Open Access Article
Open Access ArticleFrom nano-balls to nano-bowls†‡
Helena
Brake§
a,
Eugenia
Peresypkina§
 ab,
Claudia
Heindl
a,
Alexander V.
Virovets
ab,
Werner
Kremer
c and
Manfred
Scheer
ab,
Claudia
Heindl
a,
Alexander V.
Virovets
ab,
Werner
Kremer
c and
Manfred
Scheer
 *a
*a
aInstitut für Anorganische Chemie, Universität Regensburg, 93040 Regensburg, Germany. E-mail: Manfred.Scheer@ur.de
bNovosibirsk State University, Pirogova str. 2, 630090 Novosibirsk, Russia
cInstitut für Biophysik und physikalische Biochemie, Universität Regensburg, 93040 Regensburg, Germany
First published on 23rd January 2019
Abstract
Pentaphosphaferrocene [Cp*Fe(η5-P5)] in combination with Cu(I) halides is capable of a template-directed synthesis of fullerene-like spheres. Herein, we present the use of a triple decker complex as template that leads to the formation of unprecedented ‘nano-bowls’. These spherical domes resemble the truncated fullerenes Ih-C80 and represent a novel spherical arrangement in the chemistry of spherical molecules.
Introduction
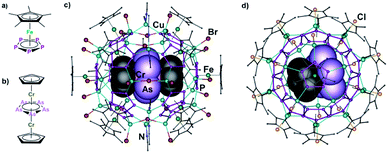
Supramolecular chemistry is one of the most fascinating topics in current research, as it is inspired by highly complex biochemical systems where the efficiency and selectivity of chemical processes are triggered by weak interactions between small subunits. During the last decades, it was successfully extended to non-biological systems. Here, the coordinative bond turned out to be an excellent tool, since it combines the advantages of both covalent bonds and weak interactions: it is relatively strong, but often weak enough to enable dynamic behaviour in solution. Hence, the self-assembly of metal salts and organic linkers has produced a wide variety of metal–organic frameworks (MOFs) on the one hand1 and discrete nano-sized supramolecules on the other hand.2 The latter often provide defined inner cavities and can be used e.g. as molecular containers.Recently, we have introduced pentaphosphaferrocene [CpRFe(η5-P5)] (CpR = Cp* = η5-C5Me5; CpBn = η5-C5(CH2Ph)5; CpBIG = η5-C5(4-nBuC6H4)5) as an outstanding five-fold symmetric organometallic building block and an auspicious alternative to the often used di- or tridentate organic linkers (Fig. 1a).3 Astonishingly, under certain conditions the cyclo-P5 ligand in combination with Cu(I) halides leads to the formation of spheres with fullerene-like topologies or beyond them.4,5 The synthesis of such nanospheres is often template-directed, thus, various molecules such as e.g. C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4f and ferrocene4d are encapsulated within these nano-balls. Among all templates, the triple decker complex [(CpCr)2(μ,η5:5-As5)] (1) is an exceptional case (Fig. 1b). Together with CuBr, it is incorporated into the 90-vertex host [{Cp*Fe(η5-P5)}12(CuBr)25(CH3CN)10] (host A), while, in the case of CuCl, a reproducible cleavage of the template takes place, and the in the free-state unstable molecule [CpCr(η5-As5)] is encapsulated into the slightly smaller 80-vertex sphere [{Cp*Fe(η5-P5)}12(CuCl)20] (host B) (Fig. 1c and d).4d Both reactions were carried out under similar conditions in a mixture of toluene/CH3CN only differing by the nature of the Cu(I) halide applied. Since not only the used Cu(I) halide affects the reaction pathway, but self-assembly processes also strongly depend on the reaction conditions used,6 the question arose in what way the applied solvent mixtures influence the reaction outcome. Addressing this issue is of general importance since it may open the way for going different pathways within one and the same reaction in supramolecular chemistry.
4f and ferrocene4d are encapsulated within these nano-balls. Among all templates, the triple decker complex [(CpCr)2(μ,η5:5-As5)] (1) is an exceptional case (Fig. 1b). Together with CuBr, it is incorporated into the 90-vertex host [{Cp*Fe(η5-P5)}12(CuBr)25(CH3CN)10] (host A), while, in the case of CuCl, a reproducible cleavage of the template takes place, and the in the free-state unstable molecule [CpCr(η5-As5)] is encapsulated into the slightly smaller 80-vertex sphere [{Cp*Fe(η5-P5)}12(CuCl)20] (host B) (Fig. 1c and d).4d Both reactions were carried out under similar conditions in a mixture of toluene/CH3CN only differing by the nature of the Cu(I) halide applied. Since not only the used Cu(I) halide affects the reaction pathway, but self-assembly processes also strongly depend on the reaction conditions used,6 the question arose in what way the applied solvent mixtures influence the reaction outcome. Addressing this issue is of general importance since it may open the way for going different pathways within one and the same reaction in supramolecular chemistry.
Herein, the solvent-dependent self-assembly of the systems containing 1, [Cp*Fe(η5-P5)] and CuX (X = Cl, Br) is demonstrated, which mainly depends on the solvent used and only to some extent on the used Cu(I) salt (formation of a 80 or 90 vertex ball). Remarkably, the change of the reaction media from toluene/CH3CN to CH2Cl2/CH3CN tunes the self-assembly to the unprecedented nano-bowls 2 with an open fullerene topology. These truncated spheres are able to incorporate the intact triple decker complexes 1.
Results and discussion
To apply the triple decker complex 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 as a template in the systems [Cp*Fe(η5-P5)] and CuX (X = Cl, Br), a solution of [Cp*Fe(η5-P5)] and 1 in CH2Cl2 is layered with a solution of CuX in CH3CN. By using CuCl and CuBr, the products 1@2a and 1@2b crystallise as dark brown rods in the monoclinic non-centrosymmetric space group Cc. The X-ray structure analysis reveals that these crystals represent the novel host–guest complexes [1]@[{Cp*Fe(η5-P5)}11{CuCl}13.45] (1@2a) and [1]@[{Cp*Fe(η5-P5)}11{CuBr}14.55] (1@2b) with an open structure resembling the truncated spheres B (Fig. 2). The 80-vertex scaffold of the spheres B contains 12 five-membered P5 ligands in a 1,2,3,4,5-coordination mode and 30 six-membered {Cu2P4} rings, whereas the halide atoms are all terminal, resembling the Ih-C80 fullerene structure (Fig. S8‡). In most cases,5c some positions of the CuBr fragments are partly vacant leading to the CuX-reduced scaffolds [{Cp*Fe(η5-P5)}12{CuBr}20−n] as was found by measuring different crystals from different batches of reaction mixtures.4b,5c,5d,8
7 as a template in the systems [Cp*Fe(η5-P5)] and CuX (X = Cl, Br), a solution of [Cp*Fe(η5-P5)] and 1 in CH2Cl2 is layered with a solution of CuX in CH3CN. By using CuCl and CuBr, the products 1@2a and 1@2b crystallise as dark brown rods in the monoclinic non-centrosymmetric space group Cc. The X-ray structure analysis reveals that these crystals represent the novel host–guest complexes [1]@[{Cp*Fe(η5-P5)}11{CuCl}13.45] (1@2a) and [1]@[{Cp*Fe(η5-P5)}11{CuBr}14.55] (1@2b) with an open structure resembling the truncated spheres B (Fig. 2). The 80-vertex scaffold of the spheres B contains 12 five-membered P5 ligands in a 1,2,3,4,5-coordination mode and 30 six-membered {Cu2P4} rings, whereas the halide atoms are all terminal, resembling the Ih-C80 fullerene structure (Fig. S8‡). In most cases,5c some positions of the CuBr fragments are partly vacant leading to the CuX-reduced scaffolds [{Cp*Fe(η5-P5)}12{CuBr}20−n] as was found by measuring different crystals from different batches of reaction mixtures.4b,5c,5d,8
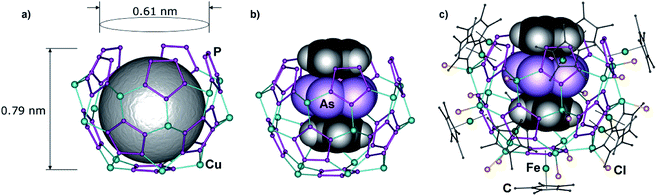
Surprisingly, in 2a and 2b, for the first time, a pentaphosphaferrocene vacancy is observed along with n minor vacancies in CuX positions. As a consequence, a {Cp*Fe(η5-P5)(CuX)5} moiety is formally cut-off when compared to the spheres B, leaving the bowl-like truncated spheres [1]@[{Cp*Fe(η5-P5)}11{CuX}15−n] (1@2a: X = Cl, n = 0.45; 1@2b: X = Br, n = 1.55) (Fig. 2). In these unprecedented nano-bowls 2, the coordination mode of the P5 rings on the upper ‘bottleneck’ part is reduced to a 1,2,3-fashion and the idealized scaffold of the supramolecule consists of 70 vertices (55P + 15Cu) arranged into 11 five-membered P5 ligands and 25 six-membered {Cu2P4} rings (Fig. 2a). The diameter of the cavity in the truncated sphere therefore amounts to a maximum width of 0.78 nm in the middle of the bowl and 0.61 nm at the bottleneck.9,10 The template, with a width of 0.78 nm at the As5 deck and 0.62 nm at the Cp deck, respectively, fits perfectly in the host cavity.10,11 The cavity is open at the top so that the template with its length of 0.99 nm is allowed to protrude from the 0.79 nm deep host but can be incorporated despite this protrusion (Fig. 2b and c).
The only comparable open-shelled assembly based on pentaphosphaferrocene observed so far is the nano-capsule [Cp*Fe(η5-P5)]2@[{Cp*Fe(η5-P5)}9(CuCl)10]2 (host C, Fig. S8‡), consisting of two open host shells each incorporating a [Cp*Fe(η5-P5)] molecule.12 However, these shells are weakly bound together to give an isolated closed capsule via π-stacking interactions between Cp* ligands of the guest molecules, as well as by a number of weak non-valent P⋯P interactions between the P5 ligands of the open shells. Therefore, this structure is not related to the ones of 1@2a,b.
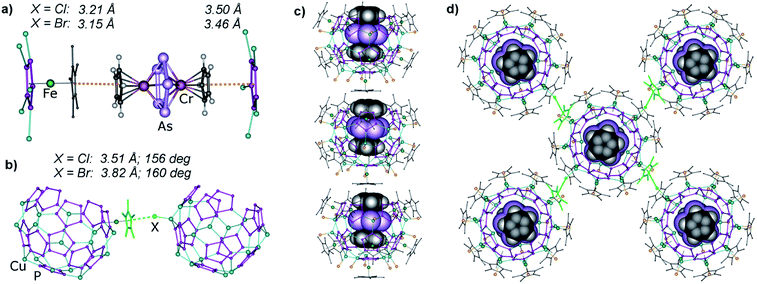
In the previously reported complex 1@A, the triple decker complex 1 forms a similar eclipsed stacking of its Cp rings with the P5 ring of the 90-vertex host A (3.56 Å).4d However, the cyclo-As5 middle deck of 1 is disordered over three positions due to weak interactions with alternating {Cu(CH3CN)2} and Br bridges of the middle part of host A (Fig. 1c). The As⋯Br distances of 4.03–4.58 Å exceed the sum of the van-der-Waals radii (3.68 Å) by far.10 In contrast, the guest molecule 1 is ordered when encapsulated in the novel nano-bowls 2. As in 1@A, the Cp ligands show an eclipsed orientation towards the cyclo-P5 rings of the host molecule, indicating π–π host–guest interactions with interplanar Cp⋯P5 distances of 3.50 Å in 1@2a and 3.46 Å in 1@2b (Fig. 3a). In the As5 middle deck, each As–As edge is arranged parallel to the corresponding cyclo-P5 ligand of the host (line-to-plane angles deviate by 0.2°–2.4° for 1@2a and by 0.2°–1.5° for 1@2b). The shortest intermolecular As⋯P contacts amounting to 3.69–3.94 Å (1@2a) and 3.78–3.89 Å (1@2b) are in the range of normal van-der-Waals contacts (3.65 Å). Therefore, the guest molecule prefers an orientation supported by van-der-Waals interactions, which is the most distant from the inner surface of the host molecule.
Interestingly, the host–guest interactions are not restricted to the encapsulation of 1 into the cavity of 2. Each guest molecule protruding from the open bowl further interacts with the Cp* ligand on the opposite side of the next host–guest assembly 1@2, resulting in unprecedented head-to-tail infinite columns (Fig. 3c). The short intra-column π–π contacts amount to 3.21 and 3.15 Å in 1@2a and 1@2b, respectively. In contrast, the aforementioned assembly C forms a nano-capsule and thus isolates the two guest molecules from extended intermolecular interactions. This behaviour might be exemplary for the future use of such systems to embed special hosts, which are much bigger, to be encapsulated in a complete sphere. Interestingly, another type of intermolecular interactions controls the packing of these columns in the solid state, namely the X⋯Cp*-specific interactions of σ–π type, also observed in some packings of other pentaphosphaferrocene-based supramolecules.13 Every bowl-like supramolecule participates in four supramolecular σ–π synthons with the four neighbouring supramolecules of other chains (Fig. 3b and d). The geometry of the σ–π synthons is in agreement with the previously reported examples and amounts to 3.48–3.55 Å (1@2a) and 3.54–4.13 Å (1@2b) for X⋯Cp* contacts and to 155.7°–157.0° (1@2a) and 158.8°–160.5° (1@2b) for Cu–X⋯Cp* angles. The earlier-noticed tendency of heavier halogens to form more obtuse angles is also valid here.
This bowl-like scaffold is observed for the first time and demonstrates that the formation of pentaphosphaferrocene-based host molecules is indeed template-directed.
Compared to [CpCr(η5-As5)]@B, the nano-bowls 1@2 are less soluble in CH2Cl2. The Br compound 1@2b is slightly more soluble in CHCl3 than in CH2Cl2, still the detection of the guest complex 1 by solution NMR spectroscopy was hampered. Therefore, both compounds 1@2 were investigated in the solid state by magic angle spinning (MAS) NMR spectroscopy. In the 1H MAS NMR spectra, a signal at 21.3 ppm is assigned to the triple decker complex 1 encapsulated in the bowl-like hosts 2. Compared to free 1 (23.8 ppm),4d the signal is shifted to higher field upon encapsulation. This effect has previously been observed for the chemical shifts of various other templates in pentaphosphaferrocene-based host systems,4b,d–g especially for the encapsulation of 1 into the 90-vertex sphere A, resulting in a similar chemical shift of 20.5 ppm.4d Moreover, two signals at 1.4 ppm and 6.7 ppm (6.8 ppm for 1@2b) can be assigned to the Cp* protons of the host, partially low-field shifted due to their proximity to the paramagnetic guest complex. Additionally, signals of low intensities can be observed in the 1H MAS NMR spectra, with chemical shifts of about 16 ppm and −13 ppm for both compounds 1@2. For 1@2b, the 31P{1H} MAS NMR spectrum shows two broad signals at 126 ppm and 70 ppm, which are due to different coordination spheres of the [Cp*Fe(η5-P5)] complex within the host 2b. For 1@2a, the 31P{1H} chemical shifts are similar and amount to 125 ppm and 74 ppm.
The products 1@2 are well soluble in pyridine, resulting in a fragmentation of the hosts. Hence, in the 1H NMR spectra in pyridine-d5, a singlet at 23.9 ppm (24.0 ppm for 1@2b) is detected corresponding to the free complex 1, next to a singlet at 1.33 ppm assigned to free [Cp*Fe(η5-P5)]. The presence of the latter is confirmed by the detection of a singlet at 150.5 ppm in the 31P{1H} NMR spectra.
Thus, the previously reported cleavage of the triple decker complex 1 in the [Cp*Fe(η5-P5)]/CuCl system is not observed when performing the reaction in CH2Cl2/CH3CN in lieu of toluene/CH3CN, but instead the novel nano-bowls 1@2 were obtained.
These results motivated us to revisit the reaction of [Cp*Fe(η5-P5)] with CuCl and 1 in toluene/CH3CN. Crystals of [CpCr(μ,η5-As5)]@[{Cp*Fe(η5-P5)}12(CuCl)20] ([CpCr(μ,η5-As5)]@B) were obtained by a slightly altered synthetic method: before being layered with a CuCl solution in CH3CN, the toluene solution of [Cp*Fe(η5-P5)] and 1 was sonicated and filtered. Both steps proved to be crucial due to the low solubility of crystalline 1 in toluene. The crystals of the product were isolated by washing with toluene/CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), since they can be redissolved in CH2Cl2/CH3CN (2
1), since they can be redissolved in CH2Cl2/CH3CN (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, previously reported method)4d and were investigated by EPR spectroscopy. The spectrum clearly showed a half-field line indicating the presence of a triplet molecule that agrees well with the calculated triplet ground state for the encapsulated 16 VE [CpCr(η5-As5)] molecule.4d In contrast, these signals were observed neither in the EPR spectrum of the intact 27 VE triple decker complex 1 nor in the EPR spectrum of the crystals of 1@2a obtained from the CH2Cl2/CH3CN reaction, which reconfirms the cleavage of the triple decker complex 1 when toluene/CH3CN is used as reaction medium. Additionally, according to the 1H NMR spectrum, the triple decker complex 1 itself stays intact when its toluene solution is sonicated and filtered, suggesting that the cleavage of 1 only occurs during the reaction with [Cp*Fe(η5-P5)] and CuCl and is not induced by the sonication.
1, previously reported method)4d and were investigated by EPR spectroscopy. The spectrum clearly showed a half-field line indicating the presence of a triplet molecule that agrees well with the calculated triplet ground state for the encapsulated 16 VE [CpCr(η5-As5)] molecule.4d In contrast, these signals were observed neither in the EPR spectrum of the intact 27 VE triple decker complex 1 nor in the EPR spectrum of the crystals of 1@2a obtained from the CH2Cl2/CH3CN reaction, which reconfirms the cleavage of the triple decker complex 1 when toluene/CH3CN is used as reaction medium. Additionally, according to the 1H NMR spectrum, the triple decker complex 1 itself stays intact when its toluene solution is sonicated and filtered, suggesting that the cleavage of 1 only occurs during the reaction with [Cp*Fe(η5-P5)] and CuCl and is not induced by the sonication.
Moreover, NMR spectroscopy of the crystalline product [CpCr(μ,η5-As5)]@B was carried out in solution, since it can sparingly be dissolved in CD2Cl2. Here, two broad signals at 120 ppm and 69 ppm in the 31P{1H} NMR spectrum as well as a signal at 19.8 ppm in the 1H NMR spectrum were detected, which can be assigned to [CpCr(η5-As5)]@B.4d This demonstrates that [CpCr(η5-As5)]@B is quite stable in a CH2Cl2 solution. On the other hand, a sharp singlet at 151.5 ppm in the 31P{1H} NMR spectrum can tentatively be assigned to free [Cp*Fe(η5-P5)]. In the 1H NMR spectrum, also a signal at 21.3 ppm is observed, which may be assigned to the intact triple decker complex 1 encapsulated in the CuCl analogue of the host A (for the CuBr analogue 1@A: 20.5 ppm, free 1: 23.8 ppm)4d or in a nano-bowl 2a (1@2a: 21.3 ppm, vide supra). Since the latter compound has a similar size and shape as [CpCr(η5-As5)]@B, a cocrystallisation of both compounds cannot be completely excluded. In both cases, in the 31P{1H} NMR spectrum, the expected signals for the hosts (CuCl analogue of host A: 68 ppm,4g host 2a: 125 ppm and 74 ppm, vide supra) would overlap with the signals of host B, so no further conclusion can be drawn. In pyridine, crystals of [CpCr(μ,η5-As5)]@B are well soluble again resulting in a fragmentation of the hosts and subsequent release of the guest molecules. Hence, in the 31P{1H} NMR spectrum, solely a singlet at 147.2 ppm is detected, assigned to free [Cp*Fe(η5-P5)]. In the 1H NMR spectrum, in pyridine-d5, a signal at 23.9 ppm is assigned to the free triple decker complex 1, whereas a very broad signal at approximately 6 ppm is also detected and might be assigned to subsequent products formed by the conversion of the released unstable complex [CpCr(η5-As5)]. These results are also in line with a cocrystallisation of [CpCr(μ,η5-As5)]@B and 1@2a in the toluene/CH3CN reaction.
Conclusions
In summary, different pathways of self-assembly in the system [(CpCr)2(μ,η5:5-As5)]/[Cp*Fe(η5-P5)]/CuX (X = Cl, Br) were addressed and the crucial role of the used solvents was demonstrated. The previously reported 1/[Cp*Fe(η5-P5)]/CuCl system in toluene/CH3CN was revisited and the cleavage of the triple decker 1 and the encapsulation of the resulting unstable 16 VE complex [CpCr(η5-As5)] into a 80-vertex sphere (B) were proven by EPR and solution NMR spectroscopy. By changing the solvent mixture toluene/CH3CN to CH2Cl2/CH3CN, unprecedented open shell bowl-like host molecules incorporating the triple decker complex 1 were obtained irrespective of the nature of the halogen: [1]@[{Cp*Fe(η5-P5)}11{CuX}15−n] (1@2a: n = 0.45; 1@2b: n = 1.55). They resemble truncated fullerene-like 80-vertex spheres B. Due to the opening in the hosts, the guest molecules can be trapped despite their protruding from the opening and still participate for the first time in π interactions not only with their own hosts, but also with the neighbouring host molecules. Thus, the supramolecular π–π columns formed in the solid state are further connected into a 3D supramolecular assembly by a system of σ–π synthons. These results do not only nicely demonstrate that supramolecular self-assembly strongly depends on the conditions applied, but also prove that the formation of spherical assemblies in the [Cp*Fe(η5-P5)]/CuX system is template-directed. Investigations regarding the transferability of these results to other triple decker complexes and guest molecules as templates are currently under way.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The European Research Council (ERC) is acknowledged for the support in the SELFPHOS AdG-339072 project. C. H. and H. B. are grateful for their PhD fellowships of the Fonds der Chemischen Industrie and of the Studienstiftung des Deutschen Volkes, respectively. Parts of this research (project I-20170135) were carried out at PETRA III at DESY, a member of the Helmholtz Association (HGF). We thank Dr O. Lorbeer for his assistance regarding the use of the beamline P11.Notes and references
- (a) M. Bosch, S. Yuan, W. Rutledge and H.-C. Zhou, Acc. Chem. Res., 2017, 50, 857–865 CrossRef CAS PubMed; (b) B. Manna, A. V. Desai and S. K. Ghosh, Dalton Trans., 2016, 45, 4060–4072 RSC; (c) L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105 CrossRef CAS PubMed; (d) O. K. Farha and J. T. Hupp, Acc. Chem. Res., 2010, 43, 1166 CrossRef CAS PubMed; (e) C. Janiak and J. K. Vieth, New J. Chem., 2010, 34, 2366 RSC; (f) J. L. C. Rowsell and O. M. Yaghi, Microporous Mesoporous Mater., 2004, 73, 3 CrossRef CAS.
- (a) J. Jiao, C. Tan, Z. Li, Y. Liu, X. Han and Y. Cui, J. Am. Chem. Soc., 2018, 140, 2251–2259 CrossRef CAS PubMed; (b) S. Bestgen, O. Fuhr, B. Breitung, V. S. K. Chakravadhanula, G. Guthausen, F. Hennrich, W. Yu, M. M. Kappes, P. W. Roesky and D. Fenske, Chem. Sci., 2017, 8, 2235–2240 RSC; (c) J. Uchida, M. Yoshio, S. Sato, H. Yokoyama, M. Fujita and T. Kato, Angew. Chem., Int. Ed., 2017, 56, 14085–14089 CrossRef CAS PubMed; (d) D. A. Roberts, B. S. Pilgrim and J. R. Nitschke, Chem. Soc. Rev., 2018, 47, 626–644 RSC; (e) N. A. Sakthivel, S. Theivendran, V. Ganeshraj, A. G. Oliver and A. Dass, J. Am. Chem. Soc., 2017, 139, 15450–15459 CrossRef CAS PubMed; (f) T. Mitra, K. E. Jelfs, M. Schmidtmann, A. Ahmed, S. Y. Chong, D. J. Adams and A. I. Cooper, Nat. Chem., 2013, 5, 276 CrossRef CAS PubMed; (g) R. W. Saalfrank and A. Scheurer, Top. Curr. Chem., 2012, 319, 125 CrossRef CAS PubMed; (h) F. J. Rizzuto and J. R. Nitschke, Nat. Chem., 2017, 9, 903–908 CrossRef CAS PubMed; (i) K. Tiefenbacher, D. Ajami and J. Rebek, Angew. Chem., Int. Ed., 2011, 50, 11805 CrossRef; (j) L. Qin, G.-J. Zhou, Y.-Z. Yu, H. Nojiri, C. Schröder, R. E. P. Winpenny and Y.-Z. Zheng, J. Am. Chem. Soc., 2017, 139, 16405–16411 CrossRef CAS PubMed.
- (a) O. J. Scherer and T. Brück, Angew. Chem., Int. Ed. Engl., 1987, 26, 59–61 Search PubMed; (b) F. Dielmann, R. Merkle, S. Heinl and M. Scheer, Z. Naturforsch., B: J. Chem. Sci., 2009, 64, 3–10 CAS; (c) S. Heinl, G. Balázs and M. Scheer, Phosphorus, Sulfur Silicon Relat. Elem., 2014, 189, 924–932 CrossRef CAS.
- (a) S. Heinl, E. V. Peresypkina, A. V. Virovets and M. Scheer, Angew. Chem., Int. Ed., 2015, 54, 13431 CrossRef CAS PubMed; (b) F. Dielmann, M. Fleischmann, C. Heindl, E. V. Peresypkina, A. V. Virovets, R. M. Gschwind and M. Scheer, Chem.–Eur. J., 2015, 21, 6208 CrossRef CAS PubMed; (c) F. Dielmann, C. Heindl, F. Hastreiter, E. V. Peresypkina, A. V. Virovets, R. M. Gschwind and M. Scheer, Angew. Chem., Int. Ed., 2014, 53, 13605 CrossRef CAS PubMed; (d) A. Schindler, C. Heindl, G. Balázs, C. Groeger, A. V. Virovets, E. V. Peresypkina and M. Scheer, Chem.–Eur. J., 2012, 18, 829 CrossRef CAS PubMed; (e) M. Scheer, A. Schindler, C. Groeger, A. V. Virovets and E. V. Peresypkina, Angew. Chem., Int. Ed., 2009, 48, 5046 CrossRef CAS PubMed; (f) M. Scheer, A. Schindler, R. Merkle, B. P. Johnson, M. Linseis, R. Winter, C. E. Anson and A. V. Virovets, J. Am. Chem. Soc., 2007, 129, 13386 CrossRef CAS PubMed; (g) J. Bai, A. V. Virovets and M. Scheer, Science, 2003, 300, 781 CrossRef CAS PubMed.
- (a) E. V. Peresypkina, C. Heindl, A. Virovets, E. Mädl, H. Brake and M. Scheer, Chem.–Eur. J., 2018, 24, 2503 CrossRef CAS PubMed; (b) C. Heindl, E. Peresypkina, A. V. Virovets, I. S. Bushmarinov, M. G. Medvedev, B. Krämer, B. Dittrich and M. Scheer, Angew. Chem., Int. Ed., 2017, 56, 13237 CrossRef CAS PubMed; (c) E. Peresypkina, C. Heindl, A. Virovets and M. Scheer, Inorganic Superspheres, in Clusters – Contemporary Insight in Structure and Bonding, ed. S. Dehnen, 2016, vol. 174, pp. 321–373 Search PubMed; (d) F. Dielmann, E. V. Peresypkina, B. Krämer, F. Hastreiter, B. P. Johnson, M. Zabel, C. Heindl and M. Scheer, Angew. Chem., Int. Ed., 2016, 55, 14833 CrossRef CAS PubMed; (e) C. Heindl, E. V. Peresypkina, A. V. Virovets, W. Kremer and M. Scheer, J. Am. Chem. Soc., 2015, 137, 10938 CrossRef CAS PubMed.
- (a) A. J. Savyasachi, O. Kotova, S. Shanmugaraju, S. J. Bradberry, G. M. Ó'Máille and T. Gunnlaugsson, Chem, 2017, 3, 764–811 CrossRef CAS; (b) K. Suzuki, M. Kawano and M. Fujita, Angew. Chem., Int. Ed., 2007, 46, 2819–2822 CrossRef CAS PubMed; (c) M. Scheer, J. Bai, B. P. Johnson, R. Merkle, A. V. Virovets and C. E. Anson, Eur. J. Inorg. Chem., 2005, 20, 4023–4026 CrossRef.
- In the course of this work, we have also determined the crystal structure of 1. For the details see ESI.‡.
- E. V. Peresypkina, C. Heindl, A. Schindler, M. Bodensteiner, A. V. Virovets and M. Scheer, Z. Kristallogr., 2014, 229, 735 CAS.
- The width of the host is measured as the average distance between opposite P atoms minus twice the van-der-Waals radius of phosphorus (rvdW(P) = 1.80 Å [ref. 9a]); the bottleneck is measured as twice the distance between the geometric centre and the closest P atom of the open top minus 2 × rvdW(P). The height of the cavity is measured as the distance between the centroid of all 10 P atoms of the bottleneck and the centroid of the P5 ligand at the bottom minus the rvdW(P)..
- (a) M. Mantina, A. C. Chamberlin, R. Valero, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. A, 2009, 113, 5806 CrossRef CAS PubMed; (b) A. Bondi, J. Phys. Chem., 1964, 68, 441 CrossRef CAS; (c) S. Alvarez, Dalton Trans., 2013, 42, 8617 RSC.
- The dimensions of the template are measured as the distances between two opposite As atoms of the middle deck and the distance between the centroids of the Cp rings, respectively, plus twice the van-der-Waals radius of As (1.85 Å) and C (1.70 Å) [ref. 9a], respectively..
- S. Welsch, C. Groeger, M. Sierka and M. Scheer, Angew. Chem., Int. Ed., 2011, 50, 1435 CrossRef CAS PubMed.
- (a) E. V. Peresypkina, A. V. Virovets and M. Scheer, Cryst. Growth Des., 2016, 16, 2335 CrossRef CAS PubMed; (b) M. Scheer, A. Schindler, J. Bai, B. P. Johnson, R. Merkle, R. Winter, A. V. Virovets, E. V. Peresypkina, V. A. Blatov, M. Sierka and H. Eckert, Chem.–Eur. J., 2010, 16, 2092–2107 CrossRef CAS PubMed.
Footnotes |
| † This paper is dedicated to Prof. W. Bensch on the occasion of his 65th birthday. |
| ‡ Electronic supplementary information (ESI) available. CCDC 1875092–1875094. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8sc05471a |
| § Authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |