Open Access Article
Open Access ArticleAqueous acid-based synthesis of lead-free tin halide perovskites with near-unity photoluminescence quantum efficiency†
Aifei
Wang
a,
Yanyan
Guo
a,
Zhaobo
Zhou
b,
Xianghong
Niu
c,
Yonggang
Wang
 d,
Faheem
Muhammad
a,
Hongbo
Li
d,
Faheem
Muhammad
a,
Hongbo
Li
 e,
Tao
Zhang
e,
Tao
Zhang
 a,
Jinlan
Wang
a,
Jinlan
Wang
 *b,
Shuming
Nie
*b,
Shuming
Nie
 *af and
Zhengtao
Deng
*af and
Zhengtao
Deng
 *a
*a
aCollege of Engineering and Applied Sciences, Nanjing National Laboratory of Microstructures, Nanjing University, Nanjing, Jiangsu 210093, P. R. China. E-mail: dengz@nju.edu.cn
bSchool of Physics, Southeast University, Nanjing 211189, P. R. China. E-mail: jlwang@seu.edu.cn
cSchool of Science, Nanjing University of Posts and Telecommunications, Nanjing 210046, People's Republic of China
dCenter for High Pressure Science and Technology Advanced Research (HPSTAR), Beijing 100949, P. R. China
eCollege of Materials Science and Engineering, Beijing Institute of Technology, Haidian District, Beijing 100081, P. R. China
fDepartments of Bioengineering, Chemistry, Electrical and Computer Engineering, and Materials Science and Engineering, University of Illinois at Urbana-Champaign, Urbana, IL, USA. E-mail: nies@illinois.edu
First published on 6th March 2019
Abstract
Recently, lead halide perovskites with outstanding emission performance have become new candidate materials for light-emitting devices and displays; however, the toxicity of lead and instability of halide perovskites remain significant challenges. Herein, we report the aqueous acid-based synthesis of highly emissive two-dimensional (2D) tin halide perovskites, (octylammonium)2SnX4 (X = Br, I, or mixtures thereof), which displayed a high absolute photoluminescence (PL) quantum yield of near-unity in the solid-state, PL emission centered at 600 nm with a broad bandwidth (136 nm), a large Stokes shift (250 nm), long-lived luminescence (τ = 3.3 μs), and zero overlap between their absorption and emission spectra. Significantly, the stability study of 2D tin halide perovskites monitored by the PL quantum yield showed no changes after 240 days of storage at room temperature under ambient air and humidity conditions. The PL emission of the 2D tin halide perovskites was tuned from yellow to deep red by controlling halide composition. Furthermore, new yellow phosphors with superior optical properties are used to fabricate UV pumped white light emitting diodes (WLEDs). We expect these results to facilitate the development of new environmentally friendly and high-performance phosphors for future lighting and display technologies.
Introduction
In recent years, solid-state lighting technology has become a cost-competitive and energy-efficient alternative to conventional electrical lighting.1 This results in great interest in the study of the chemical synthesis, luminescence properties, and engineering technology of phosphor materials. Over the past few decades, the most studied highly fluorescent solid-state semiconductor phosphor materials involved toxic heavy metals, such as cadmium selenide (CdSe) and lead selenide (PbSe), which presents a serious threat to environmental and human health.2 Alternatively, indium phosphide (InP) and copper indium sulfide (CIS) were studied as heavy-metal-free alternatives, but their luminescence properties are not significant, and indium is not abundant in the earth, so its production cost or price is very high.2–4 Recently, the outstanding performance of APbX3-type lead halide perovskites (A = Cs+, CH3NH3+; X = Cl−, Br−, I−) in photovoltaic solar cells reignited a phenomenal interest to use them also for high-performance solid-state lighting.5–9 The main advantages making them particularly attractive are the excellent optical and optoelectronic properties, the facile synthetic process at low temperatures, the low densities of trap states, and the flexibility to tune the optical bandgaps in the whole visible region.10–14 Despite the rapid development of high-performance and low-cost lead halide perovskite materials, the problem of the long-term toxicity of lead has not been solved which needs to be addressed before their large-scale and extensive applications.12,15As a result, efforts are being made to explore new alternatives for heavy metal-free and earth abundant highly fluorescent semiconductor materials.16–19 In this regard, tin is one of the major industrial metals with low toxicity, but the oxidation of divalent Sn2+ in the lead-free tin halide perovskite structure generates spontaneous vacancies and thus compromises the device performance.20 To this end, a number of strategies to address this problem are reported.21–24 The use of tetravalent tin, such as perovskite derivative Cs2SnI6, as an alternative has been studied.22,23 We have also recently developed a strategy for the synthesis of highly stable CsSnBr3 nanocages using a perfluorooctanoic acid (PFOA) post-treatment strategy.24
A family of two-dimensional (2D) perovskites is recently attracting a great deal of attention and is described by the formula (R–NH3)2An−1MnX3n+1, where R is alkyl chains, A is a small monovalent cation, M is a divalent metal and X is a halide.25,26 Such 2D perovskites are an attractive alternative to three-dimensional (3D) APbX3-type perovskites in terms of their tremendous advantages of tuning optoelectronic properties through both chemical and quantum-mechanical degrees of freedom. Each organic layer in such perovskites acts as a potential barrier confining the excitons within the MX6 octahedron layer; thereby, 2D perovskites exhibit good photoluminescence and electroluminescence even in bulk states.27–29 In addition, layered 2D perovskites have also been reported to be highly resistant to air and moisture as compared to their bulk counterparts because the hydrophobic alkyl chains block the layer of MX6 octahedrons and protect them from oxidation.30 Very recently, a 2D tin halide perovskite of (C18H35NH3)2SnBr4 with a QY of 88% has been reported using an organic based hot injection method,31 but the aqueous synthetic route to prepare 2D tin halide perovskites is still challenging due to the anticipated instability of metal halides in aqueous solution. Using solubility equilibrium between metal halides and their saturated ionic components, a 3D lead halide perovskite was synthesized in aqueous solution with a highest QY of 40%,32 but the 2D counterparts displayed a rather low QY (<10%).33,34 Despite the above-mentioned advances, the aqueous synthesis of highly fluorescent lead-free 2D metal halide perovskites has been rarely reported.
In this study, we report, for the first time, the preparation of highly emissive two-dimensional (2D) phase (OCTAm)2SnX4 (OCTAm = octylammonium cation) tin halide perovskites in acidic aqueous solution with ultra-high absolute photoluminescence (PL) quantum yield and ultra-high stability. In comparison to previous reports, the differences and significance of the current work are as follows: (i) this is the first report of the synthesis of highly fluorescent 2D tin halide perovskites with the emission centered at 600 nm with a PLQY of near-unity, large Stokes shifts, ultra-long lifetime, and good PL stability under ambient conditions. (ii) The entire synthesis process for 2D divalent tin halide perovskites was performed in acidic aqueous solution in an ambient air environment with high reproducibility and chemical yield (>80%). (iii) The PL could be tuned from yellow to dark red through controlling halide composition. (iv) The new yellow phosphor with superior optical properties was employed to fabricate UV pumped white light emitting diodes (WLEDs).
Results and discussion
2D tin perovskites with variable composition were prepared as described in the experimental section. Unlike previous reports wherein the synthesis process of tin-based perovskites required special care in inert gas environments, in our study the whole synthesis was performed in aqueous acid solution and ambient atmosphere without humidity and oxygen control, as shown in Scheme 1. Typically, we adopted octylamine as the starting cation agent and it was protonated and changed into an octylammonium (OCTAm) cation by acidification after being introduced into the system. The 2D tin perovskites were formed by a self-assembly driven crystallization process and stacked as organic–inorganic layers like 2D lead organic–inorganic perovskites.35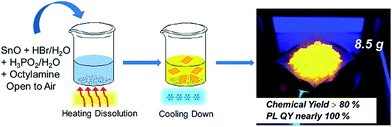 | ||
| Scheme 1 Schematic representation of the synthesis of the 2D (OCTAm)2SnBr4 by a facile aqueous acid-based synthetic method in ambient air. | ||
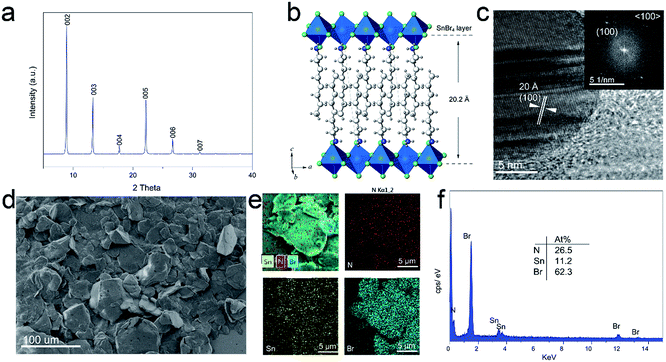
The crystalline nature of 2D perovskite crystals was confirmed by powder X-ray diffraction (PXRD) analysis. Powder X-ray diffraction (PXRD) measurements of (OCTAm)2SnBr4 showed periodic diffraction at low angles (Fig. 1a), and typical peaks resulting from atomic plane reflection of 2D perovskites at 8.9 and 13.2° [the (002) and (003) planes] indicated the horizontal growth of these compounds.36 The presence of only (001) reflections in the XRD patterns suggests a 2D structure of (OCTAm)2SnBr4, similar to that recently reported for this family of (OAm)2SnBr4.30 According to Bragg's law, the periodicity which corresponds to average layer spacing was calculated to be 19.94 Å. The anticipated structure of the 2D (OCTAm)2SnBr4 is shown in Fig. 1b, as the SnBr6 octahedral layer being separated by octylammonium. Fig. 1c shows the high-resolution transmission electron microscopy (HRTEM) image with the corresponding fast Fourier transform (FFT) of the 2D (OCTAm)2SnBr4 perovskites, and the interplanar spacing was determined to be 20 Å, which matches well with that of the (100) plane. The 2D (OCTAm)2SnBr4 perovskite layer spacing (20.2 Å) consists of a single metal halide octahedral layer which is ∼6.7 Å, and two interleaving OCTAm cation layers occupy a space of 13.5 Å. A layered morphology was observed in the scanning electron microscopy (SEM) images, as shown in Fig. 1d and S1.† The energy-dispersive X-ray spectroscopy (EDS, Fig. 1e and f) results suggest the homogeneous distribution of the elements of nitrogen, tin, and bromide in the (OCTAm)2SnBr4.
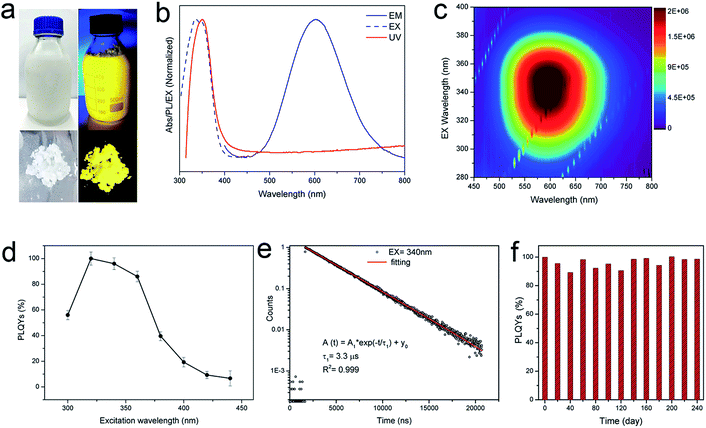
Since the procedure is so facile that the product can be readily scaled up with high reproducibility. The chemical yield of 2D (OCTAm)2SnBr4 perovskites was estimated to be >80% based on the total Sn content using an ultra-large scale (over 8.5 gram) sample, which was produced in a single batch. Furthermore, the product showed a highly bright yellow PL under UV light. The optical properties of the Sn bromide perovskites were characterized via UV-vis absorption and PL spectroscopies (Fig. 2b and c). The absorption and PL excitation results of 2D (OCTAm)2SnBr4 display similar broad spectra from 300 to 400 nm centered at around 350 nm. A plot of [F(R)hν]2versus energy indicates a direct band gap of 3.08 eV (Fig. S2†). The PL emission spectrum shows a broad peak centered at 600 nm (2.07 eV). Compared to the small Stokes shift and narrow emission caused by delocalized excitonic character in 3D perovskites, we observed the extra-large Stokes shift (∼250 nm, i.e., 1.47 eV) and broad emission (full width at half-maximum, FWHM, ∼136 nm, i.e., 467 meV) in 2D (OCTAm)2SnBr4 perovskites. According to previous reports, such a large Stokes shift and broad emission exist in both lead and tin 2D hybrid perovskites that stems from strong exciton couples in the metal-halide octahedral layer and creates transient elastic lattice distortions, viewed as self-trapping excitons.31,37 Similarly, we presume that the exciton self-trapping results in broad yellow emission in our (OCTAm)2SnBr4 materials. The 2D (OCTAm)2SnBr4 is further analyzed using three-dimensional excitation–emission matrix (EEM) fluorescence spectroscopy (Fig. 2c). There was no peak shift with the change of the excitation, suggesting that no other impurities or extra energy level existed in our samples.
2D phase (OCTAm)2SnBr4 shows excitation dependent PL QYs and the absolute PL QY was near unity (95 ± 5%) (Fig. 2d) in the solid-state. The PL QY of our 2D (OCTAm)2SnBr4 perovskite far exceeds the values reported in the case of aqueous synthesis of lead perovskites and is comparable with those of tin counterparts synthesized in organic solvents (Table S1†). It was found that there was a long PL lifetime decay of 3.3 μs (Fig. 2e), which is similar to that of the 2D (C18H35NH3)2SnBr4 perovskites. The time-resolved PL decay curve recoded at a PL emission wavelength of 600 nm and an excitation wavelength of 340 nm was easily fitted to a single exponential decay function, which suggests that the PL decay is not related to the non-radiative processes, consistent with the high PL QY of the 2D (C18H35NH3)2SnBr4.38 Moreover, there was no noticeable change in the time-resolved PL decay curve under different excitation wavelengths of 280 nm and 365 nm (Fig. S3†). In order to investigate whether H3PO2 has any effect on the quantum efficiency of the product, we used different concentrations of acid during the synthesis of (OCTAm)2SnBr4. Our findings ruled out any stabilization role of H3PO2 and no significant difference in quantum yield was observed (Fig. S4†), thus implying that the main function of H3PO2 is to avoid hydrolysis and oxidation of divalent tin. Stability of metal halide perovskites is critical for their further optical and optoelectronic applications. We found that the 2D (OCTAm)2SnBr4 exhibits high air-stability for over 240 days (Fig. 2f). It is not surprising that the (OCTAm)2SnBr4 perovskite was found to be very stable in ambient environment if we consider that the photoactive Sn–Br octahedrons are well protected by octylammonium cations. Furthermore, we embedded (OCTAm)2SnBr4 into polystyrene (PS) films (Fig. S5–S7†) that yielded a composite with superior optical clarity, bright emission (PL QY maximum = 80%) and long lifetime (3.3 μs). The high PL QY of the film is also related to the extra-large Stokes shifts, which would reduce re-absorption energy loss. These features are of vital importance for their future applications in solid-state lighting and luminescent solar concentrators (LSC).39
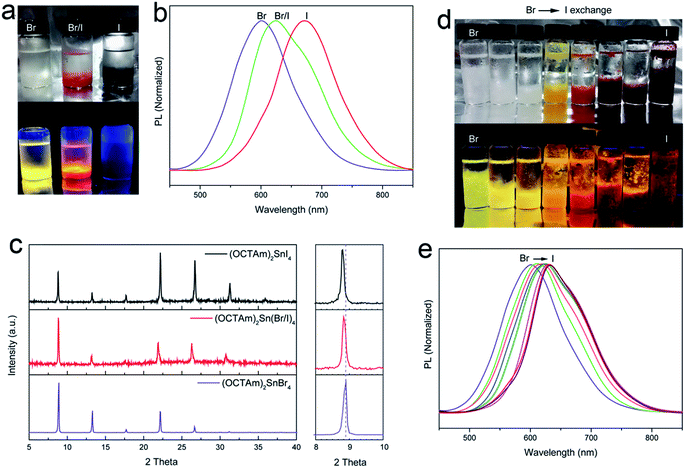
In addition to examining the tunability of 2D OCTAm-Sn hybrid perovskites, varying the halide ratio of the perovskites via in situ and post-synthesis strategies was studied. In situ protocol results in the generation of pure tin-iodide (OCTAm)2SnI4 and the various compositions of tin-bromide/iodide (OCTAm)2Sn(Br/I)4 are shown in Fig. 3a–c, which revealed that the PL emission spectrum can be tuned from 600 nm to 670 nm with a broad emission peak. The PL QY of (OCTAm)2SnI4 was determined to be 41%. Powder X-ray diffraction (PXRD) results also displayed periodic diffraction which verified a 2D layered perovskite structure in all products. The PXRD pattern of (002) planes from Br to I perovskites indicated a slight shift to lower angle due to tin-halide bond expansion.
The halide composition can also be regulated post-synthetically via an anion-exchange reaction using different halogen hydrides (Fig. 3b–e). In the anion-exchange process, pure bromide 2D OCTAm-Sn hybrid perovskites were used as the starting material; with increasing hydrogen iodide concentration the luminescence changes from yellow to deep red. This is consistent with previous reports about lead-based perovskite materials.40
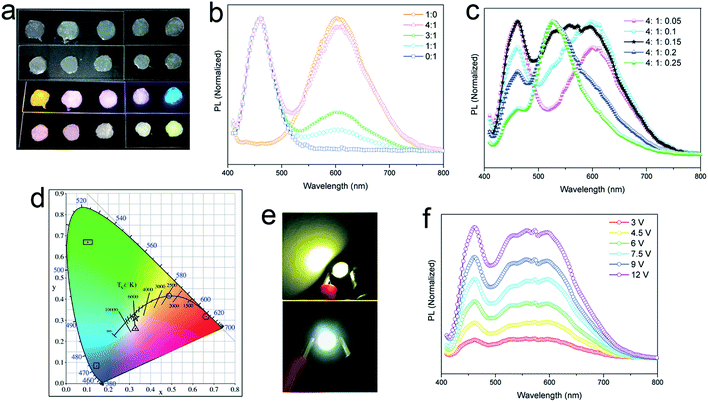
The yellow-emitting phosphor of YAG:Ce3+ is widely used in commercial WLEDs, but the deficiency of red fluorescent components in YAG:Ce3+ (centered at ∼550 nm, FWHM of 100 nm) results in a low (<80) color rendering index (CRI) in WLEDs.41 Rather than the addition of another red phosphor to broaden the emission band of the phosphor, use of any material with broad yellow emission covering also the red region can be a practical approach to improve the red component. Our lead-free 2D (OCTAm)2SnBr4 perovskites with high PL quantum yield and broadened yellow emission covering the red region motivated us to employ the obtained perovskite in WLEDs as a yellow-emitting phosphor. To validate the applicability of this material as a yellow phosphor, down conversion LEDs were fabricated. A commercial UV LED (365 nm) chip was used to optically pump different phosphor mixtures. The images of phosphor blended films and corresponding emission spectra are shown in Fig. 4a–c. A range of “warm” to “cold” white lights were attained by controlling the blending ratio between the three phosphors. The CIE color coordinates are shown in Fig. 4d. The UV pump LED of the single yellow phosphor showed a Correlated Colour Temperature (CCT) of 2500 K, a CRI of 69 and CIE coordinates of (0.488, 0.415), while with a ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by blue/yellow weight, a warm white emission with CIE coordinates of (0.33, 0.26), a CCT of 5635 and a CRI of 81 was achieved. A small amount of a green phosphor (yellow
1 by blue/yellow weight, a warm white emission with CIE coordinates of (0.33, 0.26), a CCT of 5635 and a CRI of 81 was achieved. A small amount of a green phosphor (yellow![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) blue
blue![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) green = 4
green = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) helps tune to white emission with CIE coordinates of (0.33, 0.31), a CCT of 6530 K, a CRI of 89, and an LED lamp was fabricated based on this weight ratio (Fig. 4e). Various operating voltages were used to test the color stability of the white LED (Fig. 4f). With voltage increasing from 3 V to 12 V, light intensity steadily increases without energy transfer between the three phosphors. Taken together, we envision the 2D (OCTAm)2SnBr4 perovskite as a potential yellow phosphor for display backlight applications.
1.5) helps tune to white emission with CIE coordinates of (0.33, 0.31), a CCT of 6530 K, a CRI of 89, and an LED lamp was fabricated based on this weight ratio (Fig. 4e). Various operating voltages were used to test the color stability of the white LED (Fig. 4f). With voltage increasing from 3 V to 12 V, light intensity steadily increases without energy transfer between the three phosphors. Taken together, we envision the 2D (OCTAm)2SnBr4 perovskite as a potential yellow phosphor for display backlight applications.
Conclusions
In summary, we have demonstrated facile aqueous acid-based synthesis of two-dimensional (OCTAm)2SnBr4 tin halide perovskites. The whole synthesis process was carried out in an ambient air and humidity environment, with high reproducibility and chemical yield. The obtained tin halide perovskites exhibited strong PL emission centered at 600 nm with broad FWHM (136 nm), large Stokes shifts (250 nm), high absolute PL quantum yield near unity in the solid-state, zero-overlap between their absorption and emission spectra, and an ultra-long lifetime (3.3 μs). These ultra-stable perovskite solid-state samples showed no changes after 240 days of storage at room temperature under ambient air and humidity conditions. These 2D tin perovskites act as yellow phosphors to produce optically pumped white LEDs. By a proper choice of raw sources and synthetic parameters, we anticipate that the present synthetic method can be extended for the rational design and synthesis of highly emissive, low-cost, environmentally benign, and stable metal halide perovskite phosphors, which will have broad prospects for application in the new generation of solid-state lighting and displays.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank Prof. Yuefeng Nie, Prof. Weiqiang Li, and Dr Yuguan Pan for help in acquiring the XRD spectra and helpful discussions regarding XRD analysis. This work was supported by the Natural Science Foundation of Jiangsu Province (Grant No. BK20180339 and BK20150581), the National Natural Science Foundation of China (Grant No. 51502130 and 21525311), the National Key R&D Program of China (Grant No. 2017YFA0204800), the Thousand Talents Program for Young Researchers, the Shuangchuang Program of Jiangsu Province, Fundamental Research Funds for the Central Universities (Grant No. 021314380123), and the Jiangsu Key Laboratory for Nano Technology.Notes and references
- E. F. Schubert and J. K. Kim, Science, 2005, 308, 1274–1278 CrossRef CAS PubMed.
- J. Q. Grim, L. Manna and I. Moreels, Chem. Soc. Rev., 2015, 44, 5897–5914 RSC.
- W. Shen, H. Tang, X. Yang, Z. Cao, T. Cheng, X. Wang, Z. Tan, J. You and Z. Deng, J. Mater. Chem. C, 2017, 5, 8243–8249 RSC.
- H. Li, A. G. Kanaras and L. Manna, Acc. Chem. Res., 2013, 46, 1387–1396 CrossRef CAS PubMed.
- C. C. Stoumpos and M. G. Kanatzidis, Adv. Mater., 2016, 28, 5778–5793 CrossRef CAS PubMed.
- A. R. Srimath Kandada and A. Petrozza, Acc. Chem. Res., 2016, 49, 536–544 CrossRef CAS PubMed.
- L. Pedesseau, D. Sapori, B. Traore, R. Robles, H. H. Fang, M. A. Loi, H. Tsai, W. Nie, J. C. Blancon, A. Neukirch, S. Tretiak, A. D. Mohite, C. Katan, J. Even and M. Kepenekian, ACS Nano, 2016, 10, 9776–9786 CrossRef CAS PubMed.
- H. Tan, A. Jain, O. Voznyy, X. Lan, F. P. G. de Arquer, J. Z. Fan, R. Quintero-Bermudez, M. Yuan, B. Zhang and Y. Zhao, Science, 2017, 355, 722–726 CrossRef CAS PubMed.
- Y. Zhao and K. Zhu, Chem. Soc. Rev., 2016, 45, 655–689 RSC.
- Y. Bekenstein, B. A. Koscher, S. W. Eaton, P. Yang and A. P. Alivisatos, J. Am. Chem. Soc., 2015, 137, 16008–16011 CrossRef CAS PubMed.
- Y. S. Park, S. Guo, N. S. Makarov and V. I. Klimov, ACS Nano, 2015, 9, 10386–10393 CrossRef CAS PubMed.
- W. Liu, Q. Lin, H. Li, K. Wu, I. Robel, J. M. Pietryga and V. I. Klimov, J. Am. Chem. Soc., 2016, 138, 14954–14961 CrossRef CAS PubMed.
- S. Sun, D. Yuan, Y. Xu, A. Wang and Z. Deng, ACS Nano, 2016, 10, 3648–3657 CrossRef CAS PubMed.
- C. Wang, Y. Zhang, A. Wang, Q. Wang, H. Tang, W. Shen, Z. Li and Z. Deng, Chem. Mater., 2017, 29, 2157–2166 CrossRef CAS.
- X. Yuan, S. Ji, M. C. De Siena, L. Fei, Z. Zhao, Y. Wang, H. Li, J. Zhao and D. R. Gamelin, Chem. Mater., 2017, 29, 8003–8011 CrossRef CAS.
- R. L. Z. Hoye, P. Schulz, L. T. Schelhas, A. M. Holder, K. H. Stone, J. D. Perkins, D. Vigil-Fowler, S. Siol, D. O. Scanlon, A. Zakutayev, A. Walsh, I. C. Smith, B. C. Melot, R. C. Kurchin, Y. Wang, J. Shi, F. C. Marques, J. J. Berry, W. Tumas, S. Lany, V. Stevanović, M. F. Toney and T. Buonassisi, Chem. Mater., 2017, 29, 1964–1988 CrossRef CAS.
- X. G. Zhao, J. H. Yang, Y. Fu, D. Yang, Q. Xu, L. Yu, S. H. Wei and L. Zhang, J. Am. Chem. Soc., 2017, 139, 2630–2638 CrossRef CAS PubMed.
- T. C. Jellicoe, J. M. Richter, H. F. Glass, M. Tabachnyk, R. Brady, S. E. Dutton, A. Rao, R. H. Friend, D. Credgington, N. C. Greenham and M. L. Bohm, J. Am. Chem. Soc., 2016, 138, 2941–2944 CrossRef CAS PubMed.
- P. V. Kamat, J. Bisquert and J. Buriak, ACS Energy Lett., 2017, 2, 904–905 CrossRef CAS.
- F. Wang, J. Ma, F. Xie, L. Li, J. Chen, J. Fan and N. Zhao, Adv. Funct. Mater., 2016, 26, 3417–3423 CrossRef CAS.
- K. P. Marshall, M. Walker, R. I. Walton and R. A. Hatton, Nat. Energy, 2016, 1, 16178 CrossRef CAS.
- A. Wang, X. Yan, M. Zhang, S. Sun, M. Yang, W. Shen, X. Pan, P. Wang and Z. Deng, Chem. Mater., 2016, 28, 8132–8140 CrossRef CAS.
- B. Lee, C. C. Stoumpos, N. Zhou, F. Hao, C. Malliakas, C.-Y. Yeh, T. J. Marks, M. G. Kanatzidis and R. P. H. Chang, J. Am. Chem. Soc., 2014, 136, 15379–15385 CrossRef CAS PubMed.
- A. Wang, Y. Guo, F. Muhammad and Z. Deng, Chem. Mater., 2017, 29, 6493–6501 CrossRef CAS.
- C. Huo, B. Cai, Z. Yuan, B. Ma and H. Zeng, Small Methods, 2017, 1, 1600018 CrossRef.
- M. I. Saidaminov, O. F. Mohammed and O. M. Bakr, ACS Energy Lett., 2017, 2, 889–896 CrossRef CAS.
- P. Cheng, T. Wu, J. Zhang, Y. Li, J. Liu, L. Jiang, X. Mao, R. F. Lu, W. Q. Deng and K. Han, J. Phys. Chem. Lett., 2017, 8, 4402–4406 CrossRef CAS PubMed.
- L. Mao, W. Ke, L. Pedesseau, Y. Wu, C. Katan, J. Even, M. R. Wasielewski, C. C. Stoumpos and M. G. Kanatzidis, J. Am. Chem. Soc., 2018, 140, 3775–3783 CrossRef CAS PubMed.
- A. Biswas, R. Bakthavatsalam and J. Kundu, Chem. Mater., 2017, 29, 7816–7825 CrossRef CAS.
- L. Lanzetta, J. M. Marin-Beloqui, I. Sanchez-Molina, D. Ding and S. A. Haque, ACS Energy Lett., 2017, 2, 1662–1668 CrossRef CAS.
- X. Zhang, C. Wang, Y. Zhang, X. Zhang, S. Wang, M. Lu, H. Cui, S. V. Kershaw, W. W. Yu and A. L. Rogach, ACS Energy Lett., 2018, 4, 242–248 CrossRef.
- C. Geng, S. Xu, H. Zhong, A. L. Rogach and W. Bi, Angew. Chem., Int. Ed., 2018, 57, 9650–9654 CrossRef CAS PubMed.
- E. R. Dohner, A. Jaffe, L. R. Bradshaw and H. I. Karunadasa, J. Am. Chem. Soc., 2014, 136, 13154–13157 CrossRef CAS PubMed.
- T. Y. Li, W. A. Dunlap-Shohl, Q. W. Han and D. B. Mitzi, Chem. Mater., 2017, 29, 6200–6204 CrossRef CAS.
- L. Mao, Y. Wu, C. C. Stoumpos, M. R. Wasielewski and M. G. Kanatzidis, J. Am. Chem. Soc., 2017, 139, 5210–5215 CrossRef CAS PubMed.
- D. H. Cao, C. C. Stoumpos, O. K. Farha, J. T. Hupp and M. G. Kanatzidis, J. Am. Chem. Soc., 2015, 137, 7843–7850 CrossRef CAS PubMed.
- M. D. Smith and H. I. Karunadasa, Acc. Chem. Res., 2018, 51, 619–627 CrossRef CAS PubMed.
- C. Zhou, H. Lin, Y. Tian, Z. Yuan, R. Clark, B. Chen, L. J. van de Burgt, J. C. Wang, Y. Zhou, K. Hanson, Q. J. Meisner, J. Neu, T. Besara, T. Siegrist, E. Lambers, P. Djurovich and B. Ma, Chem. Sci., 2018, 9, 586–593 RSC.
- C. S. Erickson, L. R. Bradshaw, S. McDowall, J. D. Gilbertson, D. R. Gamelin and D. L. Patrick, ACS Nano, 2014, 8, 3461–3467 CrossRef CAS PubMed.
- G. Nedelcu, L. Protesescu, S. Yakunin, M. I. Bodnarchuk, M. J. Grotevent and M. V. Kovalenko, Nano Lett., 2015, 15, 5635–5640 CrossRef CAS PubMed.
- W. Sun, Y. Jia, R. Pang, H. Li, T. Ma, D. Li, J. Fu, S. Zhang, L. Jiang and C. Li, ACS Appl. Mater. Interfaces, 2015, 7, 25219–25226 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and characterization details, an additional SEM image, additional PL decay curves, and additional pictures. See DOI: 10.1039/c9sc00453j |
| This journal is © The Royal Society of Chemistry 2019 |