Open Access Article
Open Access ArticleBoron-based stepwise dioxygen activation with 1,4,2,5-diazadiborinine†
Baolin
Wang
and
Rei
Kinjo
 *
*
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Nanyang Link 21, Singapore 637371, Singapore. E-mail: rkinjo@ntu.edu.sg
First published on 11th December 2018
Abstract
Activation of dioxygen (O2) by 1,4,2,5-diazadiborinine 1 is reported. Two boron centers in 1 undergo a formal [4 + 2] cycloaddition with O2 at room temperature affording a bicyclo[2.2.2] molecule 2 featuring a B–O–O–B unit. Treatment of 2 with an additional equivalent of 1 leads to the cleavage of the O–O bond in 2 concomitant with the formation of two B–O bonds to yield 4 involving the extremely rare B4C2N2O2 ten-membered rings. A series of these reactions demonstrate the stepwise scission of the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O π-bond and the O–O σ-bond of O2.
O π-bond and the O–O σ-bond of O2.
Introduction
Oxygen activation is of paramount importance in biological systems as represented by catalytic redox processes using oxidase and oxygenase enzymes.1 Significantly, the Fe center in the enzymes exhibits versatility in bonding with the O atom derived from O2 activation.2 To mimic such biological processes artificially, considerable attention has been paid to the isolation of metal complexes that correspond to the key intermediates in the enzyme-O2 activation process. To date, various transition metal–oxygen species such as superoxide,3 peroxide4 and metal μ-oxo species5 have been synthesized through O2 activation, and structurally characterized.Over the past decade, it has been demonstrated that main group elements serve as if they are transition metals in the activation of small molecules.6–9 Dioxygen activation has also been described by using various p-block molecules on the basis of group 13,10,11 14,12–14 and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 elements. However, in stark contrast to the extensive and detailed studies of diverse products from O2 activation by transition metals,2–5 only a handful main group protocols have achieved a complete scission of the O
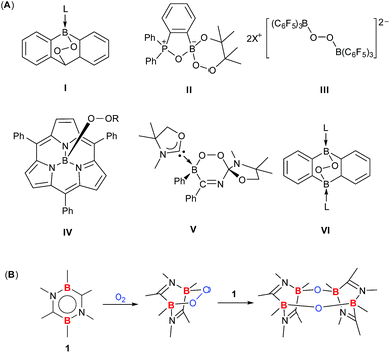
15 elements. However, in stark contrast to the extensive and detailed studies of diverse products from O2 activation by transition metals,2–5 only a handful main group protocols have achieved a complete scission of the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of O2 in distinct stepwise reactions concomitant with the isolation and full characterization of the initial and final products.10f,11i,12c,h,13a,15c Recently, a few boron compounds featuring the BOO unit were successfully isolated through the O2 activation reaction (I–VI, Fig. 1A),10 among which only III and VI possess the dibora-peroxide (B–O–O–B) moiety. To the best of our knowledge, the reactivity of dibora-peroxides III and VI has never been realized thus far.
O bond of O2 in distinct stepwise reactions concomitant with the isolation and full characterization of the initial and final products.10f,11i,12c,h,13a,15c Recently, a few boron compounds featuring the BOO unit were successfully isolated through the O2 activation reaction (I–VI, Fig. 1A),10 among which only III and VI possess the dibora-peroxide (B–O–O–B) moiety. To the best of our knowledge, the reactivity of dibora-peroxides III and VI has never been realized thus far.
 | ||
| Fig. 1 (A) Reported boron peroxides obtained through oxygen activation; (B) this work: O2 activation at the boron centers in two distinct steps. | ||
Recently, we have reported that 1,4,2,5-diazadiborinine 1 readily reacts with unsaturated bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C, and C
C, and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) and σ-bonds (C–O, B–H, Si–H, and P–H) in small molecules.16 We reasoned that the boron-centered reactivity of 1 would allow for oxygen activation only at the boron centers. Herein, we report that indeed both the O
N) and σ-bonds (C–O, B–H, Si–H, and P–H) in small molecules.16 We reasoned that the boron-centered reactivity of 1 would allow for oxygen activation only at the boron centers. Herein, we report that indeed both the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O π-bond and the O–O σ-bond of O2 can be cleaved by 1 in a stepwise manner (Fig. 1B).
O π-bond and the O–O σ-bond of O2 can be cleaved by 1 in a stepwise manner (Fig. 1B).
Results and discussion
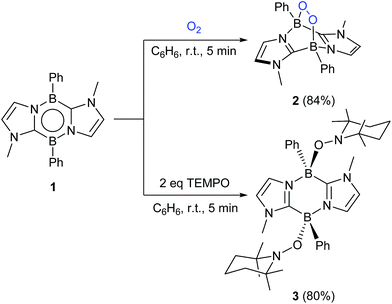
By a freeze–pump–thaw method, O2 was introduced into a Schlenk tube containing a benzene solution of 1. Within five minutes at room temperature, a white precipitate appeared concomitant with disappearance of the orange colour of 1. After work-up, 2 was obtained as a white powder in 84% yield (Scheme 1). 2 exhibits a poor solubility in benzene, acetonitrile and tetrahydrofuran but dissolves well in dichloromethane and chloroform. In the 11B NMR spectrum of 2, a sharp singlet appears at −0.4 ppm, which is shifted upfield with respect to that (18.3 ppm) of 1,16 indicating the formation of four-coordinate boron centers. The 1H NMR spectrum shows a singlet at 3.24 ppm for the methyl groups on the nitrogen atoms and two doublets at 6.60 ppm and 6.98 ppm for the CH of the imidazole rings, indicative of the center of symmetry of 2. The solid-state IR spectrum of 2 showed a characteristic peak at 964 cm−1 for the B–O stretching vibration (Fig. S20†) whereas the O–O stretching mode was detected at 953 cm−1 in the Raman spectrum (Fig. S21†), confirming the presence of B–O and O–O bonds in 2, which was further revealed by an X-ray diffraction analysis (Fig. 2).The solid-state molecular structure of 2 shows a bicyclo[2.2.2] geometry involving an endocyclic O–O unit bound to two boron atoms, indicating that 1 underwent a formal [4 + 2] cycloaddition reaction with O2. The O–O bond distance (1.507(4) Å) is slightly longer than those (1.4733(2)–1.487 (2) Å) reported for molecules featuring a B–O–O–B unit.10c,f,h The B–O bond distances (B1–O1 1.505(6) Å and B2–O2 1.495(6) Å) are similar to the related compound (1.5029(19) and 1.492(2) Å).10h The B–O–O-angles (B1–O1–O2 113.2(3)° and B2–O2–O1 112.9(3)°) are nearly identical to those (109.48(10)° and 112.41(10)°) in the endoperoxide.10h Compound 2 represents one of the rare examples of diboraperoxide derivatives.10c,f,h Note that inorganic peroxides (B–O–O) are proposed not only as the key intermediate in organic synthesis,17–19 but also as the active sites in oxidative dehydrogenation of propane catalyzed by boron nitrides.20 Moreover, recently, Linker et al. reported that an aromatic endoperoxide has proved to be a useful precursor for the generation of singlet oxygen.21
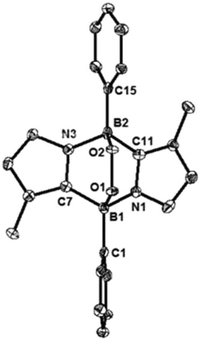
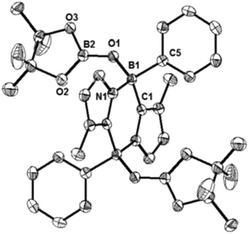
We also carried out the reaction of 1 with 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO). In benzene, 1 and two equivalents of TEMPO were mixed at room temperature, which led to a fast disappearance of the orange colour of 1, and concomitantly a white precipitate appeared. After work-up, 3 was obtained in 80% yield and fully characterized by NMR spectroscopy and X-ray diffractometry (Fig. 3). Each boron center in 3 forms a B–O bond with TEMPO, and the B–O bond distances (B1–O1: 1.500(4) Å and B2–O2: 1.480(4) Å) are similar to that of the B–O bond (1.500(4) Å) of C2H2(NCH2C6H4)2CB-TEMPO.22 Two TEMPO units are located in opposite sides with respect to the central B2C2N2 plane, probably due to the steric repulsion between the two bulky TEMPO units. This result proposes that the formation of 3 would proceed in a stepwise manner.22,23
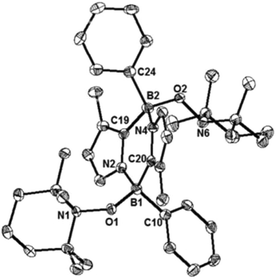
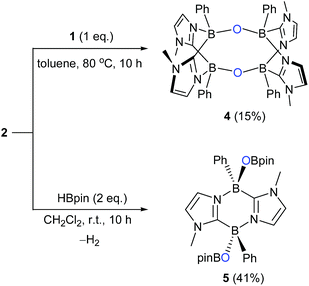
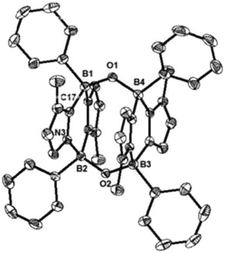
While the formation of the B–O–O–B endoperoxide 2 is reminiscent of the addition of O2 to NHC-stabilized boranthrene reported by Harman et al.,10h further examination of the complete scission of the O–O bond, in particular with the boron system, has never been achieved, which prompted us to investigate further reaction of 2 with 1. First, we observed no reaction between 2 and 1 (1 eq.) in toluene under ambient conditions. When the reaction mixture was heated at 80 °C, the orange colour of 1 disappeared gradually. After 10 h, formation of a major product 4 (Scheme 2) was detected by NMR spectroscopy, which was isolated after work-up as a light-yellow powder in 15% yield. The poor isolated yield of 4 is due to the formation of insoluble unidentified byproducts during the reaction (Fig. S8 and S9†). The 11B NMR spectrum of 4 exhibits only one singlet at −2.4 ppm, which is nearly identical to that (−0.4 ppm) of 2. The 1H NMR spectrum of 4 shows a singlet at 3.03 ppm for the Me groups on the N atoms and two doublets at 6.41 ppm and 6.15 ppm for the CH moieties of the imidazole rings. The solid-state molecular structure was unambiguously identified by an X-ray diffraction analysis (Fig. 4). Two diazadiborinine units are bridged via two B–O–B linkers, confirming that the O–O σ-bond in 2 was cleaved by 1, concomitant with the formation of two B–O bonds.
We found that compound 2 reacted with pinacolborane (HBpin) as well under ambient conditions, generating product 5 with the release of hydrogen gas. The solid-state structure of 5 revealed that along with the cleavage of the O–O bond in 2, two OBpin units were formed (Fig. 5). Interestingly, the two OBpin units are at opposite sides with respect to the central B2C2N2 ring.
Conclusions
We have shown that the two B centers of 1,4,2,5-diazadiborinine 1 readily capture O2 under ambient conditions to furnish a formal [4 + 2] cycloaddition product 2 featuring an O–O bond. The reaction of 1 with TEMPO afforded 3 bearing two B-TEMPO units. Further treatments of 2 with 1 and HBpin led to 4 and 5, respectively, via a cleavage of the O–O bond in 2. The former demonstrates complete O2 activation at the B center in two distinct steps. These results show the potential of the boron-based system for the development of a metal-free strategy to mimic metalloenzymes. The oxygen transfer reaction from 2 to other substrates is underway in our laboratory.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We are grateful to Nanyang Technological University (NTU) and the Singapore Ministry of Education (MOE2015-T2-2-032) for financial support. We thank Dr Y. Li (NTU) for assistance in X-ray analysis. We thank C. S. L. Koh and Prof. X. Y. Ling (NTU) for assistance in Raman spectroscopy analysis.Notes and references
- (a) M. Wikström, K. Krab and V. Sharma, Chem. Rev., 2018, 118, 2469 CrossRef PubMed; (b) E. Romero, J. R. G. Castellanos, G. Gadda, M. W. Fraaije and A. Mattevi, Chem. Rev., 2018, 118, 1742 CrossRef CAS PubMed.
- (a) A. J. Jasniewski and L. Que, Chem. Rev., 2018, 118, 2554 CrossRef CAS PubMed; (b) X. Huang and J. T. Groves, Chem. Rev., 2018, 118, 2491 CrossRef CAS PubMed; (c) E. G. Kovaleva and J. D. Lipscomb, Nat. Chem. Biol., 2008, 4, 186 CrossRef CAS PubMed; (d) M. Costas, M. P. Mehn, M. P. Jensen and L. Que, Chem. Rev., 2004, 104, 939 CrossRef CAS PubMed; (e) L. Que and R. Y. N. Ho, Chem. Rev., 1996, 96, 2607 CrossRef CAS PubMed.
- (a) K. Qin, C. D. Incarvito, A. L. Rheingold and K. H. Theopold, Angew. Chem., Int. Ed., 2002, 41, 2333 CrossRef CAS; (b) S. Yao, E. Bill, C. Milsmann, K. Wieghardt and M. Driess, Angew. Chem., Int. Ed., 2008, 47, 7110 CrossRef CAS PubMed; (c) J. Cho, J. Woo and W. Nam, J. Am. Chem. Soc., 2010, 132, 5958 CrossRef CAS PubMed; (d) F. Schax, S. Suhr, E. Bill, B. Braun, C. Herwig and C. Limberg, Angew. Chem., Int. Ed., 2015, 54, 1352 CrossRef CAS PubMed.
- (a) P.-C. Duan, D.-H. Manz, S. Dechert, S. Demeshko and F. Meyer, J. Am. Chem. Soc., 2018, 140, 4929 CrossRef CAS PubMed; (b) P. Holze, T. Corona, N. Frank, B. Braun-Cula, C. Herwig, A. Company and C. Limberg, Angew. Chem., Int. Ed., 2017, 56, 2307 CrossRef CAS PubMed; (c) T. Kishima, T. Matsumoto, H. Nakai, S. Hayami, T. Ohta and S. Ogo, Angew. Chem., Int. Ed., 2016, 55, 724 CrossRef CAS PubMed; (d) G. Y. Park, M. F. Qayyum, J. Woertink, K. O. Hodgson, B. Hedman, A. A. N. Sarjeant, E. I. Solomon and K. D. Karlin, J. Am. Chem. Soc., 2012, 134, 8513 CrossRef CAS PubMed; (e) J. Cho, R. Sarangi, J. Annaraj, S. Y. Kim, M. Kubo, T. Ogura, E. I. Solomon and W. Nam, Nat. Chem., 2009, 1, 568 CrossRef CAS PubMed; (f) S. Yao, Y. Xiong, M. Vogt, H. Grützmacher, C. Herwig, C. Limberg and M. Driess, Angew. Chem., Int. Ed., 2009, 48, 8107 CrossRef CAS PubMed.
- (a) X. Engelmann, S. Yao, E. R. Farquhar, T. Szilvási, U. Kuhlmann, P. Hildebrandt, M. Driess and K. Ray, Angew. Chem., Int. Ed., 2017, 56, 297 CrossRef CAS PubMed; (b) X. Dai, P. Kapoor and T. H. Warren, J. Am. Chem. Soc., 2004, 126, 4798 CrossRef CAS PubMed.
- (a) P. P. Power, Nature, 2010, 463, 171 CrossRef CAS PubMed; (b) T. Chu and G. I. Nikonov, Chem. Rev., 2018, 118, 3608 CrossRef CAS PubMed.
- (a) G. H. Spikes, J. C. Fettinger and P. P. Power, J. Am. Chem. Soc., 2005, 127, 12232 CrossRef CAS PubMed; (b) Y. Peng, B. D. Ellis, X. Wang, J. C. Fettinger and P. P. Power, Science, 2009, 325, 1668 CrossRef CAS PubMed; (c) Y. Peng, J.-D. Guo, B. D. Ellis, Z. Zhu, J. C. Fettinger, S. Nagase and P. P. Power, J. Am. Chem. Soc., 2009, 131, 16272 CrossRef CAS PubMed; (d) P. P. Power, Acc. Chem. Res., 2011, 44, 627 CrossRef CAS PubMed.
- (a) G. D. Frey, V. Lavallo, B. Donnadieu, W. W. Schoeller and G. Bertrand, Science, 2007, 316, 439 CrossRef CAS PubMed; (b) D. Martin, M. Soleilhavoup and G. Bertrand, Chem. Sci., 2011, 2, 389 RSC; (c) M. Soleilhavoup and G. Bertrand, Acc. Chem. Res., 2015, 48, 256 CrossRef CAS PubMed; (d) M. Melaimi, R. Jazzar, M. Soleilhavoup and G. Bertrand, Angew. Chem., Int. Ed., 2017, 56, 10046 CrossRef CAS PubMed.
- (a) G. C. Welch, R. R. S. Juan, J. D. Masuda and D. W. Stephan, Science, 2006, 314, 1124 CrossRef CAS PubMed; (b) D. W. Stephan, J. Am. Chem. Soc., 2015, 137, 10018 CrossRef CAS PubMed; (c) D. W. Stephan and G. Erker, Angew. Chem., Int. Ed., 2015, 54, 6400 CrossRef CAS PubMed; (d) A. J. P. Cardenas, Y. Hasegawa, G. Kehr, T. H. Warren and G. Erker, Coord. Chem. Rev., 2016, 306, 468 CrossRef CAS; (e) D. W. Stephan, Science, 2016, 354, 1248 CrossRef CAS PubMed.
- (a) T. K. Wood, W. E. Piers, B. A. Keay and M. Parvez, Angew. Chem., Int. Ed., 2009, 48, 4009 CrossRef CAS PubMed; (b) S. Porcel, G. Bouhadir, N. Saffon, L. Maron and D. Bourissou, Angew. Chem., Int. Ed., 2010, 49, 6186 CrossRef CAS PubMed; (c) J. T. Henthorn and T. Agapie, Angew. Chem., Int. Ed., 2014, 53, 12893 CrossRef CAS PubMed; (d) J. T. Henthorn, S. Lin and T. Agapie, J. Am. Chem. Soc., 2015, 137, 1458 CrossRef CAS PubMed; (e) T. Wang, G. Kehr, L. Liu, S. Grimme, C. G. Daniliuc and G. Erker, J. Am. Chem. Soc., 2016, 138, 4302 CrossRef CAS PubMed; (f) E. Tsurumaki, J. Sung, D. Kim and A. Osuka, Angew. Chem., Int. Ed., 2016, 55, 2596 CrossRef CAS PubMed; (g) L. Kong, W. Lu, Y. Li, R. Ganguly and R. Kinjo, Angew. Chem., Int. Ed., 2016, 55, 14718 CrossRef CAS PubMed; (h) J. W. Taylor, A. M. McSkimming, C. F. Guzman and W. H. Harman, J. Am. Chem. Soc., 2017, 139, 11032 CrossRef CAS PubMed; (i) X. Tao, C. G. Daniliuc, O. Janka, R. Pöttgen, R. Knitsch, M. R. Hansen, H. Eckert, M. Lübbesmeyer, A. Studer, G. Kehr and G. Erker, Angew. Chem., Int. Ed., 2017, 56, 16641 CrossRef CAS PubMed.
- (a) J. Lewinński, J. Zachara and E. Grabska, J. Am. Chem. Soc., 1996, 118, 6794 CrossRef; (b) J. Lewiński, J. Zachara, P. Goś, E. Grabska, T. Kopeć, I. Madura, W. Marciniak and I. Prowotorow, Chem.–Eur. J., 2000, 6, 3215 CrossRef; (c) N. Wiberg, T. Blank, K. Amelunxen, H. Nöth, H. Schnöckel, E. Baum, A. Purath and D. Fenske, Eur. J. Inorg. Chem., 2002, 341 CrossRef CAS; (d) H. Zhu, J. Chai, V. Jancik, H. W. Roesky, W. A. Merrill and P. P. Power, J. Am. Chem. Soc., 2005, 127, 10170 CrossRef CAS PubMed; (e) X. Li, C. Ni, H. Song and C. Cui, Chem. Commun., 2006, 1763 RSC; (f) X. Li, H. Song, L. Duan, C. Cui and H. W. Roesky, Inorg. Chem., 2006, 45, 1912 CrossRef CAS PubMed; (g) A. C. Stelzer, P. Hrobárik, T. Braun, M. Kaupp and B. Braun-Cula, Inorg. Chem., 2016, 55, 4915 CrossRef CAS PubMed; (h) B. Jana, C. Honaker and W. Uhl, J. Organomet. Chem., 2018, 856, 78 CrossRef CAS; (i) M. B. Power, W. M. Cleaver, A. W. Apblett, A. R. Barron and J. W. Ziller, Polyhedron, 1992, 11, 477 CrossRef CAS; (j) N. Wiberg, K. Amelunxen, H.-W. Lerner, H. Nöth, W. Ponikwar and H. Schwenk, J. Organomet. Chem., 1999, 574, 246 CrossRef CAS; (k) W. Uhl, S. Melle and M. Prött, Z. Anorg. Allg. Chem., 2005, 631, 1377 CrossRef CAS; (l) W. M. Cleaver and A. R. Barron, J. Am. Chem. Soc., 1989, 111, 8966 CrossRef CAS; (m) W. Uhl and B. Jana, Eur. J. Inorg. Chem., 2009, 3942 CrossRef CAS.
- (a) M. J. Fink, K. J. Haller, R. West and J. Michl, J. Am. Chem. Soc., 1984, 106, 822 CrossRef CAS; (b) M. J. Michalczyk, M. J. Fink, K. J. Haller, R. West and J. Michl, Organometallics, 1986, 5, 531 CrossRef CAS; (c) K. L. McKillop, G. R. Gillette, D. R. Powell and R. West, J. Am. Chem. Soc., 1992, 114, 5203 CrossRef CAS; (d) A. J. Millevolte, D. R. Powell, S. G. Johnson and R. West, Organometallics, 1992, 11, 1091 CrossRef CAS; (e) W. Li, N. J. Hill, A. C. Tomasik, G. Bikzhanova and R. West, Organometallics, 2006, 25, 3802 CrossRef CAS; (f) S. Yao, Y. Xiong, M. Brym and M. Driess, J. Am. Chem. Soc., 2007, 129, 7268 CrossRef CAS PubMed; (g) A. Yuasa, T. Sasamori, Y. Hosoi, Y. Furukawa and N. Tokitoh, Bull. Chem. Soc. Jpn., 2009, 82, 793 CrossRef CAS; (h) Y. Xiong, S. Yao, R. Müller, M. Kaupp and M. Driess, Nat. Chem., 2010, 2, 577 CrossRef CAS PubMed; (i) R. Rodriguez, D. Gau, T. Troadec, N. Saffon-Merceron, V. Branchadell, A. Baceiredo and T. Kato, Angew. Chem., Int. Ed., 2013, 52, 8980 CrossRef CAS PubMed; (j) Y. Wang, M. Chen, Y. Xie, P. Wei, H. F. Schaefer, P. v. R. Schleyer and G. H. Robinson, Nat. Chem., 2015, 7, 509 CrossRef CAS PubMed; (k) H. Cui, J. Zhang, Y. Tao and C. Cui, Inorg. Chem., 2016, 55, 46 CrossRef CAS PubMed.
- (a) S. Masamune, S. A. Batcheller, J. Park and W. M. Davis, J. Am. Chem. Soc., 1989, 111, 1888 CrossRef CAS; (b) M. Veith, S. Becker and V. Huch, Angew. Chem., Int. Ed. Engl., 1989, 28, 1237 CrossRef; (c) D. Ellis, P. B. Hitchcock and M. F. Lappert, J. Chem. Soc., Dalton Trans., 1992, 3397 RSC; (d) K. Kishikawa, N. Tokitoh and R. Okazaki, Chem. Lett., 1996, 25, 695 CrossRef; (e) M. Weidenbruch, M. Stürmann, H. Kilian, S. Pohl and W. Saak, Chem. Ber./Recl., 1997, 130, 735 CrossRef CAS; (f) A. Schäfer, W. Saak, M. Weidenbruch, H. Marsmann and G. Henkel, Chem. Ber./Recl., 1997, 130, 1733 CrossRef; (g) K. E. Litz, M. M. Banaszak Holl, J. W. Kampf and G. B. Carpenter, Inorg. Chem., 1998, 37, 6461 CrossRef CAS PubMed; (h) M. Veith, O. Schütt and V. Huch, Angew. Chem., Int. Ed., 2000, 39, 601 CrossRef CAS; (i) M. S. Samuel, M. C. Jennings and K. M. Baines, J. Organomet. Chem., 2001, 636, 130 CrossRef CAS; (j) Z. T. Cygan, J. E. Bender, K. E. Litz, J. W. Kampf and M. M. Banaszak Holl, Organometallics, 2002, 21, 5373 CrossRef CAS; (k) N. Tokitoh, K. Kishikawa, R. Okazaki, T. Sasamori, N. Nakata and N. Takeda, Polyhedron, 2002, 21, 563 CrossRef CAS; (l) J. T. York, V. G. Young and W. B. Tolman, Inorg. Chem., 2006, 45, 4191 CrossRef CAS PubMed; (m) I. Schranz, L. Grocholl, C. J. Carrow, L. Stahl and R. J. Staples, J. Organomet. Chem., 2008, 693, 1081 CrossRef CAS; (n) X. Wang, Y. Peng, M. M. Olmstead, J. C. Fettinger and P. P. Power, J. Am. Chem. Soc., 2009, 131, 14164 CrossRef CAS PubMed; (o) D. Yang, J. Guo, H. Wu, Y. Ding and W. Zheng, Dalton Trans., 2012, 41, 2187 RSC.
- (a) P. Brown, M. F. Mahon and K. C. Molloy, J. Chem. Soc., Chem. Commun., 1989, 1621 RSC; (b) R. W. Chorley, P. B. Hitchcock and M. F. Lappert, J. Chem. Soc., Chem. Commun., 1992, 525 RSC; (c) P. B. Hitchcock, M. F. Lappert, L. J.-M. Pierssens, A. V. Protchenko and P. G. H. Uiterweerd, Dalton Trans., 2009, 4578 RSC; (d) T. Chlupatý, Z. Padělková, A. Lyčka, J. Brusc and A. Růžička, Dalton Trans., 2012, 41, 5010 RSC; (e) T. Chlupatý, Z. Padělková, F. DeProft, R. Willem and A. Růžička, Organometallics, 2012, 31, 2203 CrossRef; (f) T. Chlupatý, Z. Růžičková, M. Horáček, M. Alonso, F. DeProft, H. Kampová, J. Brus and A. Růžička, Organometallics, 2015, 34, 606 CrossRef; (g) A. Stasch, C. M. Forsyth, C. Jones and P. C. Junk, New J. Chem., 2008, 32, 829 RSC.
- (a) E. A. V. Ebsworth, R. O. Gould, N. T. Mcmanus, D. W. H. Rankin, M. D. Walkinshaw and J. D. Whitelock, J. Organomet. Chem., 1983, 249, 227 CrossRef CAS; (b) L. Weber, G. Dembeck, H.-G. Stammler and B. Neumann, Eur. J. Inorg. Chem., 1998, 579 CrossRef CAS; (c) M. Nakamoto and K.-y. Akiba, J. Am. Chem. Soc., 1999, 121, 6958 CrossRef CAS; (d) O. J. Scherer, S. Weigel and G. Wolmershäuser, Angew. Chem., Int. Ed., 1999, 38, 3688 CrossRef CAS; (e) D. G. Ho, R. Gao, J. Celaje, H.-Y. Chung and M. Selke, Science, 2003, 302, 259 CrossRef CAS PubMed; (f) P. Mastrorilli, M. Latronico, C. F. Nobile, G. P. Suranna, F. P. Fanizzi, U. Englert and G. Ciccarella, Dalton Trans., 2004, 1117 RSC; (g) Y. Wang, Y. Xie, P. Wei, H. F. Schaefer, P. v. R. Schleyer and G. H. Robinson, J. Am. Chem. Soc., 2013, 135, 19139 CrossRef CAS PubMed; (h) B. A. Chalmers, M. Bühl, K. S. A. Arachchige, A. M. Z. Slawin and P. Kilian, J. Am. Chem. Soc., 2014, 136, 6247 CrossRef CAS PubMed; (i) D. Bockfeld, T. Bannenberg, P. G. Jones and M. Tamm, Eur. J. Inorg. Chem., 2017, 3452 CrossRef CAS; (j) N. Tokitoh, Y. Arai, T. Sasamori, R. Okazaki, S. Nagase, H. Uekusa and Y. Ohashi, J. Am. Chem. Soc., 1998, 120, 433 CrossRef CAS; (k) L. Balazs, H. J. Breunig, E. Lork, A. Soran and C. Silvestru, Inorg. Chem., 2006, 45, 2341 CrossRef CAS PubMed.
- B. Wang, Y. Li, R. Ganguly, H. Hirao and R. Kinjo, Nat. Commun., 2016, 7, 11871 CrossRef CAS PubMed.
- (a) H. C. Brown, Angew. Chem., 1980, 92, 675 CrossRef CAS; (b) H. C. Brown, Boranes in Organic Chemistry, Cornell University Press, Ithaca, NY, 1972 Search PubMed.
- (a) K. Miura, Y. Ichinose, K. Nozaki, K. Fugami, K. Oshima and K. Utimoto, Bull. Chem. Soc. Jpn., 1989, 62, 143 CrossRef CAS; (b) H. Yorimitsu and K. Oshima, Radicals in Organic Synthesis, ed. P. Renaud and M. P. Sibi, Wiley-VCH, Weinheim, 2001, vol. 1, p. 11 Search PubMed; (c) O. Cyril and R. Philippe, Chem. Rev., 2001, 101, 3415 CrossRef.
- (a) J. M. Davidson and C. Triggs, J. Chem. Soc. A, 1968, 1324 RSC; (b) R. A. Sheldon and J. K. Kochi, Activation of Molecular Oxygen by Metal Complexes, in Metal-catalyzed Oxidations of Organic Compounds, Academic Press, New York, 1981, vol. 4, p. 71 Search PubMed; (c) H. Yoshida, Y. Yamaryo, J. Ohshita and A. Kunai, Tetrahedron Lett., 2003, 44, 1541 CrossRef CAS; (d) C. Adamo, C. Amatore, I. Ciofini, A. Jutand and H. Lakmini, J. Am. Chem. Soc., 2006, 128, 6829 CrossRef CAS PubMed.
- J. T. Grant, C. A. Carrero, F. Goeltl, J. Venegas, P. Mueller, S. P. Burt, S. E. Specht, W. P. McDermott, A. Chieregato and I. Hermans, Science, 2016, 354, 1570 CrossRef CAS PubMed.
- W. Fudickar and T. Linker, Angew. Chem., Int. Ed., 2018, 57, 12971 CrossRef CAS.
- L. L. Cao and D. W. Stephan, Organometallics, 2017, 36, 3163 CrossRef CAS.
- Y. Su and R. Kinjo, Coord. Chem. Rev., 2017, 352, 346 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1873774–1873777. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8sc04624g |
| This journal is © The Royal Society of Chemistry 2019 |