Research on Innovative Areas “Hybrid Catalysis for Enabling Molecular Synthesis on Demand” (JP17H06447) from MEXT (Japan).Notes and references
-
(a) C. Aubert, O. Buisine and M. Malacria, Chem. Rev., 2002, 102, 813–834 CrossRef CAS
; (b) L. Añorbe, G. Domínguez and J. Pérez-Castells, Chem.–Eur. J., 2004, 10, 4938–4943 CrossRef PubMed
; (c) C. Bruneau, Angew. Chem., Int. Ed., 2005, 44, 2328–2334 CrossRef CAS PubMed
; (d) L. Zhang, J. Sun and S. A. Kozmin, Adv. Synth. Catal., 2006, 348, 2271–2296 CrossRef CAS
; (e) V. Michelet, P. Y. Toullec and J.-P. Genêt, Angew. Chem., Int. Ed., 2008, 47, 4268–4315 CrossRef CAS PubMed
; (f) M. Tobisu and N. Chatani, Chem. Soc. Rev., 2008, 37, 300–307 RSC
; (g) R. K. Shiroodi and V. Gevorgyan, Chem. Soc. Rev., 2013, 42, 4991–5001 RSC
; (h) C. Obradors and A. M. Echavarren, Acc. Chem. Res., 2014, 47, 902–912 CrossRef CAS PubMed
; (i) Q. Wang and M. Shi, Synlett, 2017, 28, 2230–2240 CrossRef CAS
.
-
(a) A. Correa, N. Marion, L. Fensterbank, M. Malacria, S. P. Nolan and L. Cavallo, Angew. Chem., Int. Ed., 2008, 47, 718–721 CrossRef CAS PubMed
; (b) N. Marion and S. P. Nolan, Angew. Chem., Int. Ed., 2007, 46, 2750–2752 CrossRef CAS PubMed
; (c) S. Wang, G. Zhang and L. Zhang, Synlett, 2010, 692–706 CAS
; (d) X.-Z. Shu, D. Shu, C. M. Schienebeck and W. Tang, Chem. Soc. Rev., 2012, 41, 7698–7711 RSC
; (e) B. J. Ayers and P. W. H. Chan, Synlett, 2015, 26, 1305–1339 CrossRef CAS
; (f) D. P. Day and P. W. H. Chan, Adv. Synth. Catal., 2016, 358, 1368–1384 CrossRef CAS
.
-
(a) I. Nakamura, D. Zhang and M. Terada, J. Am. Chem. Soc., 2010, 132, 7884–7886 CrossRef CAS PubMed
; (b) I. Nakamura, T. Araki, D. Zhang, Y. Kudo, E. Kwon and M. Terada, Org. Lett., 2011, 13, 3616–3619 CrossRef CAS PubMed
; (c) I. Nakamura, Y. Kudo, T. Araki, D. Zhang, E. Kwon and M. Terada, Synthesis, 2012, 44, 1542–1550 CrossRef CAS
; (d) I. Nakamura, M. Okamoto, Y. Sato and M. Terada, Angew. Chem., Int. Ed., 2012, 51, 10816–10819 CrossRef CAS PubMed
; (e) I. Nakamura, Y. Kudo and M. Terada, Angew. Chem., Int. Ed., 2013, 52, 7536–7539 CrossRef CAS PubMed
; (f) I. Nakamura, T. Onuma, D. Zhang and M. Terada, Tetrahedron Lett., 2014, 55, 1178–1182 CrossRef CAS
; (g) I. Nakamura, T. Jo, D. Zhang and M. Terada, Org. Chem. Front., 2014, 1, 914–918 RSC
; (h) I. Nakamura, Y. Sato, K. Takeda and M. Terada, Chem.–Eur. J., 2014, 20, 10214–10219 CrossRef CAS PubMed
; (i) I. Nakamura and M. Terada, Chem. Rec., 2015, 15, 429–444 CrossRef CAS
.
-
(a) I. Nakamura, S. Gima, Y. Kudo and M. Terada, Angew. Chem., Int. Ed., 2015, 54, 7154–7157 CrossRef CAS PubMed
; (b) S. Gima, I. Nakamura and M. Terada, Eur. J. Org. Chem., 2017, 4375–4378 CrossRef CAS
; (c) S. Gima, K. Shiga, M. Terada and I. Nakamura, Synlett, 2019, 30, 393–396 CrossRef CAS
.
- Selected reviews on gold catalysis:
(a) Z. Zheng, Z. Wang, Y. Wang and L. Zhang, Chem. Soc. Rev., 2016, 45, 4448–4458 RSC
; (b) A. M. Asiri and A. S. K. Hashmi, Chem. Soc. Rev., 2016, 45, 4471–4503 RSC
; (c) W. Zi and F. D. Toste, Chem. Soc. Rev., 2016, 45, 4567–4589 RSC
; (d) R. Dorel and A. M. Echavarren, Chem. Rev., 2015, 115, 9028–9072 CrossRef CAS PubMed
; (e) W. Yang and A. S. K. Hashmi, Chem. Soc. Rev., 2014, 43, 2941–2955 RSC
; (f) C. Obradors and A. M. Echavarren, Chem. Commun., 2014, 50, 16–28 RSC
; (g) H. V. Adcock and P. W. Davies, Synthesis, 2012, 44, 3401–3420 CrossRef CAS
; (h) S. Bhunia and R.-S. Liu, Pure Appl. Chem., 2012, 84, 1749–1757 CAS
; (i) B.-L. Lu, L. Dai and M. Shi, Chem. Soc. Rev., 2012, 41, 3318–3339 RSC
; (j) A. S. K. Hashmi, Angew. Chem., Int. Ed., 2010, 49, 5232–5241 CrossRef CAS PubMed
.
- Relativistic effect on gold catalysis;
(a) P. Pyykko and J. P. Desclaux, Acc. Chem. Res., 1979, 12, 276–281 CrossRef CAS
; (b) H.-C. Tai, I. Krossing, M. Seth and D. V. Deubel, Organometallics, 2004, 23, 2343–2349 CrossRef CAS
; (c) D. J. Gorin and F. D. Toste, Nature, 2007, 446, 395–403 CrossRef CAS PubMed
; (d) M. Lein, M. Rudolph, S. K. Hashmi and P. Schwerdtfeger, Organometallics, 2010, 29, 2206–2210 CrossRef CAS
and cited therein..
- Selected examples on N–O cleavage in gold catalysis,
(a) H.-S. Yeom, J.-E. Lee and S. Shin, Angew. Chem., Int. Ed., 2008, 47, 7040–7043 CrossRef CAS PubMed
; (b) L. Ye, L. Cui, G. Zhang and L. Zhang, J. Am. Chem. Soc., 2010, 132, 3258–3259 CrossRef CAS PubMed
; (c) A.-H. Zhou, Q. He, S. Chao, Y.-F. Yu, S. Liu, T. Zhao, W. Zhang, X. Lu and L.-W. Ye, Chem. Sci., 2015, 6, 1265–1271 RSC
; (d) H. Jin, L. Huang, J. Xie, M. Rudolph, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2016, 55, 794–797 CrossRef CAS PubMed
; (e) Z. Zeng, H. Jin, K. Sekine, M. Rudolph, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2018, 57, 6935–6939 CrossRef CAS
; (f) S. S. Giri and R.-S. Liu, Chem. Sci., 2018, 9, 2991–2995 RSC
; (g) R. R. Singh, M. Skaria, L.-Y. Chen, M.-J. Cheng and R.-S. Liu, Chem. Sci., 2019, 10, 1201–1206 RSC
.
-
(a) P. W. Davies and N. Martin, Org. Lett., 2009, 11, 2293–2296 CrossRef CAS PubMed
; (b) T. J. Brown, D. Weber, M. R. Gagné and R. A. Widenhoefer, J. Am. Chem. Soc., 2012, 134, 9134–9137 CrossRef CAS PubMed
; (c) L. Biasiolo, M. Trinchillo, P. Belanzoni, L. Belpassi, V. Busico, G. Ciancaleoni, A. D'Amora, A. Macchioni, F. Tarantelli and D. Zuccaccia, Chem.–Eur. J., 2014, 20, 14594–14598 CrossRef CAS PubMed
; (d) L. Biasiolo, A. Del Zotto and D. Zuccaccia, Organometallics, 2015, 34, 1759–1765 CrossRef CAS
; (e) Z. Lu, J. Han, O. E. Okoromoba, N. Shimizu, H. Amii, C. F. Tormena, G. B. Hammond and B. Xu, Org. Lett., 2017, 19, 5848–5851 CrossRef CAS PubMed
; (f) M. Gatto, A. Del Zotto, J. Segato and D. Zuccaccia, Organometallics, 2018, 37, 4685–4691 CrossRef CAS
.
- S. Orbisaglia, B. Jacques, P. Braunstein, D. Hueber, P. Pale, A. Blanc and P. de Frémont, Organometallics, 2013, 32, 4153–4164 CrossRef CAS
.
-
(a) S. Ritter, Y. Horino, J. Lex and H.-G. Schmalz, Synlett, 2006, 3309–3313 CAS
; (b) Z. Wang, Y. Wang and L. Zhang, J. Am. Chem. Soc., 2014, 136, 8887–8890 CrossRef CAS PubMed
; (c) S. Handa, S. S. Subramanium, A. A. Ruch, J. M. Tanski and L. M. Slaughter, Org. Biomol. Chem., 2015, 13, 3936–3949 RSC
; (d) Z. Wang, C. Nicolini, C. Hervieu, Y.-F. Wong, G. Zanoni and L. Zhang, J. Am. Chem. Soc., 2017, 139, 16064–16067 CrossRef CAS PubMed
; (e) X. Li, Z. Wang, X. Ma, P. Liu and L. Zhang, Org. Lett., 2017, 19, 5744–5747 CrossRef CAS PubMed
.
-
(a) R. BabaAhmadi, P. Ghanbari, N. A. Rajabi, A. S. K. Hashmi, B. F. Yates and A. Ariafard, Organometallics, 2015, 34, 3186–3195 CrossRef CAS
; (b) C. A. Gaggioli, G. Ciancaleoni, D. Zuccaccia, G. Bistoni, L. Belpassi, F. Tarantelli and P. Belanzoni, Organometallics, 2016, 35, 2275–2285 CrossRef CAS
.
-
(a) N. Gupta, S. Sharma, A. Raina, N. A. Dangroo, S. Bhushan and P. L. Sangwan, RSC Adv., 2016, 6, 106150–106159 RSC
; (b) T. J. Woltering, W. Wostl, H. Hilpert, M. Rogers-Evans, E. Pinard, A. Mayweg, M. Gobel, D. W. Banner, J. Benz, M. Travagli, M. Pollasrini, G. Marconi, E. Gabellieri, W. Guba, H. Mauser, M. Andreini, H. Jacobsen, E. Power and R. Narquizian, Bioorg. Med. Chem. Lett., 2013, 23, 4239–4243 CrossRef CAS PubMed
; (c) J. B. Chylinska, T. Urbanski and M. Mordarski, J. Med. Chem., 1963, 6, 484–487 CrossRef CAS
.
-
(a) G. H. Lonca, C. Tejo, H. L. Chan, S. Chiba and F. Gagosz, Chem. Commun., 2017, 53, 736–739 RSC
; (b) J. R. Manning and H. M. L. Davies, J. Am. Chem. Soc., 2008, 130, 8602–8603 CrossRef CAS PubMed
; (c) J. Barluenga, M. Tomas, A. Ballesteros and J.-S. Kong, Tetrahedron, 1996, 52, 3095–3106 CrossRef CAS
.
-
(a) K. V. Zavyalov, M. S. Novikov, A. F. Khlebnikov and V. V. Pakalnis, Tetrahedron, 2014, 70, 3377–3384 CrossRef CAS
; (b) A. F. Khlebnikov, M. S. Novikov, Y. G. Gorbunova, E. E. Galenko, K. I. Mikhailov, V. V. Pakalnis and M. S. Avdontceva, Beilstein J. Org. Chem., 2014, 10, 1896–1905 CrossRef PubMed
; (c) K. V. Zavyalov, M. S. Novikov, A. F. Khlebnikov and D. S. Yufit, Tetrahedron, 2013, 69, 4546–4551 CrossRef CAS
; (d) C. Francois-Endelmond, T. Carlin, P. Thuery, O. Loreau and F. Taran, Org. Lett., 2010, 12, 40–42 CrossRef CAS PubMed
; (e) J. R. Manning and H. M. L. Davies, Tetrahedron, 2008, 64, 6901–6908 CrossRef CAS PubMed
; (f) J. Barluenga, M. Tomáas, A. Bailesteros, J. S. Kong, S. Garcia-Granda and A. Aguirre, J. Chem. Soc., Chem. Commun., 1993, 217–218 RSC
; (g) R. R. Schmidt, D. Schwille and H. Wolf, Chem. Ber., 1970, 103, 2760 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental and computational details and spectral data are provided. CCDC 1892710 (2s). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc00501c |
| ‡ We have previously reported on the Cu-catalyzed rearrangements of O-propargylic phenylacetaldoximes via N–O bond cleavage to afford N-styryl epoxyimines. (a) I. Nakamura, T. Iwata, D. Zhang, M. Terada, Org. Lett., 2012, 14, 206–209. Although we have proposed the copper carbenoid species as the intermediate, it is more likely that the reaction proceeds via formal hydroamination, followed by [1,3]-oxygen rearrangement (Scheme S1†), according to our continued investigations and reports from other groups (b) D.-L. Mo, L. L. Anderson, Angew. Chem. Int. Ed., 2013, 52, 6722–6725; (c) I. Nakamura, T. Jo, Y. Ishida, H. Tashiro, M. Terada, Org. Lett., 2017, 19, 3059–3062. See also ref. 3e. Further mechanistic studies to elucidate the reaction mechanisms of the Cu-catalyzed reactions are currently underway in our laboratories. |
| § Incorporation of deuterium was not observed when the product 2d was treated with D2O in the presence of the gold catalyst. See ESI.† |
| ¶ The reaction of 1l and 1n by using gold-pyridine cocatalyst system did not afford the desired products; 1l was decomposed under the reaction conditions, while 1n was partially recovered (71%). |
| || It is alternatively possible that the intermediate 8d undergoes elimination of the gold catalyst before re-cyclization (6π-electrocyclization), of which activation energy were 6.6 and 8.6 kcal mol−1, respectively. See ESI.† |
| This journal is © The Royal Society of Chemistry 2019 |