Open Access Article
Open Access ArticleUbiquitin binds the amyloid β peptide and interferes with its clearance pathways†
F.
Bellia
a,
V.
Lanza
 a,
S.
García-Viñuales
a,
S.
García-Viñuales
 a,
I. M. M.
Ahmed
a,
I. M. M.
Ahmed
 a,
A.
Pietropaolo
a,
A.
Pietropaolo
 b,
C.
Iacobucci
b,
C.
Iacobucci
 c,
G.
Malgieri
c,
G.
Malgieri
 d,
G.
D'Abrosca
d,
G.
D'Abrosca
 d,
R.
Fattorusso
d,
R.
Fattorusso
 d,
V. G.
Nicoletti
d,
V. G.
Nicoletti
 e,
D.
Sbardella
f,
G. R.
Tundo
f,
M.
Coletta
f,
L.
Pirone
g,
E.
Pedone
e,
D.
Sbardella
f,
G. R.
Tundo
f,
M.
Coletta
f,
L.
Pirone
g,
E.
Pedone
 g,
D.
Calcagno
h,
G.
Grasso
g,
D.
Calcagno
h,
G.
Grasso
 *h and
D.
Milardi
*h and
D.
Milardi
 *a
*a
aConsiglio Nazionale delle Ricerche, Istituto di Biostrutture e Bioimmagini, Via P. Gaifami 18, 95126 Catania, Italy. E-mail: danilo.milardi@cnr.it
bDipartimento di Scienze della Salute, Università degli Studi Magna Graecia di Catanzaro, Viale Europa, 88100, Catanzaro, Italy
cDepartment of Pharmaceutical Chemistry & Bioanalytics, Institute of Pharmacy, Martin Luther University Halle-Wittenberg, 06120 Halle/Saale, Germany
dDepartment of Environmental, Biological and Pharmaceutical Sciences and Technologies, University of Campania “Luigi Vanvitelli”, Via Vivaldi 43, 81100 Caserta, Italy
eDipartimento di Scienze Biomediche e Biotecnologiche (BIOMETEC), sez. Biochimica medica, Università di Catania, Via Santa Sofia 97, 95124 Catania, Italy
fDipartimento di Scienze Cliniche e Medicina Traslazionale, Università di Roma Tor Vergata, Via Montpellier 1, 00133, Roma, Italy
gConsiglio Nazionale delle Ricerche, Istituto di Biostrutture e Bioimmagini, Via Mezzocannone, 16, Naples I-80134, Italy
hDipartimento di Scienze Chimiche, Università di Catania, V.le Andrea Doria 6, 95125 Catania, Italy. E-mail: grassog@unict.it
First published on 10th January 2019
Abstract
Several lines of evidence point to a compromised proteostasis associated with a reduction of the Ubiquitin Proteasome System (UPS) activity in patients affected by Alzheimer's Disease (AD) and suggest that the amyloid β peptide (Aβ) is an important player in the game. Inspired also by many reports, underlining the presence of ubiquitin (Ub) in the amyloid plaques of AD brains, here we set out to test whether Ub may bind the Aβ peptide and have any effect on its clearance pathways. By using an integrated array of MALDI-TOF/UPLC-HRMS, fluorescence, NMR, SPR, Microscale Thermophoresis (MST) and molecular dynamics studies, we consistently demonstrated that Aβ40 binds Ub with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry and Kd in the high micromolar range. In particular, we show that the N-terminal domain of the Aβ peptide (through residues D1, E3 and R5) interacts with the C-terminal tail of Ub (involving residues K63 and E64), inducing the central region of Aβ (14HQKLVFFAEDVGSNK28) to adopt a mixed α-helix/β-turn structure. ELISA assays, carried out in neuroblastoma cell lysates, suggest that Aβ competitively binds Ub also in the presence of the entire pool of cytosolic Ub binding proteins. Ub-bound Aβ has a lower tendency to aggregate into amyloid-like fibrils and is more slowly degraded by the Insulin Degrading Enzyme (IDE). Finally, we observe that the water soluble fragment Aβ1–16 significantly inhibits Ub chain growth reactions. These results evidence how the non-covalent interaction between Aβ peptides and Ub may have relevant effects on the regulation of the upstream events of the UPS and pave the way to future in vivo studies addressing the role played by Aβ peptide in the malfunction of proteome maintenance occurring in AD.
1 stoichiometry and Kd in the high micromolar range. In particular, we show that the N-terminal domain of the Aβ peptide (through residues D1, E3 and R5) interacts with the C-terminal tail of Ub (involving residues K63 and E64), inducing the central region of Aβ (14HQKLVFFAEDVGSNK28) to adopt a mixed α-helix/β-turn structure. ELISA assays, carried out in neuroblastoma cell lysates, suggest that Aβ competitively binds Ub also in the presence of the entire pool of cytosolic Ub binding proteins. Ub-bound Aβ has a lower tendency to aggregate into amyloid-like fibrils and is more slowly degraded by the Insulin Degrading Enzyme (IDE). Finally, we observe that the water soluble fragment Aβ1–16 significantly inhibits Ub chain growth reactions. These results evidence how the non-covalent interaction between Aβ peptides and Ub may have relevant effects on the regulation of the upstream events of the UPS and pave the way to future in vivo studies addressing the role played by Aβ peptide in the malfunction of proteome maintenance occurring in AD.
Introduction
Alzheimer's disease (AD), the most common form of dementia worldwide, is an age-related, fatal neurodegenerative disorder. A hallmark of AD is the presence of extracellular proteinaceous deposits (senile plaques) in the brain of affected people. The prevalent component of senile plaques is β-amyloid (Aβ).1 Although a firm relationship between the occurrence of different Aβ aggregates in the AD brain and the severity of the disease has not been established yet, Aβ misfolding and self-assembly are widely believed to be crucial pathogenic events in AD (amyloid hypothesis).2–4 Unfortunately, all clinical trials of amyloid-targeting drugs have failed so far5,6 suggesting that the amyloid hypothesis needs to be reconsidered. Intriguingly, several reports put in evidence that transgenic mice models of AD show an intracellular Aβ immunoreactivity7 which occurs before cognitive loss and massive amyloid plaques deposition and may be more closely associated with the disease progress.8 Therefore, studies focusing on the interplay between Aβ amyloid growth and protein clearance in the cell represent a promising arena for the design of more effective AD therapies.9The removal of misfolded and potentially toxic cytosolic proteins is mostly regulated by the ubiquitin proteasome system (UPS).10 The first component of the UPS is ubiquitin (Ub), a small protein composed of 76 amino acids, with a compact globular structure characterized by a mixed parallel/anti-parallel β-sheet packing against an α-helix.11 Degradation of misfolded proteins by the UPS occurs first by labeling a lysine residue of the substrate with a (poly)ubiquitin tag (ubiquitinylation) which is specifically identified and degraded by the proteasome.12 When the UPS is not efficient, the removal of toxic protein assemblies gets slower and, in turn, the disease may progress more rapidly.
Consistent with this hypothesis, many reports suggest that the UPS malfunction plays a significant role in Aβ accumulation and, in turn, in AD progress.13 However, the effects of Aβ on proteasome function are still under debate. In particular, on one hand, the Aβ peptide has been observed to be a proteasome inhibitor on its own;14–21 on the other hand some studies have outlined how distinct Aβ peptide assemblies may inhibit or even activate different proteasome particles.22 The controversial role of Aβ in affecting proteasome function as well as the presence of Ub-positive proteinaceous aggregates in the senile plaques of AD patients23 suggests that UPS malfunction observed in AD could be then more likely linked to a failure of its upstream components (i.e., ubiquitinylation).24
Ubiquitinylation needs the coordinated activity of three distinct types of enzyme: (i) an ATP-consuming Ub-activating enzyme (E1); (ii) a Ub-conjugating enzyme (E2) and a Ub ligase (E3).25 Furthermore, the fact that these processes are largely governed by low-affinity interactions between Ub and the various Ub-writing enzymes (i.e., E1, E2 and E3)26 envisages the possibility that an excess of Aβ might inhibit, as a decoy effect, these processes through non covalent binding to Ub.
To test this hypothesis, here we use a battery of experimental (NMR, SPR, MST, cross-linking XL ESI-MS, MALDI-TOF MS) and in silico (MD) approaches to fully characterize the interactions of Ub with the Aβ amyloid peptide in terms of stoichiometry, affinity and binding sites. Next, we employ an ELISA assay to evaluate if the interaction of Aβ with Ub is detectable in cell lysates too, thus supporting the significance of this study in a biological context. These experiments are also paralleled by ESI-MS studies addressing Aβ hydrolysis by the Insulin Degrading Enzyme (IDE), a protease which is known to be involved in the physiological amyloid clearance.27 The effects of Ub on Aβ amyloid aggregation and Ub chain growth reactions are finally investigated.
Results and discussion
NMR analysis of Ub in complex with Aβ40
In an attempt to assess if Ub binds Aβ, we first analyzed Ub/Aβ interactions in solution by NMR spectroscopy. Free 15N–13C labeled Ub was characterized by means of a standard set of triple and double resonance experiments that constituted the reference spectra. There are two naturally occurring forms of the Aβ peptide: Aβ42 and Aβ40. Although Aβ42 is known to be more prone to form amyloid aggregates, Aβ40 is produced more abundantly in the cell (Aβ40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aβ42 molar ratio is 9
Aβ42 molar ratio is 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
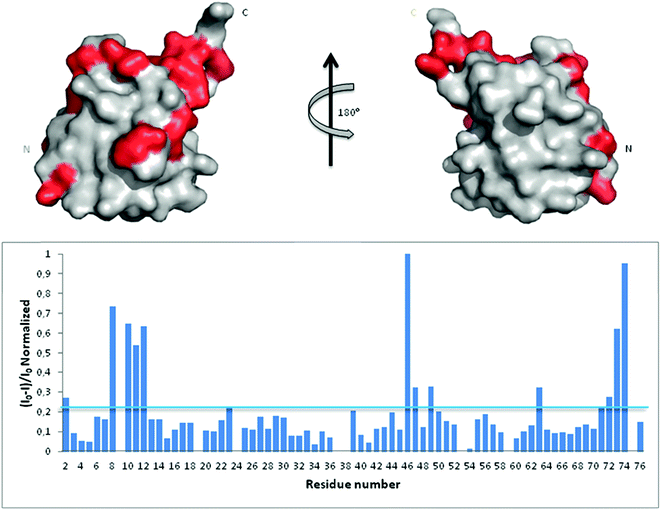
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and thus may be more conveniently used to investigate interactions with Ub;28 furthermore, the tendency of Aβ42 to form aggregates makes it very problematic to carry out NMR experiments at very high concentrations. Unlabeled Aβ40 was then added as a single aliquot of a known amount to the sample and a new set of spectra was recorded. The translational diffusion coefficient, chemical shift and intensity variations of the different nuclei in the presence and absence of Aβ were evaluated. The translational diffusion coefficient measured for Ub in the absence of the peptide is 1.33 ± 0.12 × 10−10 m2 s−1, consistent with values reported in the literature.29 The same coefficient measured in the presence of Aβ40 is 1.28 ± 0.18 × 10−10 m2 s−1. These coefficients allow us to rule out protein–protein aggregation phenomena mediated by Aβ40 within the NMR spectroscopic concentration range investigated and are in agreement with the interaction between the two molecules. The analysis of Ub HSQC after sub-stoichiometric Aβ40 addition shows that the overall Ub fold is maintained, although a number of peaks are perturbed. As protein–ligand interactions correspond often to a redistribution of the protein internal dynamics,30–33 intensity changes of the amide cross-peaks in the 2D [1H,15N]-HSQC spectra represent sensitive probes of exchange and/or relaxation rate variations of each protein residue upon interaction (see Fig. S1 in the ESI†). Perturbation of signal intensities with respect to the reference spectra as a function of ligand addition thus provides useful dynamic and conformational information about the binding event. Mapping the differences in normalized signal intensities onto the Ub structure (PDB code: 1UBQ; Fig. 1 upper panel) outlines a region influenced by the interaction with Aβ40, which involves residues belonging to the C-terminal tail and the β sheet of the molecule, leading to a loss in intensity of their HSQC cross-peaks (Fig. 1 lower panel). The residues composing the helix appear not to be involved in the interaction as their cross-peaks are less influenced by the presence of the peptide in solution.
1) and thus may be more conveniently used to investigate interactions with Ub;28 furthermore, the tendency of Aβ42 to form aggregates makes it very problematic to carry out NMR experiments at very high concentrations. Unlabeled Aβ40 was then added as a single aliquot of a known amount to the sample and a new set of spectra was recorded. The translational diffusion coefficient, chemical shift and intensity variations of the different nuclei in the presence and absence of Aβ were evaluated. The translational diffusion coefficient measured for Ub in the absence of the peptide is 1.33 ± 0.12 × 10−10 m2 s−1, consistent with values reported in the literature.29 The same coefficient measured in the presence of Aβ40 is 1.28 ± 0.18 × 10−10 m2 s−1. These coefficients allow us to rule out protein–protein aggregation phenomena mediated by Aβ40 within the NMR spectroscopic concentration range investigated and are in agreement with the interaction between the two molecules. The analysis of Ub HSQC after sub-stoichiometric Aβ40 addition shows that the overall Ub fold is maintained, although a number of peaks are perturbed. As protein–ligand interactions correspond often to a redistribution of the protein internal dynamics,30–33 intensity changes of the amide cross-peaks in the 2D [1H,15N]-HSQC spectra represent sensitive probes of exchange and/or relaxation rate variations of each protein residue upon interaction (see Fig. S1 in the ESI†). Perturbation of signal intensities with respect to the reference spectra as a function of ligand addition thus provides useful dynamic and conformational information about the binding event. Mapping the differences in normalized signal intensities onto the Ub structure (PDB code: 1UBQ; Fig. 1 upper panel) outlines a region influenced by the interaction with Aβ40, which involves residues belonging to the C-terminal tail and the β sheet of the molecule, leading to a loss in intensity of their HSQC cross-peaks (Fig. 1 lower panel). The residues composing the helix appear not to be involved in the interaction as their cross-peaks are less influenced by the presence of the peptide in solution.
Molecular modeling of Ub/Aβ complexes
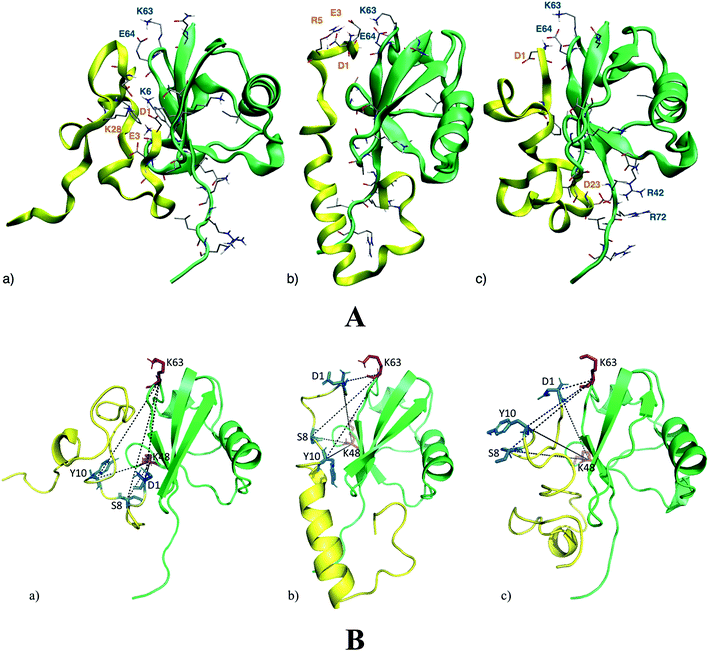
NMR analysis provides molecular details about the Ub residues directly involved in the interaction with Aβ. These data are used as a starting point to describe, at an atomistic level, the Ub-bound conformation of Aβ by molecular modeling. Upon Aβ40 interaction with Ub, three main binding poses may be disclosed (Fig. 2A). The first binding pose features a network of salt-bridges involving the N-terminal section of Aβ40, with residues D1, E3 and K28 facing the Ub K6 residue (Fig. 2A(a)). This interaction triggers hydrophobic contacts between the Ub central region and Aβ40, which mainly adopts turn conformations with a short 310 helix involving the 16KLV18 segment. At variance with the latter binding surface, in the second binding pose, the N-terminal domain of Aβ40 faces the Ub C-terminal region. Herein, Aβ40 residues D1, E3 and R5 contact Ub residues K63 and E64, perturbing the typical salt-bridge network featuring the free Ub structure (Fig. 2A(b)). The central region of Aβ40 is less prone to interact with Ub owing to the intramolecular interactions building an α-helix in the 14HQKLVFFAEDVGSNK28 segment. In the third binding pose, the Aβ40 charged N-terminal amine of D1 faces residue E64 of Ub (Fig. 2A(c)). Even in this binding pose, the salt-bridge between K63 and E64 is affected by the N-terminal amine group. Furthermore, D23 comes in contact with R42 which is close to R72, perturbing the typical network of the charged arginine residues of the Ub structure. The central region of Aβ40 is involved in a loop, forming a small β-turn, which involves the segments 16KL17 and 35MV.36 On the whole, the Aβ40 N-terminal domain interacts with both the N- and C-terminal regions of Ub triggering subtle variations in the Aβ central domain which folds in α-helical structures resembling those observed elsewhere for membrane-bound Aβ peptides.34 Notably, molecular modeling suggests that the Ub binding induces significant structural constraints along the region encompassing the hydrophobic Phe residues of Aβ.Measurement of dissociation constants (Kd) of the Aβ40/Ub complex
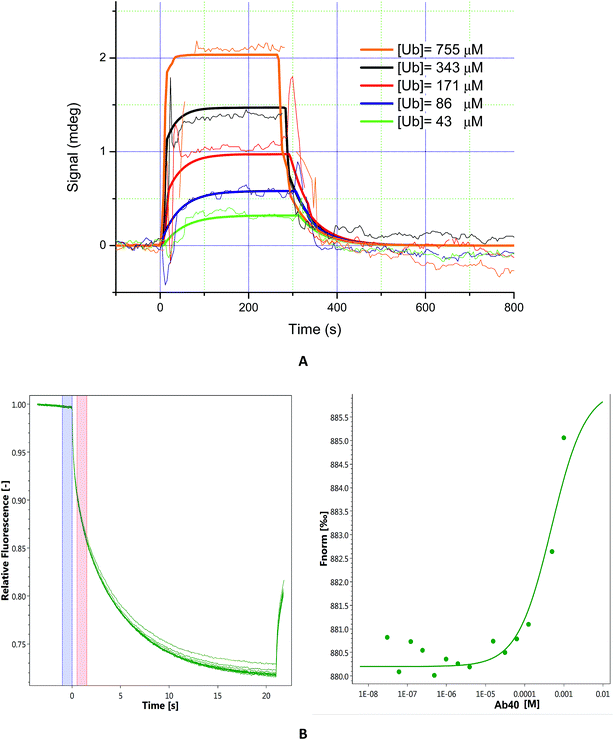
The majority of known Ub binding partners are characterized by affinities in the micro-molar range (2 μM < Kd <500 μM).41 Here, to evaluate the binding affinity of Aβ to Ub, we performed SPR analysis on immobilized Aβ40. Ub solutions were prepared by dissolving the protein in 10 mM PBS (pH 7.4)/Tween 20 (0.05%) and the injections were carried out in a serial configuration for 5 minutes at 20 μl min−1 and 30 μl min−1, respectively. Different concentration solutions of Ub (43 μM, 86 μM, 171 μM, 343 μM, and 755 μM) were prepared and after each injection a regeneration step was achieved using NaCl 2 M/NaOH 10 mM (30 μl min−1 for 3 minutes). The resulting sensograms, obtained after reference subtraction, were extracted with MP-SPR Navi Data viewer analyzed through the Trace Drawer™ software and kinetic parameters of the interaction between Ub and Aβ were calculated. Panel A of Fig. 3 represents the overlay between experimental curves and fitted ones, while in Fig. S3 in the ESI† the fitting residual value graph is shown. The fitting model adopted was “OneToOne”, which describes one monovalent ligand binding to one target. In this case, the following equation can be assumed to describe the biomolecular interactions:| dY/dt = (ka × c − kd) × Y |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fnorm = F1/F0 where F0 refers to the initial fluorescence and F1 is the fluorescence measured several seconds after the IR-laser has been turned on, when the traces of unbound and bound states can be discriminated. Plotting these values against the ligand concentration results in a typical binding isotherm providing the affinity constant value of the interaction.
Fnorm = F1/F0 where F0 refers to the initial fluorescence and F1 is the fluorescence measured several seconds after the IR-laser has been turned on, when the traces of unbound and bound states can be discriminated. Plotting these values against the ligand concentration results in a typical binding isotherm providing the affinity constant value of the interaction.
First, we carried out MST experiments to measure Kd for Ub binding to Aβ40 (see Fig. 3 panel B). Next, to rule out any possible bias due to amyloid aggregation, we also analysed Aβ1–16, a short soluble fragment that is thought to encompass the residues of the amyloid peptide involved in Ub interaction (see Fig. S4 in the ESI†). We found that Ub is able to bind both the molecules with a comparable Kd (Kd Aβ40 = 4.8 × 10−4 M and Kd Aβ1–16, = 3.4 × 10−4 M) in agreement with SPR data.
Aβ competitively binds Ub in whole cell extracts
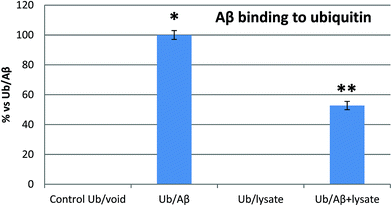
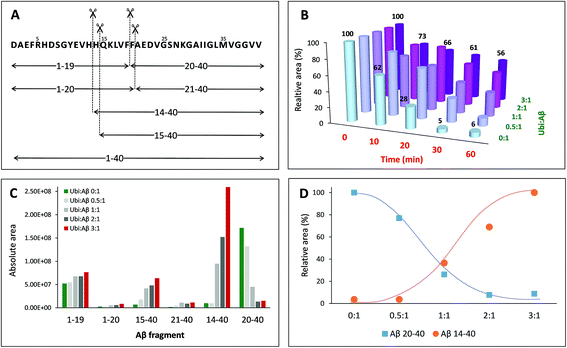
SPR and MST analysis have demonstrated that Aβ binds Ub with a binding affinity which is comparable with that observed for many other Ub binding proteins.40 Therefore, our next step was to confirm if Aβ may competitively bind Ub also in the presence of the complete pool of cellular Ub binding partners. To this aim, we investigated the interaction of Ub with the Aβ peptide in the presence of whole cell extracts from previously differentiated SHSY5Y neuroblastoma cells. Enzyme-linked immunosorbent assay (ELISA) is a convenient method widely employed to analyze protein–protein interactions in complex biological mixtures. Here, we employ an ELISA inhibition procedure, using alternatively Ub or Aβ40 as the solution binding partner to demonstrate that Aβ maintains its ability to bind Ub also in the presence of all natural cytosolic Ub binding partners (Fig. 4). The Aβ peptide binding to the Ub-coated well surface shows an overwhelming increase of the signal over the control. In this case, the presence of cell lysates reduced the Aβ40 binding to Ub by about 50%, thus demonstrating that Ub binds Aβ, albeit with a reduced signal intensity, and that this interaction is physiologically relevant, since it occurs also in the presence of the complete pool of cellular Ub binding partners. In a second control experiment, Ub binding to the Aβ40 peptide immobilized on the well surface of the ELISA plate shows a significantly increased signal too (Fig. S5 in the ESI†). As in the previous experiment, the binding is counteracted by the presence of cell lysates thus demonstrating that Aβ may competitively binds Ub also in the presence of all cytosolic components.The proteolytic activity of IDE versus Aβ40 is altered by Ub
Once established that Aβ may bind Ub in the cytosol, it is important to evaluate how this interaction may interfere with the physiological peptide clearance. The cellular homeostasis of Aβ is regulated by several proteases and degrading systems42 among which IDE gives a particularly important contribution to the clearance of amyloidogenic peptides43 including Aβ, amylin and insulin.44–47 Thus, any substance that somehow interacts with Aβ under physiological conditions could reasonably affect its IDE-mediated hydrolysis. In this context, the dose-dependent effect of Ub on the IDE-catalyzed hydrolysis of Aβ has been evaluated through a proteomic approach based on Ultra Performance Liquid Chromatography (UPLC) coupled with High Resolution Mass Spectrometry (HRMS). The enzymatic degradation of the amyloid peptide produced nine peptide fragments within 60 min of reaction (Table S3 in the ESI†). As previously described,48 in the early stage of the IDE-mediated hydrolysis, the main targeted regions encompass the hydrophobic Phe residues, the vicinal His residues, and, to a lesser extent, Asn27 and Met35 (Fig. 5A). By means of the LC-MS analysis, all complementary peptide fragments adjacent to each cleavage site have been detected. The total amount of the full length substrate (i.e., Aβ40) clearly decreases over the reaction time (Fig. 5B) such that after 20 min of reaction the concentration of Aβ40 is 28% relative to the initial value and drops down to 5% after 30 min. Such a trend is maintained when Ub was added to the reaction mixture but it is noteworthy that the rate of signal decrease is affected by Ub in a dose-dependent manner (Fig. 5B). When the Ub![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aβ40 molar ratio is 0.5
Aβ40 molar ratio is 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the residual substrate is 32% after 30 minutes with respect to the starting condition. Finally, 56% of Aβ40 is still present after 60 min of reaction at the highest Ub
1, the residual substrate is 32% after 30 minutes with respect to the starting condition. Finally, 56% of Aβ40 is still present after 60 min of reaction at the highest Ub![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aβ40 molar ratio tested (3
Aβ40 molar ratio tested (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Although the documented interaction between IDE and Ub49 does not rule out the possibility that Ub itself might somewhat affect IDE proteolytic activity, data shown in Fig. 5 represent a clear evidence in favor of an inhibitory effect by the non-covalent interaction between Ub and Aβ40 on the IDE-mediated hydrolysis of Aβ40, in accordance with other experimental approaches adopted throughout this paper. In order to elucidate the regions of the Aβ sequence mainly involved in the interaction with Ub, the amount of all peptide fragments was reported as a function of the Ub
1). Although the documented interaction between IDE and Ub49 does not rule out the possibility that Ub itself might somewhat affect IDE proteolytic activity, data shown in Fig. 5 represent a clear evidence in favor of an inhibitory effect by the non-covalent interaction between Ub and Aβ40 on the IDE-mediated hydrolysis of Aβ40, in accordance with other experimental approaches adopted throughout this paper. In order to elucidate the regions of the Aβ sequence mainly involved in the interaction with Ub, the amount of all peptide fragments was reported as a function of the Ub![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aβ40 molar ratio (Fig. 5C). In the absence of Ub (green bars) and in samples collected after 60 min of reaction, the chromatographic peaks having the higher intensity are those related to the complementary peptides Aβ1–19 and Aβ20–40, whereas Aβ1–20, Aβ21–40, Aβ14–40 and Aβ15–40 are barely detectable. Increasing amounts of Ub had a very small effect on the concentration of Aβ1–20 and Aβ21–40; only a slight increase was observed for Aβ1–19, whilst the concentration of Aβ14–40, Aβ15–40 and Aβ20–40 was greatly affected by the presence of Ub in a dose-dependent manner. In particular, the formation of Aβ15–40 (and even more of Aβ14–40) is activated by the presence of Ub, whereas the formation of Aβ20–40 is greatly inhibited proportionally to the concentration of Ub. The opposite trend, relative to the amounts of these peptide fragments formed at the various peptide
Aβ40 molar ratio (Fig. 5C). In the absence of Ub (green bars) and in samples collected after 60 min of reaction, the chromatographic peaks having the higher intensity are those related to the complementary peptides Aβ1–19 and Aβ20–40, whereas Aβ1–20, Aβ21–40, Aβ14–40 and Aβ15–40 are barely detectable. Increasing amounts of Ub had a very small effect on the concentration of Aβ1–20 and Aβ21–40; only a slight increase was observed for Aβ1–19, whilst the concentration of Aβ14–40, Aβ15–40 and Aβ20–40 was greatly affected by the presence of Ub in a dose-dependent manner. In particular, the formation of Aβ15–40 (and even more of Aβ14–40) is activated by the presence of Ub, whereas the formation of Aβ20–40 is greatly inhibited proportionally to the concentration of Ub. The opposite trend, relative to the amounts of these peptide fragments formed at the various peptide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ub ratios, is evident in Fig. 5D.
Ub ratios, is evident in Fig. 5D.
The non-covalent interaction between Aβ and Ub has a clear site-specific effect on the IDE-mediated hydrolysis of Aβ. The trend related to the formation of peptide fragments indeed suggests that Ub somehow inhibits the cleavage of the Phe19–Phe20 peptide bond. Such an effect of Ub on the degradation pathway of Aβ is in accordance with molecular models, described above (see Fig. 2), showing significant structural restraints, occurring upon Ub binding, in Aβ regions encompassing the central hydrophobic cluster (14–28 residues). The strong activation of the enzymatic hydrolysis at His13-His14 and His14-Gln15 might be considered a natural consequence of the inhibition involving the vicinal Phe residues. Indeed, Aβ14–40 and Aβ15–40 are also processed by IDE to form Aβ20–40; therefore, their own Ub-dependent accumulation is reasonably considered a consequence of the effect of Ub on the formation of Aβ20–40. It is noteworthy that the crossing point between the Ub-induced increase of Aβ14–40 and the concomitant decrease of Aβ20–40 is reached when the Ub![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aβ molar ratio is 1
Aβ molar ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This evidence indeed strongly supports the 1
1. This evidence indeed strongly supports the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of the Ub
1 stoichiometry of the Ub![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aβ complex evidenced by all other experiments (NMR, XL-MS, SPR, MST and modeling). The 1
Aβ complex evidenced by all other experiments (NMR, XL-MS, SPR, MST and modeling). The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ub
1 Ub![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
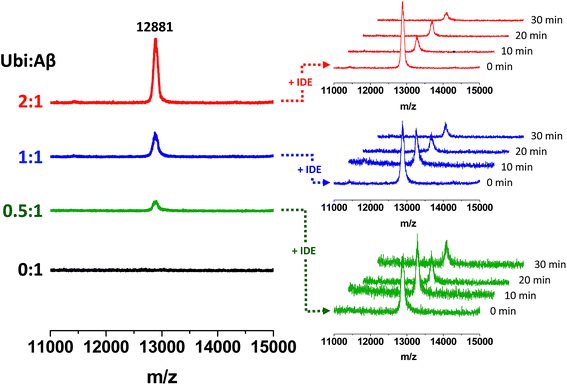
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aβ adduct has also been observed by MALDI-TOF measurements (Fig. 6). The molecular weight of the 1
Aβ adduct has also been observed by MALDI-TOF measurements (Fig. 6). The molecular weight of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct was detected in all samples containing both the amyloid peptide and Ub (Fig. 6, left graph). Moreover, the higher the Ub
1 adduct was detected in all samples containing both the amyloid peptide and Ub (Fig. 6, left graph). Moreover, the higher the Ub![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aβ molar ratio, the higher the absolute intensity of the mono-charged peak detected in linear mode, as a direct consequence of the amount of the complex formed. As a further confirmation of the peak attribution, these peaks drop down in the presence of IDE and proportionally to the reaction time, regardless of the Ub
Aβ molar ratio, the higher the absolute intensity of the mono-charged peak detected in linear mode, as a direct consequence of the amount of the complex formed. As a further confirmation of the peak attribution, these peaks drop down in the presence of IDE and proportionally to the reaction time, regardless of the Ub![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aβ molar ratio (Fig. 6, right graphs). This trend can only be ascribed to a Ub-related inhibition of Aβ hydrolysis by IDE. These results coupled also with the finding that Aβ is a proteasome inhibitor (see Fig. S6 in the ESI†) support the hypothesis that excess Aβ may significantly affect protein clearance pathways.
Aβ molar ratio (Fig. 6, right graphs). This trend can only be ascribed to a Ub-related inhibition of Aβ hydrolysis by IDE. These results coupled also with the finding that Aβ is a proteasome inhibitor (see Fig. S6 in the ESI†) support the hypothesis that excess Aβ may significantly affect protein clearance pathways.
Ub interferes with Aβ amyloid growth
It is known that Aβ aggregation starts intracellularly,50 likely during abnormal interactions with lipid membranes.51–53 In order to investigate if the interaction between the amyloid peptide and ubiquitin modifies the well-known propensity of Aβ to aggregate into amyloid-like fibers, the amyloid aggregation of Aβ was monitored by using a switch-on fluorescent dye (ThT). The experimental data obtained by the kinetic measurements were properly fitted to the theoretical aggregation models (Fig. 7) in order to calculate the kinetic parameters of the aggregation process (Table S2 in the ESI†). The amyloid-like aggregation of Aβ follows a sigmoidal trend, as previously reported.54 The lag phase of the self-induced aggregation of Aβ lasts 19.2 h. Such a value significantly increases in the presence of Ub, even when the Ub![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aβ molar ratio is 0.2
Aβ molar ratio is 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The lengthening of the lag phase is proportional to the added amount of Ub and tlag becomes more than 60 h when the Ub
1. The lengthening of the lag phase is proportional to the added amount of Ub and tlag becomes more than 60 h when the Ub![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aβ molar ratio is 3
Aβ molar ratio is 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The maximal fluorescence gain (Fmax − F0), proportional to the extent of fibril formation, has an opposite trend with respect to the concentration of Ub. The Fmax − F0 value is significantly affected by Ub when the Ub
1. The maximal fluorescence gain (Fmax − F0), proportional to the extent of fibril formation, has an opposite trend with respect to the concentration of Ub. The Fmax − F0 value is significantly affected by Ub when the Ub![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aβ molar ratio is equal or higher than 0.2
Aβ molar ratio is equal or higher than 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The Fmax − F0 value is 2.2 at the highest ratio tested (3
1. The Fmax − F0 value is 2.2 at the highest ratio tested (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). These data demonstrate that Ub delays the amyloid-type aggregation of Aβ in a dose-dependent manner and considerably reduces the extent of the fibril formation. Such an effect might be ascribed to a direct or indirect influence on the Aβ sequence covering the LVFF motif, which is involved in the mechanism of the amyloid aggregation and is a common target of many inhibitors of the Aβ aggregation.55 This hypothesis is congruent with the results of the IDE-mediated hydrolysis of Aβ in the presence of Ub.
1). These data demonstrate that Ub delays the amyloid-type aggregation of Aβ in a dose-dependent manner and considerably reduces the extent of the fibril formation. Such an effect might be ascribed to a direct or indirect influence on the Aβ sequence covering the LVFF motif, which is involved in the mechanism of the amyloid aggregation and is a common target of many inhibitors of the Aβ aggregation.55 This hypothesis is congruent with the results of the IDE-mediated hydrolysis of Aβ in the presence of Ub.
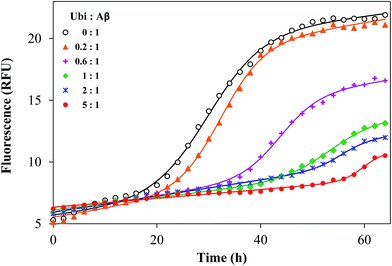 | ||
Fig. 7 Representative kinetic profiles of the aggregation of Aβ40 in the presence of ubiquitin, with the Ub![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Aβ ratio ranging from 0 Aβ ratio ranging from 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3 1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Solid lines represent the fitted curves for each kinetic profile, whose related parameters are reported in Table S2.† 1. Solid lines represent the fitted curves for each kinetic profile, whose related parameters are reported in Table S2.† | ||
Aβ1–16 inhibits Ub chain growth reactions in tube tests
In the cell, Ub chain growth is initiated by the Ub-activating enzyme E1, which adenylates the C-terminus of ubiquitin in an ATP-dependent fashion to form a high energy thioester bond by a cysteine residue. Then, E1 hands the activated Ub over to a conjugating enzyme (E2), forming an E2–Ub complex in proximity of the target protein. The final ubiquitinylation of the substrate occurs through the action of specific E3 ubiquitin ligases. Unanchored (or free) Ub chains (i.e., Ub chains that are not linked to any substrate) are normally considered very useful tools to study ubiquitinylation processes. A number of protocols have been developed to produce K48 and K63-linked free Ub chains in tube tests,56 which may be thus considered as a helpful method to single out the capacity of adverse factors in affecting the Ub-conjugation machinery.56 Due to its significant affinity for Ub, it is plausible that Aβ may interfere with the complex cascade of events leading to poly-ubiquitinylation. To address this issue, we have carried out poly-ubiquitinylation reactions in the presence of increasing concentration of Aβ. First, we tried to use the full length Aβ40 peptide, but the simultaneous formation of high molecular weight oligomeric species during the ubiquitinylation reactions masked any possible effect. Then we used a soluble Aβ1–16 peptide, a fragment that contains all peptide residues that previous experiments consistently identified as Ub binding sites. The Aβ1–16 peptide has been added with a ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 1
0.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with respect to Ub to both reaction mixtures (Fig. 8). Western Blot (WB) analysis has shown the dose-dependent inhibitory effect of the Aβ1–16 peptide on both Lys48- and Lys-63-linked ubiquitin chain elongation, being already evident at a ratio 1
2 with respect to Ub to both reaction mixtures (Fig. 8). Western Blot (WB) analysis has shown the dose-dependent inhibitory effect of the Aβ1–16 peptide on both Lys48- and Lys-63-linked ubiquitin chain elongation, being already evident at a ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of Ub/Aβ1–16. These data have further demonstrated that the interaction of Ub and Aβ hinders the regions close to the lysine 63 and 48.
1 of Ub/Aβ1–16. These data have further demonstrated that the interaction of Ub and Aβ hinders the regions close to the lysine 63 and 48.
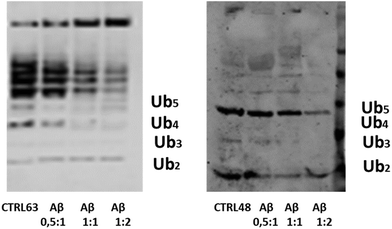 | ||
Fig. 8 The WB analysis of Ub reactions in the presence of the Aβ1-16 peptide, with ratios of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1 1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with respect to the Ub concentration. 2 with respect to the Ub concentration. | ||
Conclusions
Although accumulation of extracellular amyloid plaques in the brain is widely considered a hallmark of AD, a direct relationship between amyloid load and cognitive decline has never been unequivocally demonstrated. On the other hand, it is becoming increasingly evident that an abnormal rise of intracellular Aβ levels may be better correlated with neuronal loss and disease progression. Unfortunately, our knowledge of the intracellular chemistry of Aβ is largely incomplete and there is an urgent need for a more detailed description of the role played by Aβ in the pathological failure of cytosolic protein clearance supervisors like UPS and IDE.Here, we show that Aβ has a relatively significant affinity for Ub. It forms 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 protein–protein complexes mostly involving the C-terminal tail and the β1/β2 loop of Ub. Next, consistent with many reports showing that Ub is often recognized by binding proteins through the hydrophobic surface encompassing residues Leu8, Ile44, His 68 and Val 70,57 non-covalent interactions involving the central hydrophobic cluster of Aβ40 and the Ub Ile44 were also observed. The residue D1 of the peptide was found to be linked to the K48 and K63 residues of Ub. These two Ub residues were also found to be in contact with residues S8 and Y10 of Aβ40. The N-terminal segment of Aβ40 (residues D1 E3 and R5) interacts with the Ub C-terminal domain and in particular with residues Lys63 and Glu64. Consistent with the observation that Lys48 and Lys63 residues are buried upon interaction with the N-terminal domain of Aβ, we have also observed that the water soluble fragment Aβ1–16 significantly inhibits Ub chain growth in tube tests. It is noteworthy that the Aβ/Ub interaction resembles non covalent contacts between Ub and Ub binding domains (UBDs) which are important players in Ub chain growth and signaling. For example, most of the known UBDs are predominantly α-helical and bind the Ile44 hydrophobic patch of Ub.58 Moreover, most of the UBDs exhibit binding affinities (Kd) in the range between 2 and 500 μM.41 Next, Aβ40 may assume an α-helical structure in its central hydrophobic cluster (residues 14–20) upon interaction with Ub. Besides these hydrophobic contacts, salt bridges connecting the D1 residue of Aβ40 with K63 and K48 of Ub are also evident. This coupled electrostatic/hydrophobic interaction likely explains the evident binding affinity of Aβ for Ub and may explain why the peptide is a competitive Ub binding protein. Furthermore, while on one side Aβ binding to Ub compromises its ability to recognize physiological binding partners, on the other side it significantly influences peptide secondary structure and ability to self-assemble into amyloid-like aggregates and to be degraded by IDE. The reduced propensity of Aβ40 to aggregate into amyloid fibrils is ascribable to the increased α-helical content observed in the Ub-bound peptide. This ability of Ub to mold poorly structured peptides has been already observed in previous reports.30 Remarkably, Ub/Aβ interactions also have a site-specific effect on the IDE-mediated degradation process of the amyloid peptide with possible consequences on the toxicity of the hydrolyzed Aβ fragments.59 Conclusively, these results evidence how the non-covalent interaction between Aβ40 and Ub may have important consequences in the regulation of the upstream events of the UPS and in IDE-mediated clearance pathways. These results may hopefully pave the way to future studies addressing the multifaceted role played by the altered Aβ homeostasis occurring in AD.
1 protein–protein complexes mostly involving the C-terminal tail and the β1/β2 loop of Ub. Next, consistent with many reports showing that Ub is often recognized by binding proteins through the hydrophobic surface encompassing residues Leu8, Ile44, His 68 and Val 70,57 non-covalent interactions involving the central hydrophobic cluster of Aβ40 and the Ub Ile44 were also observed. The residue D1 of the peptide was found to be linked to the K48 and K63 residues of Ub. These two Ub residues were also found to be in contact with residues S8 and Y10 of Aβ40. The N-terminal segment of Aβ40 (residues D1 E3 and R5) interacts with the Ub C-terminal domain and in particular with residues Lys63 and Glu64. Consistent with the observation that Lys48 and Lys63 residues are buried upon interaction with the N-terminal domain of Aβ, we have also observed that the water soluble fragment Aβ1–16 significantly inhibits Ub chain growth in tube tests. It is noteworthy that the Aβ/Ub interaction resembles non covalent contacts between Ub and Ub binding domains (UBDs) which are important players in Ub chain growth and signaling. For example, most of the known UBDs are predominantly α-helical and bind the Ile44 hydrophobic patch of Ub.58 Moreover, most of the UBDs exhibit binding affinities (Kd) in the range between 2 and 500 μM.41 Next, Aβ40 may assume an α-helical structure in its central hydrophobic cluster (residues 14–20) upon interaction with Ub. Besides these hydrophobic contacts, salt bridges connecting the D1 residue of Aβ40 with K63 and K48 of Ub are also evident. This coupled electrostatic/hydrophobic interaction likely explains the evident binding affinity of Aβ for Ub and may explain why the peptide is a competitive Ub binding protein. Furthermore, while on one side Aβ binding to Ub compromises its ability to recognize physiological binding partners, on the other side it significantly influences peptide secondary structure and ability to self-assemble into amyloid-like aggregates and to be degraded by IDE. The reduced propensity of Aβ40 to aggregate into amyloid fibrils is ascribable to the increased α-helical content observed in the Ub-bound peptide. This ability of Ub to mold poorly structured peptides has been already observed in previous reports.30 Remarkably, Ub/Aβ interactions also have a site-specific effect on the IDE-mediated degradation process of the amyloid peptide with possible consequences on the toxicity of the hydrolyzed Aβ fragments.59 Conclusively, these results evidence how the non-covalent interaction between Aβ40 and Ub may have important consequences in the regulation of the upstream events of the UPS and in IDE-mediated clearance pathways. These results may hopefully pave the way to future studies addressing the multifaceted role played by the altered Aβ homeostasis occurring in AD.
Author contribution
F. B. performed IDE-mediated hydrolysis studies; V. L. and S. G. V. performed polyubiquitination reactions; I. M. M. A. performed MALDI-TOF studies; A. P. performed molecular simulations; C. I. performed cross-linking studies; G. M., G. D. A and R. F. performed NMR studies; V. G. N. performed ELISA assays; D. S. and G. R. T. contributed to cell studies; M. C. wrote the paper; D. C. performed SPR experiments; L. P. and E. P. performed MST experiments; G. G. analyzed SPR data and designed the research; D. M. designed the research and wrote the paper.Conflicts of interest
There are non conflicts of interest to declare.Acknowledgements
This work was financially supported by the Italian MiUR (PRIN 20157WZM8A). C. I. is grateful to Prof. Andrea Sinz for continuous support.References
- D. J. Selkoe, Neuron, 1991, 6, 487–498 CrossRef CAS PubMed.
- E. Terzi, G. Hölzemann and J. Seelig, Biochemistry, 1997, 36, 14845–14852 CrossRef CAS PubMed.
- B. A. Yankner, L. K. Duffy and D. A. Kirschner, Science, 1990, 250, 279–282 CrossRef CAS PubMed.
- J. A. Hardy and G. A. Higgins, Science, 1992, 256, 184 CrossRef CAS PubMed.
- I. Benilova, E. Karran and B. De Strooper, Nat. Neurosci., 2012, 15, 349 CrossRef CAS PubMed.
- K. P. Kepp, Prog. Neurobiol., 2016, 143, 36–60 CrossRef CAS PubMed.
- O. Wirths and T. A. Bayer, Life Sci., 2012, 91, 1148–1152 CrossRef CAS PubMed.
- C. Schmitz, B. P. Rutten, A. Pielen, S. Schäfer, O. Wirths, G. Tremp, C. Czech, V. Blanchard, G. Multhaup and P. Rezaie, Am. J. Pathol., 2004, 164, 1495–1502 CrossRef PubMed.
- G. Grasso, A. M. Santoro, V. Lanza, D. Sbardella, G. R. Tundo, C. Ciaccio, S. Marini, M. Coletta and D. Milardi, Coord. Chem. Rev., 2017, 347, 1–22 CrossRef CAS.
- A. Ciechanover and Y. T. Kwon, Exp. Mol. Med., 2015, 47, e147 CrossRef CAS PubMed.
- S. Vijay-Kumar, C. E. Bugg and W. J. Cook, J. Mol. Biol., 1987, 194, 531–544 CrossRef CAS PubMed.
- C. M. Pickart and D. Fushman, Curr. Opin. Chem. Biol., 2004, 8, 610–616 CrossRef CAS PubMed.
- D. A. Nijholt, T. R. de Graaf, E. S. van Haastert, A. O. Oliveira, C. R. Berkers, R. Zwart, H. Ovaa, F. Baas, J. J. Hoozemans and W. Scheper, Cell Death Differ., 2011, 18, 1071 CrossRef CAS PubMed.
- L. Gregori, C. Fuchs, M. E. Figueiredo-Pereira, W. E. Van Nostrand and D. Goldgaber, J. Biol. Chem., 1995, 270, 19702–19708 CrossRef CAS PubMed.
- L. Gregori, J. F. Hainfeld, M. N. Simon and D. Goldgaber, J. Biol. Chem., 1997, 272, 58–62 CrossRef CAS PubMed.
- M. L. Salon, L. Pasquini, M. B. Moreno, J. M. Pasquini and E. Soto, Exp. Neurol., 2003, 180, 131–143 CrossRef.
- S. Song, S.-Y. Kim, Y.-M. Hong, D.-G. Jo, J.-Y. Lee, S. M. Shim, C.-W. Chung, S. J. Seo, Y. J. Yoo and J.-Y. Koh, Mol. Cell, 2003, 12, 553–563 CrossRef CAS PubMed.
- S. Oh, H. S. Hong, E. Hwang, H. J. Sim, W. Lee, S. J. Shin and I. Mook-Jung, Mech. Ageing Dev., 2005, 126, 1292–1299 CrossRef CAS PubMed.
- C. G. Almeida, R. H. Takahashi and G. K. Gouras, J. Neurosci., 2006, 26, 4277–4288 CrossRef CAS PubMed.
- B. P. Tseng, K. N. Green, J. L. Chan, M. Blurton-Jones and F. M. LaFerla, Neurobiol. Aging, 2008, 29, 1607–1618 CrossRef CAS PubMed.
- X. Zhao and J. Yang, ACS Chem. Neurosci., 2010, 1, 655–660 CrossRef CAS PubMed.
- A. V. Morozov, A. A. Kulikova, T. M. Astakhova, V. A. Mitkevich, K. M. Burnysheva, A. A. Adzhubei, P. A. Erokhov, M. B. Evgen'ev, N. P. Sharova and V. L. Karpov, J. Alzheimer's Dis., 2016, 54, 763–776 CAS.
- J. Lowe, A. Blanchard, K. Morrell, G. Lennox, L. Reynolds, M. Billett, M. Landon and R. J. Mayer, J. Pathol., 1988, 155, 9–15 CrossRef CAS PubMed.
- A. Ciechanover and P. Brundin, Neuron, 2003, 40, 427–446 CrossRef CAS PubMed.
- A. Hershko and A. Ciechanover, Annu. Rev. Biochem., 1998, 67, 425–479 CrossRef CAS PubMed.
- J. D. Wright, P. D. Mace and C. L. Day, Trends Biochem. Sci., 2016, 41, 924–937 CrossRef CAS PubMed.
- G. R. Tundo, D. Sbardella, C. Ciaccio, G. Grasso, M. Gioia, A. Coletta, F. Polticelli, D. Di Pierro, D. Milardi and P. Van Endert, Crit. Rev. Biochem. Mol. Biol., 2017, 52, 554–582 CrossRef CAS PubMed.
- M. F. Sciacca, V. Romanucci, A. Zarrelli, I. Monaco, F. Lolicato, N. Spinella, C. Galati, G. Grasso, L. D'Urso and M. Romeo, ACS Chem. Neurosci., 2017, 8, 1767–1778 CrossRef CAS PubMed.
- G. Arena, R. Fattorusso, G. Grasso, G. I. Grasso, C. Isernia, G. Malgieri, D. Milardi and E. Rizzarelli, Chem.–Eur. J., 2011, 17, 11596–11603 CrossRef CAS PubMed.
- V. Lanza, A. Travaglia, G. Malgieri, R. Fattorusso, G. Grasso, G. Di Natale, V. Zito, G. Arena, D. Milardi and E. Rizzarelli, Chem.–Eur. J., 2016, 22, 17767–17775 CrossRef CAS PubMed.
- T. Ebendal, J. Neurosci. Res., 1992, 32, 461–470 CrossRef CAS PubMed.
- J. S. Elam, S. T. Thomas, S. P. Holloway, A. B. Taylor and P. J. Hart, Adv. Protein Chem., 2002, 60, 151–219 CrossRef CAS PubMed.
- L. Aloe, L. Bracci-Laudiero, S. Bonini, L. Manni and L. Aloe, Allergy, 1997, 52, 883–994 CrossRef CAS PubMed.
- S. Vivekanandan, J. R. Brender, S. Y. Lee and A. Ramamoorthy, Biochem. Biophys. Res. Commun., 2011, 411, 312–316 CrossRef CAS PubMed.
- A. Sinz, Mass Spectrom. Rev., 2006, 25, 663–682 CrossRef CAS PubMed.
- J. Rappsilber, J. Struct. Biol., 2011, 173, 530–540 CrossRef CAS PubMed.
- A. Leitner, T. Walzthoeni, A. Kahraman, F. Herzog, O. Rinner, M. Beck and R. Aebersold, Molecular & Cellular Proteomics, 2010 Search PubMed.
- C. Iacobucci, S. Reale and F. De Angelis, ChemBioChem, 2013, 14, 181–183 CrossRef CAS PubMed.
- M. Q. Müller, F. Dreiocker, C. H. Ihling, M. Schäfer and A. Sinz, Anal. Chem., 2010, 82, 6958–6968 CrossRef PubMed.
- C. Iacobucci, M. Götze, C. H. Ihling, C. Piotrowski, C. Arlt, M. Schäfer, C. Hage, R. Schmidt and A. Sinz, Nat. Protoc., 2018, 13, 2864–2889 CrossRef CAS PubMed.
- K. Sokratous, L. V. Roach, D. Channing, J. Strachan, J. Long, M. S. Searle, R. Layfield and N. J. Oldham, J. Am. Chem. Soc., 2012, 134, 6416–6424 CrossRef CAS PubMed.
- R. J Baranello, K. L Bharani, V. Padmaraju, N. Chopra, D. K. Lahiri, N. H. Greig, M. A. Pappolla and K. Sambamurti, Curr. Alzheimer Res., 2015, 12, 32–46 CrossRef.
- L. A. McCord, W. G. Liang, E. Dowdell, V. Kalas, R. J. Hoey, A. Koide, S. Koide and W.-J. Tang, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 13827–13832 CrossRef CAS PubMed.
- F. Bellia and G. Grasso, J. Mass Spectrom., 2014, 49, 274–279 CrossRef CAS PubMed.
- F. Bellia, A. Pietropaolo and G. Grasso, J. Mass Spectrom., 2013, 48, 135–140 CrossRef CAS PubMed.
- G. R. Tundo, E. Di Muzio, C. Ciaccio, D. Sbardella, D. Di Pierro, F. Polticelli, M. Coletta and S. Marini, The FEBS J., 2016, 283, 3755–3770 CrossRef CAS PubMed.
- G. Tundo, C. Ciaccio, D. Sbardella, M. Boraso, B. Viviani, M. Coletta and S. Marini, PloS One, 2012, 7, e34376 CrossRef CAS PubMed.
- V. Lanza, F. Bellia and E. Rizzarelli, Coord. Chem. Rev., 2018, 369, 1–14 CrossRef CAS.
- G. Grasso, E. Rizzarelli and G. Spoto, Biochim. Biophys. Acta, Proteins Proteomics, 2008, 1784, 1122–1126 CrossRef CAS PubMed.
- S. Oddo, A. Caccamo, L. Tran, M. P. Lambert, C. G. Glabe, W. L. Klein and F. M. LaFerla, J. Biol. Chem., 2006, 281, 1599–1604 CrossRef CAS PubMed.
- S. A. Kotler, P. Walsh, J. R. Brender and A. Ramamoorthy, Chem. Soc. Rev., 2014, 43, 6692–6700 RSC.
- M. F. M. Sciacca, C. Tempra, F. Scollo, D. Milardi and C. La Rosa, Biochim. Biophys. Acta, Biomembr., 2018, 1860, 1625–1638 CrossRef CAS PubMed.
- F. Scollo, C. Tempra, F. Lolicato, M. F. Sciacca, A. Raudino, D. Milardi and C. La Rosa, J. Phys. Chem. Lett., 2018, 9, 5125–5129 CrossRef CAS PubMed.
- G. I. Grasso, G. Arena, F. Bellia, E. Rizzarelli and G. Vecchio, J. Inorg. Biochem., 2014, 131, 56–63 CrossRef CAS PubMed.
- V. Oliveri, S. Zimbone, M. L. Giuffrida, F. Bellia, M. F. Tomasello and G. Vecchio, Chem.–Eur. J., 2018, 24, 6349–6353 CrossRef CAS PubMed.
- C. M. Pickart and S. Raasi, in Methods in Enzymology, Academic Press, 2005, vol. 399, pp. 21–36 Search PubMed.
- D. Komander and M. Rape, Annu. Rev. Biochem., 2012, 81, 203–229 CrossRef CAS PubMed.
- J. H. Hurley, S. Lee and G. Prag, Biochem. J., 2006, 399, 361–372 CrossRef CAS PubMed.
- A. Zhang, W. Qi, T. A. Good and E. J. Fernandez, Biophys. J., 2009, 96, 1091–1104 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8sc03394c |
| This journal is © The Royal Society of Chemistry 2019 |