Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Comment on Enantioselective total synthesis of (−)-colchicine, (+)-demecolcinone and metacolchicine: determination of the absolute configurations of the latter two alkaloids by B. Chen, X. Liu, Y.-J. Hu, D.-M. Zhang, L. Deng, J. Lu, L. Min, W.-C. Ye and C.-C. Li, Chem. Sci., 2017, 8, 4961–4966
Reinhard W.
Hoffmann
 *a,
Hans-Günther
Schmalz
*a,
Hans-Günther
Schmalz
 b,
Ulrich
Koert
b,
Ulrich
Koert
 a and
Gregory K.
Pierens
a and
Gregory K.
Pierens
 c
c
aFachbereich Chemie der Philipps Universität Marburg, Hans Meerwein Str. 4, D-35032 Marburg, Germany. E-mail: rwho@chemie.uni-marburg.de
bDepartment of Chemistry, University of Cologne, Greinstrasse 4, D-50939 Koeln, Germany
cThe Centre for Advanced Imaging, The University of Queensland, Building 57, Research Road, St. Lucia, Queensland 4072, Australia
First published on 18th December 2018
Abstract
We note that some features of the NMR spectra deposited for the purported isomeric metacolchicines (Chem. Sci.8, 4961 (2017)) are not compatible with the assignment of those isomers as being atrop-diastereomers. We suggest that there are no such isomeric metacolchicines as reported. The differences in the spectra could rather be a consequence of a (precedented) monomer/dimer equilibrium of metacolchicine in solution.
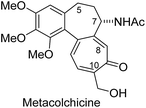
Metacolchicine has been isolated and characterized in 2011.1 Its structure assignment rests on 1H and 13C NMR spectra including 1H–1H-COSY, HMBC und NOE investigations. The authors fully assigned the spectra.
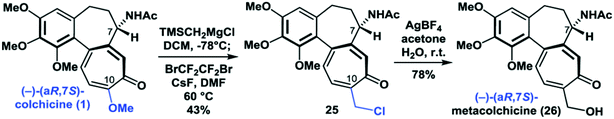
Recently approaches to the synthesis of metacolchicine were published by Li.2 The Li group used a complex reaction sequence to arrive at compound 26 which did not fully match the data reported for metacolchicine.
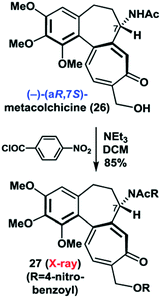
This non-identity apparently was reason to prepare the bis-4-nitrobenzoate of 26 in order to subject it to X-ray crystallographic analysis. This revealed that compound 26 possesses the structure assigned to metacolchicine.
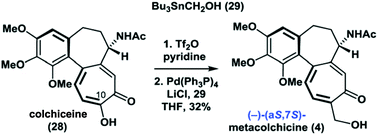
In the course of their efforts to synthesize metacolchicine the Li group explored a second route originating from colchiceine which furnished compound 4. The spectral data of the latter were claimed to be identical to those of natural metacolchicine.3
These findings suggested that there are two different compounds with the constitution of metacolchicine. Li and his coauthors address these, more or less by default, as being stereoisomers, i.e. atrop-diastereomers, 26 = (aR,7S) and 4 = (aS,7S). Unfortunately they did not provide any evidence (such as CD-spectra cf.4) for this interpretation.
This interpretation appears daring, as the rotational barrier at the axis of colchicine and colchicine-derivatives (which should include metacolchicine) is ≤90 kJ mol−1 (ref. 5) rendering atrop-diastereomers short-lived. Unfortunately Li and coworkers do not report information on any thermal interconversion of 26 and 4. Atrop-diastereomers can be isolated and studied, though, in the case of iso-colchicine-derivatives.4
In the search for features which are characteristic of individual atropisomers we noted the 1H NMR signal position of H-7 in the isocolchicines:4,6 In the (aR,7S)-isomer it appears at 4.6 ppm, whereas in the (aS,7S)-isomers it resonates at ≥5.0 ppm. This difference is caused by the position of H-7 over the aromatic ring in the (aR,7S)-isomer, whereas after rotation at the axis and concomitant inversion of the cycloheptane ring H-7 enjoys a position remote from the aromatic ring in the (aS,7S)-isomers. This should apply as well to colchicine-derivatives, where the same conformation-based determining factors prevail. Li and coworkers report that the signal of H-7 resonates in both compound 26 and 4 at 4.6 ppm. Hence, both compounds belong to the same (aR,7S)-family. This finding then renders the thesis that 26 and 4 are atrop-diastereomers untenable.
By the same token, the claim2 that colchiceine would exist in solution as the (aS,7S)-atropisomer is contrasted by the chemical shift of H-7 in colchiceine which resonates at δ = 4.7 ppm.
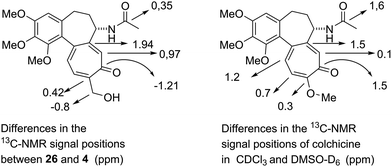
When the two isomeric metacolchicines are not atrop-diastereomers, what else are they? Any consideration along these lines has to start from the differences in the NMR spectra, which are summarized in Table 1:
| Atom | 26 | 4 | Δδ (ppm) |
|---|---|---|---|
| 13 C NMR | |||
| C-7a | 153.68 | 151.74 | 1.94 |
| C-8 | 135.36 | 134.39 | 0.97 |
| C-9 | 185.68 | 186.89 | −1.21 |
| C-10 | 150.95 | 150.53 | 0.42 |
| C-11 | 134.22 | 134.39 | −0.17 |
| CH3CO | 170.00 | 169.65 | 0.35 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| 1 H NMR | |||
| H-6α | 1.92 | 1.8 | 0.12 |
| H-8 | 7.35 | 6.88 | 0.47 |
The pattern of the 13C NMR differences turned out to be not compatible with the assumption, that compound 4 might be an iso-colchicine type compound, that could arise by the synthetic route employed to generate 4 (Nor was this pattern in line with the differences displayed by atrop-diasteromeric iso-colchicide derivatives7).
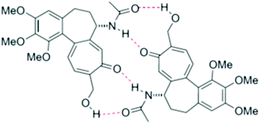
In view of the fact that the spectral differences between 26 and 4 are rather small, we found ourselves eventually confronted with the question: are they real? Could it be that the small differences in the NMR spectra are simply caused by solvent or concentration effects? This appeared the more probable, given the solvent and concentration effects reported for the 1H- and 13C NMR spectra of colchicine.8 These effects are due to a monomer/hydrogen-bonded dimer equilibrium. This dimer formation should be even more facile in the case of metacolchicine, due to two additional hydrogen bonds:
Looking at the 13C NMR spectra, we compared the differences recorded for 26vs.4 with the differences reported for colchicine in CDCl3 (high dimer content) and DMSO-D6 (low dimer content).
It is remarkable that essentially the same atoms on the eastern side of the molecule are involved in these differences. Moreover, the (small) magnitude of the effects is very similar. Encouraged by these coincidences we examined the 1H NMR spectra. In the case of 26 and 4 we noted that the signals of H-8 and H-6α are broadened compared to the other signals. This points to the involvement of H-8 and H-6α in some dynamic process. In the colchicine case8 it is exactly the signals of H-8 and H-6α that show a conspicuous concentration dependence of their chemical shifts. These signals are the only ones in the 1H NMR spectra of 26 and 4 that display recognizable differences in chemical shift, cf. also,3 the direction of which points to the solution of 26 being the more highly concentrated one (the latter fact is substantiated by looking at the relative size of the solvent peak in the spectra).
We therefore conclude that a concentration-dependent monomer/dimer equilibrium of metacolchicine could explain the differences recorded for the samples 4 and 26. This explanation would obviate the postulate that 4 and 26 represent stable atrop-diastereomers, a postulate which appears not to be tenable (see above). We should nevertheless stress, that the hypothesis of the monomer/dimer equilibrium of metacolchicine, while plausible, still lacks experimental proof which we cannot provide without authentic samples.
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. Kitajima, A. Tanaka, N. Kogure and H. Takayama, Heterocycles, 2011, 83, 1903–1907 CrossRef CAS.
- B. Chen, X. Liu, Y.-J. Hu, D.-M. Zhang, L. Deng, J. Lu, L. Min, W.-C. Ye and C.-C. Li, Chem. Sci., 2017, 8, 4961–4966 RSC.
- According to the spectra deposited in the ESI2 this holds for the 13C NMR data, whereas in the 1H NMR spectrum the signal of H-8 is off by 0.42 ppm.
- M. Cavazza, M. Zandomeneghi and F. Pietra, Tetrahedron Lett., 2000, 41, 9129–9133 CrossRef CAS.
- U. Berg, H. Bladh and K. Mpampos, Org. Biomol. Chem., 2004, 2, 2125–2130 RSC.
- W. Gaffield, R. E. Lundin and R. M. Horowitz, J. Chem. Soc., Chem. Commun., 1984, 610–612 RSC.
- M. Cavazza, M. Zandomeneghi and F. Pietra, J. Chem. Soc., Perkin Trans. 1, 2002, 560–564 RSC.
- G. K. Pierens, T. K. Venkatachalan and D. C. Reutens, Sci. Rep., 2017, 7, 5605 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2019 |