Two studies comparing students’ explanations of an oxidation–reduction reaction after viewing a single computer animation: the effect of varying the complexity of visual images and depicting water molecules
Martin H.
Cole
a,
Deborah P.
Rosenthal
b and
Michael J.
Sanger
 *c
*c
aPhysical Sciences Department, Holyoke Community College, Holyoke, MA 01040, USA
bPhysical Science Department, Bakersfield College, Bakersfield, CA 93305, USA
cDepartment of Chemistry, Middle Tennessee State University, Murfreesboro, TN 37132, USA. E-mail: michael.sanger@mtsu.edu
First published on 22nd May 2019
Abstract
This paper describes two studies comparing students’ explanations of an oxidation–reduction reaction after viewing the chemical demonstration and one of two different particulate-level computer animations. In the first study, the two animations differed primarily in the complexity of the visual images. Students viewing the more simplified animation provided more correct explanations regarding the identity of water and nitrate ions in the animations, the absence of ion pairs, the correct ratios of silver to nitrate ions and silver ions to copper atoms, the electron transfer process, size changes in the atoms and ions as the reaction occurred, the source of blue colour in solution, and the driving force for the reaction. Students viewing the more simplified animation also wrote more correct balanced chemical equations for the reaction compared to students viewing the more complex animation. Students in the first study also noted that the more simplified animation did not depict extraneous information (camera angle changes, the overabundance of water molecules), and did depict relevant information (atom and ion charges, the number of electrons transferred, the source of the blue colour). In the second study, the two animations differed only by whether water molecules were shown or omitted from the animation. Students’ explanations for most concepts were similar for these two groups of students; however, students viewing the animation with water molecules omitted were better able to identify nitrate ions in the animation. The only difference the students in the second study noticed between the two animations is the presence or absence of water molecules, but these student did not agree as to whether showing or omitting water molecules was more beneficial. The results of the two studies together suggest that showing or omitting water molecules in the animations had a limited effect on students’ explanations of the oxidation–reduction process.
Introduction
Electrochemistry and its related topics can be very difficult for students to understand, and several researchers have identified students’ misconceptions and difficulties related to galvanic and electrolytic cells (Garnett and Treagust, 1992b; Sanger and Greenbowe, 1997a; Schmidt et al., 2007; Supasorn, 2015; Loh and Subramaniam, 2018; Tsaparlis, 2018), oxidation–reduction reactions (Garnett and Treagust, 1992a; De Jong et al., 1995; Rosenthal and Sanger, 2012; Brandriet and Bretz, 2014; Lu et al., 2018, 2019), and the electrical conductivity of aqueous solutions (Sanger and Greenbowe, 1997b; Lu and Bi, 2016; Nyachwaya, 2016; Lu et al., 2019). One reason why these concepts may be difficult for students to understand is that explanations of these processes require students to focus their attention on the dynamic processes involving the movement of electrons and other charged particles (Rosenthal and Sanger, 2012; Loh and Subramaniam, 2018; Tsaparlis, 2018). Previous research studies have also shown that the use of computer animations or simulations at the particulate level can help students improve their conceptual understanding of these electrochemistry concepts (Sanger and Greenbowe, 2000; Yang et al., 2003; Talib et al., 2005; Osman and Lee, 2014; Kelly et al., 2017).Several chemical education researchers have demonstrated that the use of computer animations depicting chemical phenomena at the particulate level can improve students’ conceptual understanding of these phenomena on topics other than electrochemistry, especially their particulate-level understanding (Williamson and Abraham, 1995; Russell et al., 1997; Sanger et al., 2000; Sanger et al., 2001; Ardac and Akaygun, 2004; Kelly et al., 2004; Tasker and Dalton, 2006; Kelly and Jones, 2007, 2008; Sanger, 2009; Gregorious et al., 2010a, 2010b; Antonoglou et al., 2011; Williamson et al., 2012; Ryoo et al., 2018). In addition, research studies have shown that learning interventions using computer animations/simulations of chemical phenomena at the particulate level improved students’ spatial abilities (Williamson et al., 2013; Al-Balushi et al., 2017).
In 1998, Roy Tasker described his work on the VisChem Project, a series of three-dimensional computer animations depicting the particulate behaviour of atoms, molecules, and ions (Tasker, 1998). The animations created and subsequently disseminated by this group used an evidence-based, multimedia information-processing model to probe students’ mental models regarding the particulate behaviour of the chemicals before showing them these animations. Tasker (1998, 2005) also pointed out (with some pretty egregious examples) that there were several other chemistry computer animations available at the time that incorrectly depicted the particulate behaviour of atoms, molecules, and ions, and cautioned that the use of these incorrect animations could foster or even generate student misconceptions. However, no research studies were described in which students’ interpretations of these two types of animations were directly analysed and compared with respect to the formation of correct or incorrect conceptions.
Kelly and Jones (2008) asked students to describe the process of dissolving solid sodium chloride in water at the particulate level after viewing a chemical demonstration, and then again after viewing two different particulate-level computer animations depicting the process. Both animations were deemed to have good production quality, correct content, and good illustrations of key features of the dissolving process. One of these animations (created by VisChem) focused on the dynamics and energetics of the dissolving process, while the other animation (created by Prentice Hall) focused on the structural features of the dissolving process. Although Kelly and Jones did not provide a detailed comparison of the students’ responses after viewing the two different animations, they did note that students viewing the Prentice Hall animation made changes to their self-generated solution pictures while those viewing the VisChem animation made changes to their conceptions of the dissolving process. In general, the authors found that the combination of animations led to improved (but not perfectly correct) particulate-level explanations of the dissolving process in the main areas of structure and function. However, incorrect conceptions remained and students often demonstrated difficulty incorporating the depictions shown in the animations into their mental models.
Kelly et al. (2017) asked students to compare two different animations of the same oxidation–reduction reaction of silver nitrate and copper metal: One of them was created to depict the correct electron transfer mechanism while the other was purposefully animated to be inaccurate, depicting “molecules” of silver nitrate reacting with the copper metal and the nitrate ions and one copper ion leaving as a copper(II) nitrate “molecule”. Students created particulate-level drawings and were asked to explain their conceptions of the reaction during interviews. This research study showed that even though most students identified the scientifically accurate animation as being the more correct animation, about half of the students revised their drawings and conceptual explanations of the process to fit the inaccurate animation; fewer changed their drawings/conceptions to match the more complex but more accurate animation. In addition, the vast majority of students thought that both animations were correct and would be useful for understanding the oxidation–reduction process, showing a general inability to determine whether the animations were scientifically correct or not. It should be noted that the more accurate animation used by these researchers is the same animation we used as the more complex animation in this paper and other publications by Rosenthal and Sanger (2012, 2013a, 2013b). This animation was designed by Roy Tasker for the VisChem Project. The research described in this paper is also different from the work of Kelly et al. (2017) in that all animations used in this paper were designed and animated to be scientifically accurate.
Rosenthal and Sanger (2012) asked students to view the non-narrated versions of two different animations of the same copper metal–silver nitrate oxidation–reduction reaction and to explain their understanding of the oxidation–reduction reaction based on their interpretation of these animations. The goal of this study was to identify the common misconceptions exhibited by students regarding the oxidation–reduction process and the difficulty some students had in correctly interpreting the visual images depicted in both animations. Many of the common errors found in this study (misidentifying the depicted water molecules and nitrate ions, predicting incorrect ion charges, incorrect silver to nitrate ion and silver to copper ratios, etc.) served as the basis for comparing students’ conceptual understanding of the oxidation–reduction process after viewing the two animations in Rosenthal and Sanger's subsequent studies (2013a, 2013b), including this paper.
Using the misconceptions and misinterpretations identified in their first study, Rosenthal and Sanger (2013a) compared how the order of viewing the two non-narrated animations affected the participants’ particulate-level explanations of the oxidation–reduction reaction. The results of this study showed that participants who viewed the more complex animation followed by the more simplified animation gave better explanations of the oxidation–reduction process than the participants who viewed the more simplified animation followed by the more complex animation. As one student explained, “the [more complex] one would get their attention and get them interested and get them wondering, and then the [more simplified] one would break it down.” (Rosenthal and Sanger, 2013a, p. 335). A recent study (Chen et al., 2016) comparing students’ learning gains from simulations using a simplified versus a more complex (scale-realistic) astronomical model of planetary distances showed that students had better conceptual gains for scale-neutral questions when using the more complex simulation followed by the more simplified simulation than when using the more simplified simulation followed by the more complex model. These researchers hypothesised that the attractiveness of the simple model may prevent further learning from the more complex model. In a subsequent study, Rosenthal and Sanger (2013b) examined how viewing one of the animations affected the students’ ensuing explanations of the other animation. Viewing the more complex animation before viewing the more simplified animation had no significant impact on students’ explanations of the more simplified animation. Viewing the more simplified animation before viewing the more complex animation, however, improved students’ explanations of the more complex animation. The researchers hypothesised that the more simplified animation provided more iconic information that helped the students interpret the symbolic information in the more complex animation. When students viewed the more simplified animation followed by the more complex animation, it impaired their ability to explain the source of the blue colour in the solution. Rosenthal and Sanger (2013b) felt the viewers of the more complex animation expected the colour change depicted in the more simplified animation to be shown in the more complex one and became confused when that didn’t happen.
Research questions
Even though our research group has made several comparisons of students’ responses after viewing the more simplified or more complex animations, we have yet to fully describe a direct comparison of students’ responses after viewing a single animation. The goal of the first study in this paper is to describe the differences in students’ conceptual understanding of this oxidation–reduction process after viewing either the more simplified or the more complex animation used in previous studies (Rosenthal and Sanger, 2012, 2013a, 2013b) and the important differences the students perceived between the attributes of these two animations that have many different features, but differ primarily in the visual complexity depicted in the animations.Research Question 1: How does viewing a computer animation with differing levels of visual complexity affect students’ conceptual understanding of the oxidation–reduction process depicted in these animations?
Research Question 2: After viewing both animations, what do students think are the important differences between how the two animations depict visual information regarding the oxidation–reduction process?
The results of the first study (and our previous research investigations) found significant differences in the way students interpreted the two particulate-level animations, but because these animations depicted so much information in very different ways, it was difficult to attribute which of these many differences were responsible for the differences in the students’ responses identified in these studies. The impetus for the second study in this paper was to create two different animations that differ only by a single variable (showing or omitting the potentially distracting water molecules in the animation) and to determine whether this single variable will affect students’ particulate-level explanations of the oxidation–reduction process. Participants in this study were also asked to describe the important differences they perceived between the attributes of the two animations.
Research Question 3: How does viewing a computer animation that shows or omits water molecules affect students’ conceptual understanding of the oxidation–reduction process depicted in these animations?
Research Question 4: After viewing both animations, what do students think are the important differences between how the two animations depict visual information regarding the oxidation–reduction process?
Students in both studies were asked, after viewing both animations in their respective studies, which of the two animations (or combination of the two) was helpful in learning about oxidation–reduction reactions, and which animations should be shown to future students to help them learn this information. The student responses regarding which animations should be used in the future were compared.
Research Question 5: After viewing both animations, which animations do the students believe should be used in the future to teach students about the oxidation–reduction reaction depicted in these animations?
Theoretical framework
Underlying the theoretical framework of this study is the idea that there are three distinct, but related representations used by chemists to describe chemical reactions and other phenomena (Tasker, 2005; Johnstone, 2006, 2010; Gilbert and Treagust, 2009; Talanquer, 2011). The macroscopic representation describes qualitative observations made by chemists using their five senses while the particulate representation describes the properties and interactions of atoms, molecules, and ions. Symbols (numbers, chemical symbols, chemical formulas, balanced equations, etc.), the basis of the third representation used by chemists, represent more abstract concepts. One's ability to transform one form of representation to another is described as representational competence (Sanger, 2009), and this skill is critically important if students are to understand many complex chemical phenomena (Thomas, 2017). Assisting students in the development of representational competence is the reason why many particulate-level computer animations are used in the chemistry classroom (Suits and Sanger, 2013).The explanation for the effectiveness of computer animations as educational tools comes from Mayer's cognitive theory of multimedia learning (Mayer, 2009). This theory was adapted by Mayer from Paivio's dual-coding theory (Paivio, 1986) and Baddeley's model of working memory (Baddeley, 1986). Mayer's cognitive theory of multimedia learning posits that learners have two separate cognitive channels used for processing visual (pictorial) and auditory (verbal) information, that learners have limited processing capabilities for each channel, and that learners are actively engaged in learning by attending to relevant information, organizing information into their mental schema, and integrating this new knowledge with their pre-existing knowledge. Mayer's theory also incorporates cognitive load theory (Sweller, 1994; Sweller and Chandler, 1994), which states that if the cognitive load of a learning event exceeds the limits of the learner's working memory, then learning will be negatively impacted. Although intrinsic cognitive load is based on the content to be learned, extraneous cognitive load is a function of how the instructional lesson is presented and any load imposed on the lesson based on the instructional design of the lesson wastes cognitive resources without improving learning (Lee et al., 2006). Although Mayer's theory does not apply solely to computer animations (i.e., it is valid for static pictures as well), it is certainly relevant to animations.
Several researchers have published summaries or meta-analyses on the effectiveness of computer animations. Sanger (2009) summarised several chemistry-specific education research studies involving computer animations and found that animations can be more effective than no additional instruction, traditional instruction involving no static pictures, and instruction involving static pictures similar to the animations. In addition, Sanger noted that animations can provide instructors with a way to introduce the particulate level of chemistry to students in the classroom, but they can also cause pedagogical problems if students misinterpret the visual images used in these animations. Höffler and Leutner (2007) performed a meta-analysis comparing the instructional effectiveness of computer animations with limited interactivity to static pictures. They found that lessons using animations had an instructional advantage over similar lessons involving static images, and that this superiority of animations over static images also applied to animations whose depicted motions were relevant to the information to be learned (i.e., not just decorative) and whose learning goals involved declarative or problem-solving knowledge. In a subsequent meta-analysis comparing computer animations to static pictures, Berney and Betrancourt (2016) also found that instruction involving computer animations was superior to comparable instruction involving static pictures. In addition, they found that this animation effect was present for factual and conceptual knowledge; when students were asked to remember, understand, or apply information; when the animations allowed no student control or interactivity; and when audio or no narrations were provided. This study also found a medium effect size (0.773) for research studies specifically involving chemistry content. Both meta-analyses also noted that individual learner characteristics, such as spatial ability or prior knowledge, can impact the effectiveness of instruction involving animations or static pictures.
Lowe and co-workers (Lowe, 2004; Lowe and Boucheix, 2008; Lowe, 2014) observed that the design and creation of many educational computer animations has been led more by intuition than research-based evidence, and that almost all of these animations focused on providing a time-faithful (“behaviourally realistic”) presentation of information to the learner. They also noted that existing theories related to learning with animations (e.g., Mayer, 2009) treated animations the same way they treated static pictures and focused solely on the cognitive aspects of learning. Lowe and Boucheix (2008) proposed a new model for learning with animations, the Animation Processing Model, that focuses on both the perceptual and cognitive processes used by learners and on both bottom-up (stimulus driven) and top-down (knowledge driven) contributions to information processing. This model has five hierarchical stages, although novices in the content area tend to focus on bottom-up strategies involving the first three levels. In Stage 1, novices parse the presented information and focus on creating “event units” consisting of a visual object and its associated behaviours; in Stage 2, novices form relationships between the event units from Stage 1, condensing them into more extensive dynamic “micro chunks” that are still isolated in space and time; Stage 3 involves integrating these micro chunks across space and time to create a well-structured internal characterisation of the animation. Stage 3 requires learners to have domain-specific knowledge that is needed to help structure the developing mental model, prevent learners from misinterpreting the information presented, and reduce the extent to which the learners are susceptible to irrelevant but salient perceptual cues (Lowe and Boucheix, 2008).
The differential effect on learning that can occur due to the use of more or less visually complex images in the computer animations can either be explained by the coherence principle from Mayer's cognitive theory of multimedia learning (Mayer, 2009) or cognitive load theory (Sweller, 1994; Sweller and Chandler, 1994). Mayer's coherence principle states that students learn more effectively when extraneous material or information is excluded from the animation. These “seductive details” (as Mayer calls them) represent information that might prove to be interesting to the learner, but does not provide any relevant information for learning the content of interest. As such, these seductive details serve to catch and divert the learner's attention away from more relevant information being presented in the animation (Garner et al., 1989; Moreno and Mayer, 2000; Harp and Maslich, 2005). But what if the information presented by the more complex images in a computer animation is not extraneous and is relevant to the content to be learned? Relevant information can also overload a learner's working memory if the intrinsic cognitive load of the lesson or the extraneous cognitive load introduced by relevant but complex images in the computer animations become too high (Sweller, 1994).
Lastly, the implementation and interpretation of the research studies in this paper has been informed by variation theory (Bussey et al., 2013; Kelly, 2014). Variation theory is used to explain why different learners who experience the same instructional lesson often learn different things and at different levels. This theory focuses on which instructional features a learner pays attention to since these features and the meaning placed on them determine an individual's perception of a given learning event and the important aspects within it. Any research study informed by variation theory must examine three aspects of learning (intended, enacted, and lived objects of learning) and their relationships. The intended object of learning considers the instructor's perspective and represents what the instructor intends students to learn from the lesson. The enacted object of learning represents what actually happened during the lesson and is dictated by the interactions of the students, the instructor, and the instructional materials. The lived object of learning focuses on the student's perception of the lesson and what the student was actually able to learn from the lesson. Bussey et al. (2013) have modified the variation theory to include the effects of the learner's prior knowledge and skills (which affects what information students perceive and attend to, impacting the lived object of learning) and the effects of the instructional materials design (which affects how the instructional lesson is presented, impacting the enacted object of learning). Previous research on learning with animations have noted that the individual differences associated with the learner's spatial ability or prior knowledge can have a large impact on the effectiveness of learning using animations (Höffler and Leutner, 2007; Lowe and Boucheix, 2008; Berney and Betrancourt, 2016).
All of the animations used in these studies were created to teach the same chemistry concepts related to oxidation–reduction reactions, but use different visual images to convey this information to the learner. The intended objects of learning in both studies are represented by the content questions posed to the participants; these represent the information the researchers (as instructors) expected students to learn from viewing each of the animations. The lived objects of learning were measured by the participant's responses to these content questions and the extent to which they learned the content the researchers had intended them to learn from the animations (Research Questions 1 and 3). The enacted object of learning, which is concerned with the students’ perceived differences (variations) in the lessons provided by the two different animations, was measured by asking the participants to compare the two different animations and discuss how they are different (Research Questions 2, 4, and 5). Although the differences that students deem to be important (critical features) may not be the same as those identified by the researchers or animation designers, they allow us to focus on the students’ perceptions of the learning environment and what information they think was important and necessary for learning to occur.
Study 1: comparing student responses to the more simplified versus the more complex animation
Visual complexity study – methods
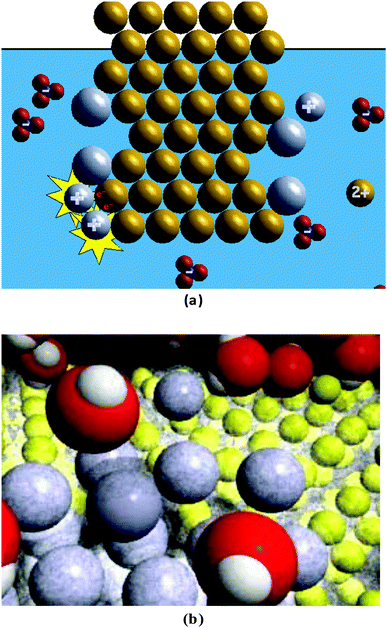
Fig. 1b provides a screen shot of the more complex (MC) animation, which was created by Roy Tasker as part of the VisChem project (Tasker and Dalton, 2006) and used with his permission. The animation contains several yellow spheres in a 3-D pattern in front of a black background representing the copper metal. In between and surrounding each of the yellow spheres (copper nucleus and core electrons) is a light grey “fuzziness” (valence electrons). The cluster of yellow/light grey spheres is surrounded by several red spheres with two white spheres attached to them (water molecules). Among the red/white shapes are a few grey spheres (silver ions). Occasionally, a cluster with a blue sphere surrounded by three red spheres (nitrate ions) appears. During the animation, a grey sphere moves towards the yellow/light grey cluster. When they touch, a transparent light grey sphere encompasses the grey sphere (electron transfer) and the grey/light grey sphere stays attached to the yellow/light grey cluster. At another place on the yellow/light grey cluster, a yellow sphere loses its light grey “fuzziness” and leaves the yellow/light grey cluster, mixing among the red/white shapes. For every yellow sphere leaving the cluster, two grey spheres attach to the cluster.
 | ||
| Fig. 2 The interview questions used during the semi-structured interviews in the visual complexity study (based on information presented in Rosenthal and Sanger, 2012). | ||
Visual complexity study – results for research question 1
After viewing either the more simplified or the more complex animation, students’ explanations were compared for the following concepts: identifying water in the animation, identifying nitrate ions in the animation, recognizing the absence of ion pairs in solution, recognizing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of silver and nitrate ions, recognizing a 2
1 ratio of silver and nitrate ions, recognizing a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reacting ratio of silver ions and copper atoms, explaining the electron transfer process, recognizing size changes of the silver ion and copper atom, identifying the source of the blue colour in solution, recognizing that water is not the driving force for this reaction, and writing a balanced equation for the reaction. A summary of the statistical data for these comparisons is given in Table 1.
1 reacting ratio of silver ions and copper atoms, explaining the electron transfer process, recognizing size changes of the silver ion and copper atom, identifying the source of the blue colour in solution, recognizing that water is not the driving force for this reaction, and writing a balanced equation for the reaction. A summary of the statistical data for these comparisons is given in Table 1.
| Animation effect | Covariate | |||||||
|---|---|---|---|---|---|---|---|---|
| Concept | df | F value | p value | MC scores (st. dev.) | MS scores (st. dev.) | df | F value | p value |
| a p < 0.05 corresponds to a significant difference between explanations of the two animations. b p < 0.05 corresponds to a significant relationship between the dependent variable and the covariate. c A test of proportions (z-test) was performed due to a lack of variability in the responses from one of the student groups. | ||||||||
| Identifying water | 1, 53 | 7.660 | 0.008a | 0.621 (0.075) | 0.923 (0.079) | — | — | — |
| Identifying nitrate ions | 1, 53 | 18.162 | 0.000a | 0.448 (0.077) | 0.923 (0.081) | — | — | — |
| Absence of ion pairs | 1, 52 | 20.738 | 0.000a | 0.414 (0.077) | 0.923 (0.081) | 1, 52 | 0.010 | 0.921 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 silver–nitrate ratio (interviews) 1 silver–nitrate ratio (interviews) |
1, 52 | 43.793 | 0.000a | 0.000 (0.064) | 0.615 (0.067) | 1, 52 | 0.085 | 0.771 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 silver–nitrate ratio (balanced equations) 1 silver–nitrate ratio (balanced equations) |
1, 52 | 7.479 | 0.009a | 0.578 (0.068) | 0.856 (0.072) | 1, 52 | 18.309 | 0.000b |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 silver–copper ratio (interviews)c 1 silver–copper ratio (interviews)c |
— | 7.416 | 0.000a | 0.000 (0.135) | 1.000 (0.135) | — | — | — |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 silver–copper ratio (balanced equations) 1 silver–copper ratio (balanced equations) |
1, 52 | 28.129 | 0.000a | 0.279 (0.067) | 0.804 (0.071) | 1, 52 | 15.781 | 0.000b |
| Electron transfer process | 1, 52 | 251.190 | 0.000a | 0.357 (0.117) | 3.102 (0.124) | 1, 52 | 65.541 | 0.000b |
| Atom/ion size changes | 1, 53 | 39.237 | 0.000a | 0.552 (0.138) | 1.808 (0.146) | — | — | — |
| Source of the blue colour in solution | 1, 52 | 27.581 | 0.000a | 0.797 (0.100) | 1.573 (0.106) | 1, 52 | 67.760 | 0.000b |
| Water is not the driving force (ignoring nitrates) | 1, 52 | 13.016 | 0.000a | 0.630 (0.068) | 0.990 (0.072) | 1, 52 | 0.686 | 0.411 |
| Water is not the driving force (including nitrates) | 1, 52 | 41.626 | 0.000a | 0.360 (0.065) | 0.983 (0.069) | 1, 52 | 2.193 | 0.145 |
| Writing a balanced equation for the reaction | 1, 52 | 9.027 | 0.004a | 14.88 (0.680) | 17.87 (0.719) | 1, 52 | 3.158 | 0.066 |
The fact that several of the covariate scores are statistically significant indicates that the students’ prior knowledge of oxidation–reduction reactions affected their interpretations of the animations, which is consistent with previous research showing that prior knowledge can affect learning using animations (Höffler and Leutner, 2007; Lowe and Boucheix, 2008; Berney and Betrancourt, 2016).
After viewing the more simplified (MS) animation, many students felt confident that the silver or copper(II) ions would not be associated with the nitrate ions. However, those students who misidentified the red/white clusters in the more complex animation (MC) as nitrate ions confused the interactions of silver or copper(II) ions with water molecules as representing ion pairs in solution.
Interviewer: Is that what you thought silver nitrate would look like in the container?
MS Student: Uh, no.
Interviewer: What did you think it would look like?
MS Student: I thought the silvers and the nitrates would be connected.
MC Student: Silver came in with the reds and whites, so silver nitrate came in and then it goes to the copper… We see two things come together so it has to be copper and nitrate.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 ratio of silver and nitrate ions.
Roughly 20% of students in both groups explicitly mentioned a 1
1 ratio of silver and nitrate ions.
Roughly 20% of students in both groups explicitly mentioned a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 silver-to-nitrate ratio in the chemical demonstration interviews. Of the students who viewed the more simplified animation, 62% specifically mentioned this 1
1 silver-to-nitrate ratio in the chemical demonstration interviews. Of the students who viewed the more simplified animation, 62% specifically mentioned this 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in their interviews; 0% of the students viewing the more complex animation mentioned the proper ratio, F(1, 52) = 43.793, p < 0.001. For the 38% of students viewing the more simplified animation who did not mention a 1
1 ratio in their interviews; 0% of the students viewing the more complex animation mentioned the proper ratio, F(1, 52) = 43.793, p < 0.001. For the 38% of students viewing the more simplified animation who did not mention a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, the interview transcripts showed no mention of the silver-to-nitrate ratio at all. In the group of students viewing the more complex animation, 11 students (38%) believed that there were more silver ions than nitrate ions. The more complex animation shows fewer nitrate ions compared to the silver ions (presumably since the nitrate ions are spectators and could be omitted from the more complex animation for clarity); however, this depiction does seem to have affected students’ conceptual understanding of the chemical system. Another 38% of the students who viewed the more complex animation believed that there were more nitrate ions than silver ions. These students seemed to misinterpret the red/white shapes (water molecules) as nitrate ions, and as a result saw many more of these “nitrate ions” compared to silver ions.
1 ratio, the interview transcripts showed no mention of the silver-to-nitrate ratio at all. In the group of students viewing the more complex animation, 11 students (38%) believed that there were more silver ions than nitrate ions. The more complex animation shows fewer nitrate ions compared to the silver ions (presumably since the nitrate ions are spectators and could be omitted from the more complex animation for clarity); however, this depiction does seem to have affected students’ conceptual understanding of the chemical system. Another 38% of the students who viewed the more complex animation believed that there were more nitrate ions than silver ions. These students seemed to misinterpret the red/white shapes (water molecules) as nitrate ions, and as a result saw many more of these “nitrate ions” compared to silver ions.
Interviewer: [What are the] red/white things?
MC Student: I say they’re nitrates.
Interviewer: Let's talk about ratios. How many silvers and how many nitrates should we have together?
MC Student: One silver, two nitrates.
Interviewer: Does it [animation] have equal amounts?
MC Student: A lot more nitrates than silvers.
It is possible that some of the students who failed to explicitly mention the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio still believed that the silver and nitrate ions were present in equal amounts. So, we decided to evaluate the chemical formulas for silver nitrate in their self-generated balanced equations. For the chemical demonstration part of the interview, 77% of students viewing the more simplified animation and 55% of students viewing the more complex animation wrote the formula for silver nitrate as ‘AgNO3’. After viewing the animations, 92% of students seeing the more simplified animation and 59% of students viewing the more complex animation wrote balanced equations showing a 1
1 ratio still believed that the silver and nitrate ions were present in equal amounts. So, we decided to evaluate the chemical formulas for silver nitrate in their self-generated balanced equations. For the chemical demonstration part of the interview, 77% of students viewing the more simplified animation and 55% of students viewing the more complex animation wrote the formula for silver nitrate as ‘AgNO3’. After viewing the animations, 92% of students seeing the more simplified animation and 59% of students viewing the more complex animation wrote balanced equations showing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 silver-to-nitrate ratio, F(1, 52) = 7.479, p = 0.009.
1 silver-to-nitrate ratio, F(1, 52) = 7.479, p = 0.009.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 reacting ratio of silver ions and copper atoms.
About 23% of students viewing the more simplified animation and 0% of students viewing the more complex animation explicitly mentioned a 2
1 reacting ratio of silver ions and copper atoms.
About 23% of students viewing the more simplified animation and 0% of students viewing the more complex animation explicitly mentioned a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reacting ratio for the silver ions and the copper atoms in the chemical demonstration interviews. After viewing the animations, 100% of students viewing the more simplified animation and 0% of students viewing the more complex animation specifically mentioned this 2
1 reacting ratio for the silver ions and the copper atoms in the chemical demonstration interviews. After viewing the animations, 100% of students viewing the more simplified animation and 0% of students viewing the more complex animation specifically mentioned this 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in their interviews. Because there was no variability in the responses from the more complex group (i.e., every student gave the same answers before and after viewing the animation), an ANCOVA score could not be calculated. So, we performed a test of proportions instead, z = 7.416, p < 0.001.
1 ratio in their interviews. Because there was no variability in the responses from the more complex group (i.e., every student gave the same answers before and after viewing the animation), an ANCOVA score could not be calculated. So, we performed a test of proportions instead, z = 7.416, p < 0.001.
The more simplified animation depicts two silver ions and one copper atom undergoing electron transfer at the same time and place while the more complex animation depicts the reduction of two silver ions and the oxidation of one copper atom as happening at different times and on different places on the copper metal surface. These results suggest that the realistic depiction of the oxidation–reduction process shown in the more complex animation confused students regarding the relative number of silver ions and copper atoms involved in the oxidation–reduction reaction. This difference could also be explained by Lowe's Animation Processing Model (Lowe and Boucheix, 2008; Lowe, 2014)—since the more simplified animation showed the silver ion–copper atom reaction happening at the same place and time in the animation, interpreting this ratio only required students to work at Stage 2, but since the more complex animation showed this reaction happening at different times and at different spots of the copper surface, students would have to have been working at Stage 3 in order to integrate these micro chunks from Stage 2 across space and time to create a well-structured internal characterisation of this ratio from the animation.
Some of the students viewing the more complex animation thought the reacting ratio was close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a few thought it was close to 2
1, a few thought it was close to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 but weren’t sure and told us they were guessing, and others thought it might be 3
1 but weren’t sure and told us they were guessing, and others thought it might be 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 5
1 or 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
Interviewer: Can you tell the relative amounts? How many coppers or silvers [reacting]?
MC Student: It looks like at least two silvers to one copper, at least.
Interviewer: Guess or obvious?
MC Student: Not real obvious. They show [a] cluster of silver and every once in a while a copper comes out.
Since it is possible for students to believe in the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reacting ratio without explicitly mentioning it, we evaluated the reacting ratios in their self-generated balanced equations. For the chemical demonstration part of the interview, 23% of students viewing the more simplified animation and 14% of students viewing the more complex animation wrote a 2
1 reacting ratio without explicitly mentioning it, we evaluated the reacting ratios in their self-generated balanced equations. For the chemical demonstration part of the interview, 23% of students viewing the more simplified animation and 14% of students viewing the more complex animation wrote a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reacting ratio for silver nitrate and copper metal. After viewing the animations, 85% of students viewing the more simplified animation and 24% of students viewing the more complex animation showed a 2
1 reacting ratio for silver nitrate and copper metal. After viewing the animations, 85% of students viewing the more simplified animation and 24% of students viewing the more complex animation showed a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reacting ratio for silver nitrate and copper metal, F(1, 52) = 28.129, p < 0.001.
1 reacting ratio for silver nitrate and copper metal, F(1, 52) = 28.129, p < 0.001.
MC Student: The water is taking away the copper ion to make copper nitrate, and silver just stays solid. Silver is getting dropped off; silver is bundling up together. If silver is getting compacted, it must be solid. Copper is attracting the silver and once the silver is compact, the copper is taken away by the water and nitrogen [nitrate] is floating around too so that makes copper nitrate. It looks like the silver nitrate is trading out to be copper nitrate. So it would be copper nitrate and silver solid.
MS Student: You see two silver atoms react with the copper, and one copper is released that has a +2 charge. So that must mean that two electrons were donated from the copper, one to each silver.
It appears that explicitly showing the number of electrons being transferred and the charges on the atoms and the ions, both before and after the reaction, helped students better understand the electron transfer process.
Interviewer: Anything happen to the size [of the silver ion when it attached to the copper surface]?
MC Student: Maybe, if you count the almost transparent casing, but it is not real clear… but it could have gotten bigger.
Interviewer: Yes or no?
MC Student: I didn’t see it get bigger.
MC Student: I think the nitrate and the copper bonds together is causing the blue colour to form, [be]cause if you look at silver and nitrate [red/white cluster], nothing is formed until we switch it and you have copper and nitrate. That still leaves the question, what is that blue thing? I think a foreign object…
Interviewer: Does the animation show why the solution turns blue?
MC Student: No, [be]cause you only saw that one molecule with the blue in it. You did not see how it was formed. It just floated by. Hi, Bye.
MC Student: It [animation] makes it look like the water is doing everything. Causing the reaction – bringing silver to copper.
MC Student: Now the reaction is occurring… Now, the nitrates [red/white clusters] go for the copper. We started with copper solid. Nitrates are coming in, taking them [points to copper ions] and stacking them [points to silver atoms] up.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio for the reactants and for the products than those viewing the more complex animation.
1 stoichiometric ratio for the reactants and for the products than those viewing the more complex animation.
Visual complexity study – results for research question 2
After viewing both animations, students were asked to provide a list of strengths and weaknesses for the two animations, and how the animations were different from each other. These responses were compiled and categorized to create list of common ideas expressed (in one form or another) by at least 10% of the total population. These common ideas include: The usefulness of the computer animations, the complexity of the computer animations, depicting water molecules, showing the charges of the atoms and ions, showing the electrons as particles, and recognizing the source of the blue colour in solution.MS Student: It helped give an idea of what is going on at the atomic level… it is hard to imagine what is going on, even though she [our instructor] told us but if she had shown this, it would be easier to remember. But I have never been in chemistry class where any animation was shown. I think that is why I have such a hard time with even simple things like writing equations because you can imagine a periodic table all day long, but I can’t imagine what is going on [at the molecular level].
MS Student: If we could study all the reactions we study in chemistry with animations like that we would all be making A's.
MC Student: [The more complex animation] confused me. I think it would make me think, though. It would actually, like, challenge me to think about what's going on instead of… we are always being spoon-fed information. And… that demo actually made me, like… I seriously had to sit here and think and now you know, like, we strung it all together… I am definitely going to remember this tomorrow. I am not going to forget it when I walk out that door.
MC Student: It is very attractive, but swoopy and moving around and too hard to follow, with too much stuff going on.
MC Student: It showed what was happening, but did not explain it.
MC Student: …this 3D one might be more helpful with commentary or someone narrating it, explaining, because it is obviously more involved and accurate because it shows the Brownian motion of the copper – all the atoms moving because it shows the atoms moving, [be]cause they are not at absolute zero and it shows the electron clouds. But the problem is, it shows it all at once and the camera never stops moving and it is hard to pick out the relevant information… [be]cause I don’t really feel like I am gaining anything by the camera orbiting around, but on the other hand you kind of have to zoom in on parts.
Five students (9%) mentioned that Tasker's animation was difficult to follow because the camera angle was constantly changing throughout the animation, and they also implied that the combination of changing camera angles and the number of objects being depicted on the screen overloaded their thought processes.
MC Student: It is a nice animation to watch. Then it moves.
Interviewer: Oh, the camera moves?
MC Student: Yeah, the viewing angle is weird. It looks like the camera is trying to get what is happening over the entire surface of the copper wire in as short of time as possible and if it would focus on one area, you would see copper is leaving and silver is coming, but it is trying to get too much …
MS Student: The only thing I don’t like is this one does not show the water. If you could just take this one and show water molecules in it.
MS Student: Maybe put waters [molecules in the animation], but it would be too confusing. Just make sure to say the blue background is water.
The more complex animation did depict the water molecules, showing that aqueous solutions (like silver nitrate solution in this reaction) are composed of a large number of water molecules compared to the number of ions dissolved in the water. Although eight students (15%) felt that depicting so many water molecules was a strength, eight students (15%) felt that including so many water molecules confused them. Some students who incorrectly identified the red/white clusters as nitrate ions also claimed that there were too many nitrate ions in the system based on the formula for silver nitrate. The identity of the red/white clusters is explained in the audio portion of Tasker's animation that was disabled as part of this study.
MC Student: They could have done with less water. It is not confusing; it is distracting… It [the solution] has a lot of water. I get that they are trying to get that across.
Interviewer: Is there anything you would do to fix that?
MC Student: Take out all the water molecules. It is a good animation for really advanced [students] if you already understand the concepts, but if it is the first time you encounter the reaction and have not talked about it, it is really confusing.
MC Student: The main thing again is that there is just seems to be a surplus of nitrate, which is not realistic in regards to the equation itself. So it skews my perception. I would presume there is a lot more nitrate needed if I had not seen the bottle with silver nitrate written on it. I might write six N-O-3 to one A-G, but it looks like 25.
MS Student: Main thing about [the animation] is the charges. It showed exactly which was gaining and losing [electrons], and the states, and the ratio.
Interviewer: What is your general feeling about the animation? Is it helpful?
MS Student: It was helpful in some parts. We got our equation right [be]cause we looked at it.
Interviewer: What part of the animation helped with the equation?
MS Student: We could not remember if the nitrate was a minus one or minus two charge.
The more complex animation does not explicitly label the charges on the atoms or ions, and six students (11%) felt that this was a definite weakness of the animation. Some of these students also felt that the lack of charges also prevented them from understanding the chemical reaction and hindered their ability to write a balanced equation for this reaction.
MC Student: I think this one makes it harder for me to understand, [be]cause… there are not charges depicted in this animation.
Interviewer: Balanced equation? Does this animation make you change your answer?
MC Student: I don’t believe so. The only issue that remains is the charges. If I knew the charges of silver and copper, I would know charges of nitrate and that would change my subscripts.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 silver–copper stoichiometry.
1 silver–copper stoichiometry.
MS Student: I liked where you can see the electrons moving. In the demonstration, you do not see the molecules moving out, so it kind of helps you understand what is going on… For me, watching the two silver hit before the copper was released with the exchange of electrons and watch it get bigger and smaller really helped me visual[ise] what is going on in the beaker.
For the more complex animation, four students (7%) felt that the depiction of the electron cloud for the valence electrons in copper metal was more realistic. However, four students (7%) also believed that the way this animation depicted the electron transfer process was vague or confusing. The student quote below shows that he recognized the realistic nature of depicting bulk copper metal using a field of electrons, and that the electron transfer process occurred because electrons were transferred from copper atoms to silver ions. However, he could not determine how many electrons were transferred during the oxidation–reduction process.
MC Student: It is more realistic picture, as far as showing the packing structure. Copper solids here.
Interviewer: Did it help you with any charges, or phases, or sizes?
MC Student: It showed that there was a charge change, but not what it was. There was just a white cloud. It confirms to me that we learned in class how electrons can just float everywhere, [be]cause each copper atom does not have its own cloud. The cloud surrounding the whole solid.
Recognizing the source of the blue colour in solution
Thirteen students (24%) believed that the more simplified animation was more helpful in enabling them to recognize aqueous copper(II) ions as the source of the blue colour in this solution. Some of these students also noted that the animation helped them determine that it was the copper(II) ion and not the copper(II) nitrate that caused the blue colour. None of the students mentioned this as a strength or weakness for the more complex animation.MS Student: It also showed as copper was released, the water changed colour.
MS Student: I noticed colour change. Animation of copper not bonding to nitrate helps.
Study 2: comparing student responses to the animation depicting water molecules versus the animation omitting water molecules
Water molecules study – methods
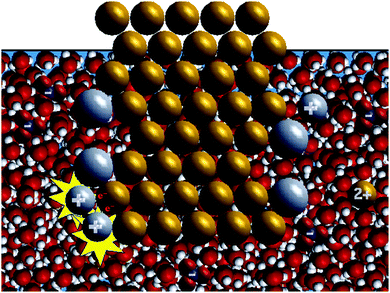 | ||
| Fig. 3 Screen shot for the animation showing water molecules used in the water molecules study. The animation in Fig. 1a was used as the water-omitted animation in the water molecules study. | ||
The Qualtrics platform allowed the participants to view the video demonstration and animations as many times as they wanted during the survey. The surveys were designed so that when a participant completed a section of the survey, they could not return to it. Students accessed the surveys with a web address provided by Qualtrics using desktop computers in the third author's office for the students participating in the semi-structured interviews or in a computer lab for students in the survey group. Qualtrics collected and tabulated individual student responses into one report for later data analysis.
After viewing the demonstration, one group of students (8 interview and 28 survey students) watched the 24-second non-narrated animation of the copper metal–silver nitrate reaction showing water molecules and answered the questions in Part 2 of the interview protocol in Fig. 4, followed by viewing the 24-second non-narrated animation with water molecules omitted and answering the interview questions in Part 3 of the protocol. The other group of students (7 interview and 29 survey students) viewed the animations in the reverse order and answered the same questions in Parts 2 and 3 of the interview protocol. The students were allowed to watch the video demonstration and the two animations as many times as they wanted. Part 4 of the interview protocol asked students to indicate which animation they thought was more useful in understanding the oxidation–reduction process. They were also asked which animation they would show in class and if they chose both, which animation would they show first.
Water molecules study – results for research question 3 (after viewing one animation)
After viewing one of the animations, students’ answers to the questions in Part 2 of Fig. 4 were analysed for the following concepts: identifying the nitrate ions in the animations, the presence of ion pairs in the solution, describing the electron transfer process, explaining the driving force, recognizing size changes of the silver ion and copper atom, recognizing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of silver and nitrate ions, recognizing a 2
1 ratio of silver and nitrate ions, recognizing a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reacting ratio of silver ions and copper atoms, and writing a balanced equation for the reaction. A summary of the statistical data for these comparisons is given in Table 2. In contrast to the visual complexity study, only one comparison was found to be significant: identifying nitrate ions.
1 reacting ratio of silver ions and copper atoms, and writing a balanced equation for the reaction. A summary of the statistical data for these comparisons is given in Table 2. In contrast to the visual complexity study, only one comparison was found to be significant: identifying nitrate ions.
| Concept | df | F value | p value |
|---|---|---|---|
| a p < 0.05 corresponds to a significant difference between student responses to questions about the two animations. | |||
| Identifying nitrate ions | 1, 71 | 6.387 | 0.014a |
| Presence of ion pairs | 1, 71 | 0.878 | 0.352 |
| Electron transfer process | 1, 71 | 0.412 | 0.532 |
| Driving force | 1, 71 | 0.000 | 1.000 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 silver/nitrate ratio 1 silver/nitrate ratio |
1, 71 | 0.000 | 1.000 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 silver/copper ratio 1 silver/copper ratio |
1, 71 | 0.235 | 0.629 |
| Writing a balanced equation for the reaction | 1, 71 | 3.075 | 0.084 |
WS Student: [T]he water molecules made it more difficult to focus and find the nitrate molecules.
WS Student: With all of the water molecules, it became confusing to differentiate the ions from the water molecules. It became very busy.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of silver and nitrate ions (F(1, 71) = 0.000, p = 1.000), recognizing a 2
1 ratio of silver and nitrate ions (F(1, 71) = 0.000, p = 1.000), recognizing a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reacting ratio of silver ions and copper atoms (F(1, 71) = 0.235, p = 0.629), and writing a balanced equation for the reaction (F(1, 71) = 3.075, p = 0.084). This is in stark contrast to what was found in the visual complexity study, in which students’ explanations for all of the concepts were better for the students viewing the more simplified animation that did not depict water molecules. While the animations in the visual complexity study differed by several aspects (water molecules shown or not, charges shown or not, electrons depicted as particles or clouds, etc.), the animations in this study differed only by the presence or absence of water molecules. The results of this study seem to indicate that it was not showing or omitting the water molecules in the visual complexity study that led to the significant differences in students’ explanations of those two animations for any concept except identifying nitrate ions.
1 reacting ratio of silver ions and copper atoms (F(1, 71) = 0.235, p = 0.629), and writing a balanced equation for the reaction (F(1, 71) = 3.075, p = 0.084). This is in stark contrast to what was found in the visual complexity study, in which students’ explanations for all of the concepts were better for the students viewing the more simplified animation that did not depict water molecules. While the animations in the visual complexity study differed by several aspects (water molecules shown or not, charges shown or not, electrons depicted as particles or clouds, etc.), the animations in this study differed only by the presence or absence of water molecules. The results of this study seem to indicate that it was not showing or omitting the water molecules in the visual complexity study that led to the significant differences in students’ explanations of those two animations for any concept except identifying nitrate ions.
Water molecules study – results for research question 3 (after viewing both animations)
The students’ answers after viewing both animations were also analysed for the following concepts: identifying the nitrate ions in the animations, the presence of ion pairs present in the solution, describing the electron transfer process, explaining the driving force, recognizing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of silver and nitrate ions, recognizing a 2
1 ratio of silver and nitrate ions, recognizing a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reacting ratio of silver ions and copper atoms, and writing a balanced equation for the reaction. The statistical results of these comparisons appear in Table 3. None of these comparisons were statistically significant, and this suggests that there was no order effect (i.e., the order in which students viewed the animations did not significantly change their answers); this is also in direct contrast to the order effect studies performed using the animations from the visual complexity study (Rosenthal and Sanger, 2013a, 2013b).
1 reacting ratio of silver ions and copper atoms, and writing a balanced equation for the reaction. The statistical results of these comparisons appear in Table 3. None of these comparisons were statistically significant, and this suggests that there was no order effect (i.e., the order in which students viewed the animations did not significantly change their answers); this is also in direct contrast to the order effect studies performed using the animations from the visual complexity study (Rosenthal and Sanger, 2013a, 2013b).
| Concept | df | F value | p value |
|---|---|---|---|
| Identifying nitrate ions | 1, 70 | 0.400 | 0.529 |
| Presence of ion pairs | 1, 70 | 0.034 | 0.855 |
| Electron transfer process | 1, 70 | 0.586 | 0.447 |
| Driving force | 1, 70 | 1.045 | 0.310 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 silver/nitrate ratio 1 silver/nitrate ratio |
1, 70 | 0.680 | 0.412 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 silver/copper ratio 1 silver/copper ratio |
1, 70 | 0.090 | 0.766 |
| Writing a balanced equation for the reaction | 1, 70 | 0.230 | 0.633 |
Students’ explanations for the driving force question were analysed and four major themes emerged from this analysis (Phelps, 1994). These themes for the driving force of this reaction were: Differences in the electronegativity of copper and silver was the driving force, the reaction occurs to reach stability (ΔG is the driving force), the nitrate ions were the driving force, or some other process/concept (e.g., collision theory) was the driving force. Examples of student quotes illustrating each category are listed below.
Electronegativity. WS Student: Because copper is more [sic] electronegative than silver.
WO Student: Silver is more electronegative than copper in this reaction…
Stability (ΔG). WS Student: The reaction occurs because the copper metal and the silver nitrate want to be as stable as possible.
WO Student: The two substances are trying to reach equilibrium and become as stable as possible.
Nitrate ions. WS Student: The reaction occurs because the affinity between the nitrate and the copper ions is stronger than the affinity between the aluminium [sic] and the nitrate ions.
WO Student: It occurs because the Nitrate reacts with the Copper forming Copper Nitrate on the solid.
Other. WS Student: it is reacting magnetically to the silver nitrate.
WO Student: silver is gains [sic] an electron and copper is losing one.
Table 4 contains a summary of the students’ explanations for the driving force in both groups after viewing the chemical demonstration, the first animation, and the second animation. Most of the students provided explanations in the ‘Other’ category. The number and identity of the students in each group providing electronegativity arguments (the “correct” answer) stayed the same throughout the experiment; these five students did not change their answers during the study, and no other students changed their answers from an incorrect answer to the correct answer as part of this study, which explains why no significant difference was seen with the ANOVA comparison of correct versus incorrect answers.
| Category | WS first, WO second | WO first, WS second | ||||
|---|---|---|---|---|---|---|
| Demo | Anim #1 | Anim #2 | Demo | Anim #1 | Anim #2 | |
| Electronegativity | 3 | 3 | 3 | 2 | 2 | 2 |
| Stability (ΔG) | 5 | 5 | 4 | 8 | 8 | 6 |
| Nitrate ions | 8 | 4 | 4 | 5 | 3 | 7 |
| Other | 20 | 24 | 25 | 21 | 23 | 21 |
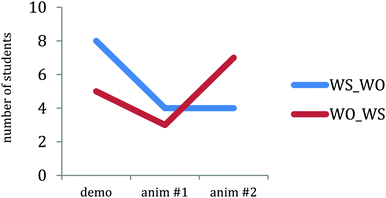
Fig. 5 shows a plot of the number of students in each group claiming that the nitrate ions were the driving forces for this reaction. After viewing the first animation, fewer students in both groups made statements claiming that the nitrate ions were the driving force for the reaction. However, there is an order effect after viewing the second animation: After initially viewing the animation with water molecules, none of the students in the WS_WO group changed their driving force answers to reference the nitrate ions after viewing the animation without water molecules; however, four students in the WO_WS group who initially viewed the animation without water molecules changed their driving force answers to include nitrate ions after viewing the animation with water molecules (z = 2.06, p = 0.02). This result is consistent with the result found by Rosenthal and Sanger (2013a), which showed that 90% of students viewing more complex animation followed by the more simplified animation in Fig. 1 correctly identified the nitrate ions, while only 46% of students viewing the more simplified animation followed by more complex animation correctly identified the nitrate ions. A quote from one of the WO_WS students who changed their answers after viewing the animation with water molecules appears below:
WO_WS Student: [After viewing the animation without water molecules] …random motion and collisions are driving this reaction, nitrates seem to be bystanders.
Same student: [After viewing the animation with water molecules] It looks like the nitrate facilitated the collision of copper and silver reacting.
Water molecules study – results for research question 4
After viewing both animations, the students only mentioned one difference between the two animations: whether the water molecules were shown or omitted from the animation. This difference was mentioned by every student who discussed how the two animations differed from each other. It is interesting to note that some students considered showing the water molecules to be an advantage while others thought that it was a disadvantage. When asked which animation helped them understand the oxidation–reduction process better, more students (52) said they preferred the animation without water molecules, while fewer students (19) preferred the animation showing water molecules; only one student left this question blank (χ2(1) = 14.222, p < 0.001). The first two comments below came from students who considered the animation omitting water molecules to be more useful in understanding the oxidation–reduction process and the last comment came from a student who believed that the animation showing water molecules was more useful.WS/WO Student: There was too much red and small with dots [in the WS animation] to be able to clearly see what was going on. Picking out [t]he Nitrogen was way more difficult with water represented in the animation.
WO/WS Student: With all of the water molecules, it became confusing to differentiate the ions from the water molecules. It became very busy.
WO/WS Student: It [WS animation] shows the H2O ions [sic] carrying the nitrate and silver ions towards the copper and bumping the copper out and then picking up the copper and leaving with it.
Results for research question 5 – which animations should be shown in class?
Students in both the visual complexity and the water molecules studies were asked which animation they thought should be shown in future classes when teaching about oxidation–reduction reactions. The results for this question appear in Table 5. For the visual complexity study, 14 of the 55 students (25%) felt that only the animation without water molecules should be shown, but none of these students felt that only the animation with water molecules should be shown or that neither animation should be shown. The other 75% felt that both animations should be shown. Of these students, 26 of the 41 (63%) said that the animation without water molecules should be shown first while the other 15 students (37%) felt that the animation with water molecules should be shown first. For the water molecules study, 18 of the 72 (25%) felt that only the animation without water molecules should be shown, 12 out of the 72 (17%) felt that only the animation with water molecules should be shown, and 4 of the 72 (6%) felt that neither animation should be shown. The other 53% felt that both animations should be shown: 17 of those 38 students (45%) felt that the animation without water molecules should be shown first while the other 21 (55%) felt that the animation with water molecules should be shown first. The distribution of students choosing each option was found to be significantly different for the students in the two studies (χ2(4) = 17.42, p = 0.002).| Study | Neither | WO only | WS only | WO then WS | WS then WO |
|---|---|---|---|---|---|
| Visual complexity study (N = 55) | 0 | 14 | 0 | 26 | 15 |
| Water molecules study (N = 72) | 4 | 18 | 12 | 17 | 21 |
An analysis of the residual scores showed that fewer students in the visual complexity study and more students in the water molecules study felt that the instructor should show only the animation with water molecules present to students studying oxidation–reduction reactions. For students in the visual complexity study, the water-shown animation (the “more complex” animation by Tasker) and the water-omitted animation (the “more simplified” animation by Sanger) had several differences in addition to whether water molecules were shown or not (ion charges shown or absent, electrons were shown as a cloud or as particles, colour change of solution shown or absent, static or moving camera angles, etc.). For the students in the water molecules study, however, the only difference between the two animations was whether the water molecules were shown or absent. Based on the results of the chi-square analysis, it appears that simply adding water molecules to the water-omitted animation in the water molecules study did not cause students to think that showing this animation alone would negatively affect future students’ abilities to learn about oxidation–reduction reactions. However, the many differences between the two animations in the visual complexity study (including showing or omitting water molecules) did cause these students to believe that showing the water-omitted animation alone would negatively affect future students’ abilities to learn about oxidation–reduction reactions.
Conclusions
In the first study described in this paper (visual complexity study), students’ explanations of the oxidation–reduction reaction occurring between aqueous silver nitrate and solid copper metal were compared after viewing the chemical demonstration and either a more simplified or more complex animation depicting the same chemical reaction at the particulate level. The statistical analyses showed that students viewing the more simplified animation were able to provide more correct explanations than students viewing the more complex animation related to the absence of ion pairs, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of silver and nitrate ions, a 2
1 ratio of silver and nitrate ions, a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reacting ratio of silver ions and copper atoms, the electron transfer process, the size changes of atoms and ions as the reaction occurs, the source of the blue colour in solution, and the fact that water was not driving this reaction to occur. In addition, students were better at identifying the depictions of water and nitrate ions in the more simplified animation compared to the more complex animation. Students viewing the more simplified animation also provided more correct self-generated balanced equations than students viewing the more complex animation, suggesting that the more simplified animation may have been better at helping students’ develop their representational competence (Sanger, 2009; Thomas, 2017) as they converted the particulate-level images they had seen into a symbolic-level balanced chemical equation. In general, it appears that instruction including the more simplified animation may have provided a more useful learning environment (enacted object of learning) that helped students develop a more robust mental model (lived object of learning) of the oxidation–reduction process (Bussey et al., 2013).
1 reacting ratio of silver ions and copper atoms, the electron transfer process, the size changes of atoms and ions as the reaction occurs, the source of the blue colour in solution, and the fact that water was not driving this reaction to occur. In addition, students were better at identifying the depictions of water and nitrate ions in the more simplified animation compared to the more complex animation. Students viewing the more simplified animation also provided more correct self-generated balanced equations than students viewing the more complex animation, suggesting that the more simplified animation may have been better at helping students’ develop their representational competence (Sanger, 2009; Thomas, 2017) as they converted the particulate-level images they had seen into a symbolic-level balanced chemical equation. In general, it appears that instruction including the more simplified animation may have provided a more useful learning environment (enacted object of learning) that helped students develop a more robust mental model (lived object of learning) of the oxidation–reduction process (Bussey et al., 2013).
To probe the differences in the two learning environments created by these animations, the visual complexity study also analysed comments from students regarding what they believed made the two different animations useful in understanding the oxidation–reduction reaction. These student comments suggested that one reason why they had difficulty interpreting the more complex animation is that it depicted extraneous material that distracted them from paying attention to the important information being shown. In particular, the changes in camera angle and the overabundance of water molecules in this animation were mentioned by students as major sources of distraction. Student comments also suggested that the more simplified animation provided more explicit (and more useful) depictions of information that was vital in understanding the oxidation–reduction reaction. These depictions included explicitly labelling the atom and ion charges, clearly showing the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of silver and nitrate ions and the 2
1 ratio of silver and nitrate ions and the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reacting ratio of silver ions and copper atoms, explicitly showing the size changes of the atoms and ions as the reaction occurs, clearly showing the number of electrons transferred, and showing that the blue colour of the solution became darker after each copper(II) ion was released. Some of these concepts were also depicted by the more complex animation (e.g., the 2
1 reacting ratio of silver ions and copper atoms, explicitly showing the size changes of the atoms and ions as the reaction occurs, clearly showing the number of electrons transferred, and showing that the blue colour of the solution became darker after each copper(II) ion was released. Some of these concepts were also depicted by the more complex animation (e.g., the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reacting ratio and the size changes), but because of the complexity of this animation, students were often unable to see or correctly interpret this information. Some of these concepts, however, were not depicted by the more complex animation (e.g., atom/ion charges, the number of electrons transferred, the source of the blue colour); only one of these concepts appeared to be incorrectly depicted—the more complex animation showed more silver ions compared to nitrate ions in the reaction; presumably, this was done to reduce the complexity of the animation by showing fewer of the nitrate ions that are spectators in this reaction. Unfortunately, students noticed this simplification and it seemed to confuse or mislead some of those students. The more simplified animation showed each oxidation–reduction event occurring in the same spot and at the same time, compared to the more complex animation that showed these events as happening on different spots on the copper surface and at different times. As a result, for some concepts (such as the 2
1 reacting ratio and the size changes), but because of the complexity of this animation, students were often unable to see or correctly interpret this information. Some of these concepts, however, were not depicted by the more complex animation (e.g., atom/ion charges, the number of electrons transferred, the source of the blue colour); only one of these concepts appeared to be incorrectly depicted—the more complex animation showed more silver ions compared to nitrate ions in the reaction; presumably, this was done to reduce the complexity of the animation by showing fewer of the nitrate ions that are spectators in this reaction. Unfortunately, students noticed this simplification and it seemed to confuse or mislead some of those students. The more simplified animation showed each oxidation–reduction event occurring in the same spot and at the same time, compared to the more complex animation that showed these events as happening on different spots on the copper surface and at different times. As a result, for some concepts (such as the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reacting ratio of the silver ions and copper atoms and the electron transfer process) the more complex animation required students to work at a higher level in the Animation Processing Model (Lowe and Boucheix, 2008; Lowe, 2014) to understand the same concepts than the more simplified animation did, and that could explain why students were more successful in learning from the more simplified versus the more complex animation for those concepts.
1 reacting ratio of the silver ions and copper atoms and the electron transfer process) the more complex animation required students to work at a higher level in the Animation Processing Model (Lowe and Boucheix, 2008; Lowe, 2014) to understand the same concepts than the more simplified animation did, and that could explain why students were more successful in learning from the more simplified versus the more complex animation for those concepts.
Kelly et al. (2017) also asked students to provide particulate-level explanations (and drawings) of this oxidation–reduction reaction after viewing the same VisChem animation that was used in the visual complexity study (the more complex animation). This provides an opportunity to compare the results of two different research studies using the same animation; it should be noted that Kelly's study used the narrated version of this animation in their study while our study used a non-narrated version of the same animation. Kelly et al. (2017) noted that students often conflated the macroscopic properties of the chemical demonstration and the behaviour of the particles in the oxidation–reduction process, and students often depicted these macroscopic properties in their particulate-level drawings of the chemical reaction. They also observed that students struggled with trying to depict the macroscopic colour change of the solution in their particulate drawings. In general, they noted that, “…some students found it challenging to understand what the more complicated EEA [VisChem animation] depicted without a macroscopic connection” (Kelly et al., 2017, p. 591). Students in the visual complexity study of this paper also seemed to have difficulty interpreting what was causing the colour change in the VisChem animation, and it appears that showing the solution colour change at the macroscopic level in the more simplified animation in the visual complexity study appeared to provide scaffolding that helped students interpret the particulate-level changes responsible for the macroscopic-level colour change (Sanger, 2009; Thomas, 2017).
Students’ explanations of water's possible behaviours in this reaction range from present but not involved in the process (“watching”) to present and involved but not driving the reaction (“assisting”) to present and involved and driving the reaction (“causing”). The goal of animations depicting this reaction should be to help students see that water molecules are present and involved but not driving the reaction. Kelly et al. (2017) found that after viewing both animations (the non-VisChem one showing water molecules as “watching” and the VisChem animation showing water molecules as “assisting”), students were more likely to draw pictures showing water molecules “watching”. Some students justified this idea by stating that since the water molecules do not appear in the symbolic balanced equation then they could not be involved in the particulate-level chemical reaction. The visual complexity study in this paper and other research studies (Tasker and Dalton, 2006; Rosenthal and Sanger, 2012, 2013a, 2013b), on the other hand, have noted that the way this VisChem animation depicts hydrated water molecules and their behaviours during this reaction actually caused students to believe that water molecules were the driving force for (“causing”) this reaction.
The results of the visual complexity study showed that students had much more success (and less difficulty) in interpreting the images presented in the more simplified animation compared to the more complex animation. However, these two animations depict the same copper metal–silver nitrate oxidation–reduction reaction in very different ways—including showing or omitting water molecules, the colour changes of the solution as the reaction occurs, the charges of the ions in solution, correct silver to nitrate ratio, and changes in the camera angle; depicting the oxidation–reduction process as an event that occurs at the same place and time or showing it happening in different places and at different times; showing the electrons as particles or as a sphere of “fuzziness”; etc. Since there are so many differences in the way these animations depicted the oxidation–reduction process, it is impossible to determine from the visual complexity study alone which of these differences had a significant impact on students’ abilities to interpret the images depicted in these animations and which of these differences were largely irrelevant. The goal of the water molecules study in this paper was to determine whether one of these factors (showing or omitting the water molecules in the animation) significantly affected students’ abilities to understand the oxidation–reduction process.
The second study described in this paper (water molecules study) examined student responses to questions about the copper metal–silver nitrate oxidation–reduction reaction after viewing a particulate-level animation of the reaction that differed only by showing or omitting water molecules. Analysis of the data indicated there were no differences between student responses for the two groups to questions about the presence of ion pairs in solution, the electron transfer process, recognizing the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of silver ion and nitrate ion, recognizing the 2
1 ratio of silver ion and nitrate ion, recognizing the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reacting ratio of silver ions and copper atoms, and in writing a balanced equation for the reaction. The only significant difference between the responses from the two groups occurred when trying to identify the nitrate ions. Students viewing the animation showing water molecules had more difficulty correctly identifying the nitrate ions. The water molecules were depicted as a red sphere with two white spheres and the nitrate ions were depicted as a blue sphere with three red spheres. Both molecules were in motion during the animation and this “sea of red” would, undoubtedly, be a distraction when attempting to find and correctly identify the nitrate ions in the animation. Both studies presented in this paper found that having water molecules present confused students about the correct identity of the nitrate ions, and in both studies students misinterpreted the red-white water molecules as nitrate ions and attributed the motions and behaviours of the water molecules to the nitrate ions. Rosenthal and Sanger (2013a, 2013b) found significant order effects when using the two animations in the visual complexity study. In the water molecules study, however, no significant order effects were seen for any of the questions. Based on the results of the water molecules study, it seems reasonable to conclude that the significant order effects found by Rosenthal and Sanger (2013a, 2013b) were not the result of the presence or absence of water molecules depicted in the two animations.
1 reacting ratio of silver ions and copper atoms, and in writing a balanced equation for the reaction. The only significant difference between the responses from the two groups occurred when trying to identify the nitrate ions. Students viewing the animation showing water molecules had more difficulty correctly identifying the nitrate ions. The water molecules were depicted as a red sphere with two white spheres and the nitrate ions were depicted as a blue sphere with three red spheres. Both molecules were in motion during the animation and this “sea of red” would, undoubtedly, be a distraction when attempting to find and correctly identify the nitrate ions in the animation. Both studies presented in this paper found that having water molecules present confused students about the correct identity of the nitrate ions, and in both studies students misinterpreted the red-white water molecules as nitrate ions and attributed the motions and behaviours of the water molecules to the nitrate ions. Rosenthal and Sanger (2013a, 2013b) found significant order effects when using the two animations in the visual complexity study. In the water molecules study, however, no significant order effects were seen for any of the questions. Based on the results of the water molecules study, it seems reasonable to conclude that the significant order effects found by Rosenthal and Sanger (2013a, 2013b) were not the result of the presence or absence of water molecules depicted in the two animations.
In the water molecules study, we identified four categories for the students’ explanations of the driving force for this reaction: electronegativity arguments, stability (ΔG) arguments, viewing nitrate ions as the driving forces, and other miscellaneous arguments (including blank answers, “I don’t know”, and descriptions of what happened in the oxidation–reduction process and not why it happened). Although no significant order effects were found in the water molecules study for the correct answers, a significant order effect was found for one of the incorrect student responses as to why the reaction occurred (nitrate ions as the driving force). After viewing either animation, students were less likely to attribute nitrate ions as the driving force than they were before viewing either animation. Students who viewed the animation with water molecules shown first and then saw the animation with water molecules omitted second did not change their beliefs about nitrate's role in this reaction. However, students who viewed the animation with water molecules omitted first were more likely to suggest nitrates were driving the reaction after viewing the animation with water molecules shown. It is likely that this order effect is due to the increased likelihood that students viewing the animation showing the red and white water molecules would confuse these water molecules with nitrate ions and would attribute the behaviours of these water molecules to the nitrate ions.
Students in the water molecules study only identified one difference between the two animations—the presence or absence of water molecules. Unlike the visual complexity study, in which most student comments about the differences between the two animations seemed to favour a single animation, these comments seemed to have mixed results as to whether showing or omitting the water molecules would be more useful to future students. This result seems to suggest that showing or omitting water molecules in the two animations used in the visual complexity study is probably not responsible for students’ opinions regarding which animation would be more useful to future students.
Students in both studies of this paper preferred the animation without water molecules over the animation showing water molecules. Students in the visual complexity study identified several issues that caused them to favour the more simplified animation over the more complex animation: it was less complex, it didn’t show water molecules, it showed ion charges, it showed electrons as particles, and it showed the blue colour of the solution. Students from both studies felt that the animation that did not show water molecules was less confusing and less distracting than the animation showing water molecules. While both groups had more students who would show only the animation without water molecules than those who would show only the animation with water molecules, when given a choice as to which animation(s) they would show in class, the majority of students in both studies suggested that both animations be shown. The students felt that showing both animations provided a more complete picture of the copper metal–silver nitrate oxidation–reduction at the particulate level. This is consistent with the results from Kelly et al. (2017), which found that, even when one of the animations was purposefully animated to be inaccurate, students still saw merit in both animations and felt that future students could learn from both animations. Students in the visual complexity study were less likely to suggest showing only the animation without water molecules compared to students in the water molecules study. One reason for this difference could be that the animations in the visual complexity study differ by more than just whether water molecules were shown or omitted, and it could be those other differences that made the students in the visual complexity study less likely to recommend showing only the animation with water molecules.
Limitations and implications
One major limitation to both of these studies is that the animations were used without the supporting narration. Mayer's multimedia learning theory (Mayer, 2009), as well as Paivio's dual coding theory (Paivio, 1986) and Baddeley's working memory model (Baddeley, 1986), asserts that students will learn better if provided information using both the visual and verbal channels (animations with narration) rather than using only the visual channel (non-narrated animation). Results from the visual complexity study suggest that students were better at interpreting the more simplified animation without the assistance of narration, but had difficulty interpreting the more complex animation without narration. It is possible that many of the differences in the students’ explanations found in the visual complexity study may disappear or at least be diminished if the students had viewed the animations with narration, but further study would be needed to test this assertion. Kelly et al. (2017) found that students still experienced difficulty interpreting the VisChem animation used in the visual complexity study even with the narration included. These results suggest that animators should carefully consider the narrations that accompany any animation to ensure that these narrations assist student learning by focusing on and explaining the relevant information (scaffolding), and minimizing or downplaying the effects of extraneous information depicted by the animation. Several researchers studying animations have observed that learning with animations can affect (Williamson et al., 2013; Al-Balushi et al., 2017) or be affected by (Höffler and Leutner, 2007; Lowe and Boucheix, 2008; Berney and Betrancourt, 2016) the learner's spatial ability. Therefore, additional research studies should be performed to see whether the students’ spatial abilities would affect (or be affected by) their interpretations of the animations used in this study.Based on student-supplied quotes from the visual complexity study, Mayer's cognitive theory of multimedia (Mayer, 2009) can be used to explain why students who viewed the more simplified animation may have provided more correct and more complete explanations of the oxidation–reduction reaction depicted in the two animations than those who viewed the more complex animation. First, the more complex animation depicted extraneous material (the presence of water molecules and changing camera angles) that may have distracted students from identifying and attending to the important information being shown and overloading the students’ visual working memory with extraneous information, consistent with Mayer's coherence principle (Mayer, 2009). Second, the more simplified animation depicted more explicit images (ion charges, electrons as particles, solution colour changes) that may have helped students in selecting and organizing relevant images in their active cognitive processing of the presented material (Mayer, 2009). Other researchers (Chen et al., 2016; Kelly et al., 2017) have found that simpler animations/simulations are more attractive to students than similar but more complicated animations/simulations, even when they impart more incorrect or incomplete knowledge to the learner compared to the more complex models. Chen et al. (2016) also postulated that a simpler model may pose obstacles for further student progress because it “anchors students’ understanding, and they appear reluctant to change their conceptualization when exposed to a model that requires a higher cognitive load”. This suggestion makes it even more imperative that designers of simpler animated sequences ensure that they depict scientifically accurate information and that simplifications requiring the use of incorrect or incomplete images or animated events should be avoided. The Animation Processing Model (Lowe and Boucheix, 2008; Lowe, 2014) can also be used to explain differences in students’ abilities to interpret these two animations. Lowe and Boucheix (2008) noted that learners tend to be most affected by the negative aspects of an animation's dynamic motions if they are novices in the content area and if the animation's images are very complex. More complex images can affect both the learner's ability to correctly interpret the depicted images/motions and their ability to work with the large cognitive demands associated with the additional, more complex images. This is especially true for animations that are designed to be temporally and spatial consistent with the animated system (behaviourally realistic) because a learner can be distracted by irrelevant moving objects that catch the learner's attention but provide little relevant information to understand the concept of interest. In the case of the more complex animation, the constant movement of the water molecules may catch the student's attention but their motions provide little to no useful information to explain the electron transfer process between the silver ions and the copper atom in the oxidation–reduction reaction depicted by the animation. Since the more simplified animation does not show the water molecules, the students are less likely to be distracted from the interactions of the silver ions and the copper atoms in the animation.
In the water molecules study, the only difference in the visual images presented by the two animations was the presence or absence of the potentially distracting water molecules. The water molecules study showed that the addition of the water molecules in the animation had a very limited impact on students’ understanding of the oxidation–reduction process occurring in the animations, and only seemed to affect their ability to correctly identify the red-white objects as water molecules and not nitrate ions. This result implies that whatever difficulty students in the visual complexity study had in interpreting the visual images of the more complex animation, the presence of water molecules in the animation did not have a huge distracting effect on these interpretations. Future research studies should investigate the other differences between the two animations in the visual complexity study to determine which of these differences are responsible for the differences in the students’ explanations of the oxidation–reduction process. Our research group is currently working on another study that is investigating the effect of showing or omitting the ion charges and on showing valence electrons as particles or as an outer shell on an atom.
Conflicts of interest
There are no conflicts to declare.References
- Al-Balushi S. M., Al-Musawi A. S., Ambusaidi A. K. and Al-Hajri F. H., (2017), The effectiveness of interacting with scientific animations in chemistry using mobile devices on grade 12 students’ spatial ability and scientific reasoning skills, J. Sci. Educ. Technol., 26, 70–81.
- Antonoglou L. D., Charistos N. D. and Sigalas M. P., (2011), Design, development and implementation of a technology enhanced hybrid course on molecular symmetry: students' outcomes and attitudes, Chem. Educ. Res. Pract., 12, 454–468.
- Ardac D. and Akaygun S., (2004), Effectiveness of multimedia-based instruction that emphasizes molecular representations on students' understanding of chemical change, J. Res. Sci. Teach., 41, 317–337.
- Baddeley A. D., (1986), Working memory, Oxford: Oxford University Press.
- Berney S. and Betrancourt M., (2016), Does animation enhance learning? A meta-analysis, Comput. Educ., 101, 150–167.
- Boo H. K., (1998), Students’ understandings of chemical bonds and the energetics of chemical reactions, J. Res. Sci. Teach., 35, 569–581.
- Brandriet A. R. and Bretz S. L., (2014), Measuring meta-ignorance through the lens of confidence: examining students’ redox misconceptions about oxidation numbers, charge, and electron transfer, Chem. Educ. Res. Pract., 15, 729–746.
- Bussey T. J., Orgill M. K. and Crippen K. J., (2013), Variation theory: a theory of learning and a useful theoretical framework for chemical education research, Chem. Educ. Res. Pract., 14, 9–22.
- Butts B. and Smith R., (1987), HSC chemistry students' understanding of the structure and properties of molecular and ionic compounds, Res. Sci. Educ., 17, 192–201.
- Chen C., Schneps M. H. and Sonnert G., (2016), Order matters: sequencing scale-realistic versus simplified models to improve science learning, J. Sci. Educ. Technol., 25, 806–823.
- De Jong O., Acampo J. and Verdonk A., (1995), Problems in teaching the topic of redox reactions: actions and conceptions of chemistry teachers, J. Res. Sci. Teach., 32, 1097–1110.
- Garner R., Gillingham M. G. and White C. S., (1989), Effects of “seductive details” on macroprocessing and microprocessing in adults and children, Cogn. Instr., 6, 41–57.
- Garnett P. J. and Treagust D. F., (1992a), Conceptual difficulties experienced by senior high school students of electrochemistry: electric circuits and oxidation-reduction equations, J. Res. Sci. Teach., 29, 121–149.
- Garnett P. J. and Treagust D. F., (1992b), Conceptual difficulties experienced by senior high school students of electrochemistry: electrochemical (galvanic) and electrolytic cells, J. Res. Sci. Teach., 29, 1079–1099.
- Gilbert J. K. and Treagust D. F., (2009), Towards a coherent model for macro, submicro, and symbolic representations in chemical education, in Gilbert J. K. and Treagust D. F. (ed.), Models and modeling in science education: multiple representations in chemical education, Dordrecht: Springer-Verlag, pp. 333–350.
- Glaser B. G. and Strauss A. L., (1967), The discovery of grounded theory: strategies for qualitative research, New York: Aldine.
- Gregorious R. M., Santos R., Dano J. B. and Guiterrez J. J., (2010a), Can animations effectively substitute for traditional teaching methods? Part I: preparation and testing materials, Chem. Educ. Res. Pract., 11, 253–261.
- Gregorious R. M., Santos R., Dano J. B. and Guiterrez J. J., (2010b), Can animations effectively substitute for traditional teaching methods? Part II: potential differentiated learning, Chem. Educ. Res. Pract., 11, 262–266.
- Harp S. F. and Maslich A. A., (2005), The consequences of including seductive details during lecture, Teach. Psych., 32, 100–103.
- Höffler T. N. and Leutner D., (2007), Instructional animation versus static pictures: a meta-analysis, Learn. Instr., 17, 722–738.
- Johnstone A. H., (2006), Chemical education research in Glasgow in perspective, Chem. Educ. Res. Pract., 7, 49–63.
- Johnstone A. H., (2010), You can’t get there from here, J. Chem. Educ., 87, 22–29.
- Kelly R. M., (2014), Using variation theory with metacognitive monitoring to develop insights into how students learn from molecular visualizations, J. Chem. Educ., 91, 1152–1161.
- Kelly R. M. and Jones L. L., (2007), Exploring how different features of animations of sodium chloride dissolution affect students' explanations, J. Sci. Educ. Technol., 85, 303–309.
- Kelly R. M. and Jones L. L., (2008), Investigating students’ ability to transfer ideas learned from molecular animations to the dissolution process, J. Chem. Educ., 85, 303–309.
- Kelly R. M., Phelps A. J. and Sanger M. J., (2004), The effects of a computer animation on students’ conceptual understanding of a can-crushing demonstration at the macroscopic, microscopic, and symbolic levels, Chem. Educ., 9, 184–189.
- Kelly R. M., Akaygun S., Hansen S. J. R. and Villalta-Cerdas A., (2017), The effect that comparing molecular animations of varying accuracy has on students’ submicroscopic explanations, Chem. Educ. Res. Pract., 18, 582–600.
- Lee H., Plass J. L. and Homer B. D., (2006), Optimizing cognitive load for learning from computer-based science simulations, J. Educ. Psych., 98, 902–913.
- Liu X. and Lesniak K., (2006), Progression in children's understanding of the matter concept from elementary to high school, J. Res. Sci. Teach., 43, 320–347.
- Loh A. S. L. and Subramaniam R., (2018), Mapping the knowledge structure exhibited by a cohort of students based on their understanding of how a galvanic cell produces energy, J. Res. Sci. Teach., 55, 777–809.
- Lowe R. K., (2004), Animations and learning: value for money? in Atkinson R., McBeath C., Jonas-Dwyer D. and Phillips R. (ed.), Beyond the comfort zone: Proceedings of the 21st ASCILITE Conference, Perth: ACSILITE, pp. 558–561.
- Lowe R. K., (2014), Dynamic visualizations: a two-edged sword?, in Huang W. (ed.), Handbook of human centric visualization, New York: Springer, pp. 581–604.
- Lowe R. K. and Boucheix J.-M., (2008), Learning from animated diagrams: how are mental models built? in Stapleton G., Howse J., and Lee J. (ed.), Diagrammatic representation and inference, Berlin: Springer, pp. 266–281.
- Lu S. and Bi H., (2016), Development of a measurement instrument to assess students’ electrolyte conceptual understanding, Chem. Educ. Res. Pract., 17, 1030–1040.
- Lu S., Bi H. and Liu X., (2018), The effects of explanation-driven inquiry on students’ conceptual understanding of redox, Int. J. Sci. Educ., 40, 1857–1873.
- Lu S., Bi H. and Liu X., (2019), A phenomenographic study of 10th grade students’ understanding of electrolytes, Chem. Educ. Res. Pract., 20, 204–212.
- Mayer R. E., (2009), Multimedia learning, Cambridge, UK: Cambridge University Press.
- Moreno R. and Mayer R. E., (2000), A coherence effect in multimedia learning: the case for minimizing irrelevant sounds in the design of multimedia instructional messages, J. Educ. Psych., 92, 117–125.
- Nyachwaya J. M., (2016), General chemistry students’ conceptual understanding and language fluency: acid–base neutralization and conductometry, Chem. Educ. Res. Pract., 17, 509–522.
- Nyachwaya J. M., Mohamed A.-R., Roehrig G. H., Wood N. B., Kern A. L. and Schneider J. L., (2011), The development of an open-ended drawing tool: an alternative diagnostic tool for assessing students' understanding of the particulate nature of matter, Chem. Educ. Res. Pract., 12, 121–132.
- Orgill M. K., (2007), Phenomenography, in Bodner G. M. and Orgill M. K. (ed.), Theoretical frameworks for research in chemistry/science education, Upper Saddle River, NJ: Prentice Hall, pp. 132–151.
- Osman K. and Lee T. T., (2014), Impact of interactive multimedia module with pedagogical agents on students’ understanding and motivation in the learning of electrochemistry, Int. J. Sci. Math. Educ., 12, 395–421.
- Paivio A., (1986), Mental representations: a dual coding approach, New York: Oxford University Press.
- Phelps A. J., (1994), Qualitative methodologies in chemical education research: challenging comfortable paradigms, J. Chem. Educ., 71, 191–194.
- Qualtrics [software], (2015), Provo, UT, USA: Qualtrics, retrieved from http://www.qualtrics.com.
- Rosenthal D. P. and Sanger M. J., (2012), Student misinterpretations and misconceptions based on their explanations of two computer animations of varying complexity depicting the same oxidation–reduction reaction, Chem. Educ. Res. Pract., 13, 471–483.
- Rosenthal D. P. and Sanger M. J., (2013a), How does the order of viewing two computer animations of the same oxidation-reduction reaction affect students’ particulate-level explanations? in Suits J. P. and Sanger M. J. (ed.), Pedagogic roles of animations and simulations in chemistry courses, Washington, DC: American Chemical Society, vol. 1142, pp. 313–340.
- Rosenthal D. P. and Sanger M. J., (2013b), How does viewing one computer animation affect students’ interpretations of another animation depicting the same oxidation–reduction reaction? Chem. Educ. Res. Pract., 14, 286–296.
- Russell J. W., Kozma R. B., Jones T., Wykoff J., Marx N. and Davis J., (1997), Use of simultaneous-synchronized macroscopic, microscopic, and symbolic representations to enhance the teaching and learning of chemical concepts, J. Chem. Educ., 74, 330–334.
- Ryoo K., Bedell K. and Swearingen A., (2018), Promoting linguistically diverse students’ short-term and long-term understanding of chemical phenomena using visualizations, J. Sci. Educ. Technol., 27, 508–522.
- Sanger M. J., (2009), Computer animations of chemical processes at the molecular level, in Pienta N. J., Cooper M. M. and Greenbowe T. J. (ed.), Chemist's guide to effective teaching, Upper Saddle River, NJ: Prentice Hall, vol. II, pp. 198–211.
- Sanger M. J. and Greenbowe T. J., (1997a), Common student misconceptions in electrochemistry: galvanic, electrolytic, and concentration cells, J. Res. Sci. Teach., 34, 377–398.
- Sanger M. J. and Greenbowe T. J., (1997b), Students’ misconceptions in electrochemistry: current flow in electrolyte solutions and the salt bridge, J. Chem. Educ., 74, 819–823.
- Sanger M. J. and Greenbowe T. J., (2000), Addressing student misconceptions concerning electron flow in aqueous solutions with instruction including computer animations and conceptual change strategies, Int. J. Sci. Educ., 22, 521–537.
- Sanger M. J. and Phelps A. J., (2007), What are students thinking when they pick their answer?: a content analysis of students' explanations of gas properties, J. Chem. Educ., 84, 870–874.
- Sanger M. J., Phelps A. J. and Fienhold J., (2000), Using a computer animation to improve students’ conceptual understanding of a can-crushing demonstration, J. Chem. Educ., 77, 1517–1520.
- Sanger M. J., Brecheisen D. M. and Hynek B. M., (2001), Can computer animations affect college biology students’ conceptions about diffusion and osmosis? Am. Biol. Teach., 63, 104–109.
- Schmidt H.-J., Marohn A. and Harrison A. G., (2007), Factors that prevent learning in electrochemistry, J. Res. Sci. Teach., 44, 258–283.
- Smith K. J. and Metz P. A., (1996), Evaluating student understanding of solution chemistry through microscopic representations, J. Chem. Educ., 73, 233–235.
- Smith K. C. and Nakhleh M. B., (2011), University students’ conceptions of bonding and melting and dissolving phenomena, Chem. Educ. Res. Pract., 12, 398–408.
- Suits J. P. and Sanger M. J., (2013), Dynamic visualizations in chemistry courses, in Suits J. P. and Sanger M. J. (ed.), Pedagogic roles of animations and simulations in chemistry courses, Washington, DC: American Chemical Society, vol. 1142, pp. 1–13.
- Supasorn S., (2015), Grade 12 students’ conceptual understanding and mental models of galvanic cells before and after learning by using small-scale experiments in conjunction with a model kit, Chem. Educ. Res. Pract., 16, 393–407.
- Sweller J., (1994), Cognitive load theory, learning difficulty and instructional design, Learn. Instr., 4, 295–312.
- Sweller J. and Chandler P., (1994), Why some material is difficult to learn, Cogn. Instr., 12, 185–233.
- Talanquer V., (2011), Macro, submicro, and symbolic: the many faces of the chemistry “triplet”, Int. J. Sci. Educ., 33, 179–195.
- Talib O., Matthews R. and Secombe M., (2005), Computer-animated instruction and students’ conceptual change in electrochemistry: preliminary qualitative analysis, Int. Educ. J., 5, 29–42.
- Tasker R., (1998), The VisChem project: molecular level animations in chemistry—Potential and caution, UniServe Science News, 9, 12–16.
- Tasker R., (2005), Using multimedia to visualize the molecular world: educational theory into practice, in Pienta N. J., Cooper M. M. and Greenbowe T. J. (ed.), Chemist's guide to effective teaching, Upper Saddle River, NJ: Prentice Hall, vol. I, pp. 195–211.
- Tasker R. and Dalton R., (2006), Research into practice: visualisation of the molecular world using animations, Chem. Educ. Res. Pract., 7, 141–159.
- Thomas G. P., (2017), ‘Triangulation’: an expression for stimulating metacognitive reflection regarding the use of ‘triplet’ representations for chemistry learning, Chem. Educ. Res. Pract., 18, 533–548.
- Tien L. T., Teichert M. A. and Rickey D., (2007), Effectiveness of a MORE laboratory module in prompting students to revise their molecular-level ideas about solutions, J. Chem. Educ., 84, 175–181.
- Tsaparlis G., (2018), Teaching and learning electrochemistry, Isr. J. Chem., 58, 1–16.
- Williamson V. M. and Abraham M. R., (1995), The effects of computer animation on the particulate mental models of college chemistry students, J. Res. Sci. Teach., 32, 521–534.
- Williamson V. M., Lane S. M., Gilbreath T., Tasker R., Ashkenazi G., Williamson K. C. and Macfarlane R. D., (2012), The effect of viewing order of macroscopic and particulate visualizations on students’ particulate explanations, J. Chem. Educ., 89, 979–987.
- Williamson V. M., Watkins J. T. and Williamson III K. C., (2013), The effect of student-constructed animations versus storyboards on students’ mental rotation ability, equilibrium content knowledge, and attitudes, in Suits J. P. and Sanger M. J. (ed.), Pedagogic roles of animations and simulations in chemistry courses, Washington, DC: American Chemical Society, vol. 1142, pp. 293–311.
- Yang E., Andre T., Greenbowe T. J. and Tibell L., (2003), Spatial ability and the impact of visualization/animation on learning electrochemistry, Int. J. Sci. Educ., 25, 329–349.
| This journal is © The Royal Society of Chemistry 2019 |