Open Access Article
Open Access ArticleRepresentational challenges in animated chemistry: self-generated animations as a means to encourage students’ reflections on sub-micro processes in laboratory exercises
Astrid
Berg
 *,
Daniel
Orraryd
*,
Daniel
Orraryd
 ,
Alma Jahic
Pettersson
,
Alma Jahic
Pettersson
 and
Magnus
Hultén
and
Magnus
Hultén

Department of Social and Welfare Studies, University of Linköping, Sweden. E-mail: astrid.berg@liu.se
First published on 10th May 2019
Abstract
A central aspect of learning chemistry is learning to relate observations of phenomena to models of the sub-microscopic level of matter, and hence being able to explain the observable phenomena. However, research shows that students have difficulties discerning and comprehending the meaning of the sub-micro level and its models, and that practical work in its traditional form fails to help students to discern the relation between observations and models. Consequently, there is a strong call for new teaching activities to address these issues. This paper emerges from a growing number of studies showing that learning is supported when students are set to cooperatively create their own multimodal representations of science phenomena. In this paper, we explore the approach of letting students create their own stop-motion animation as a means to explain observations during practical work. The students’ work of producing a phenomenon in the laboratory and creating an animation was recorded (audio–video) to capture students’ verbal and non-verbal interactions and use of resources. Data was analysed using a thematic content analysis with a deductive approach aimed at identifying the aspects of chemistry content that are being reasoned. The analysis showed that the task enabled students to engage in reasoning concerning both the observations and the sub-micro-level models, and how they relate to each other. The task also enabled students to reason about features of the representation that are needed to make sense of both the observational and sub-microscopic aspects of a phenomenon, as well as reflecting upon the meaning of a model.
Introduction
Relating observations of a chemical phenomenon to causal explanations at the sub-microscopic level is at the heart of what chemists do (Taber, 2013). This includes generating and evaluating visualisations of events taking place at a sub-micro level, which are crucial in framing thinking and reasoning, and necessary for constructing theoretical explanations of observed phenomena (Kozma and Russell, 1997; Kozma et al., 2000; Ainsworth et al., 2011).A number of studies show that students have difficulties in understanding and relating chemical phenomena at the macroscopic, submicroscopic and symbolic levels (Lijnse et al., 1990; Kozma, 2003; Chittleborough and Treagust, 2008; Gilbert and Treagust, 2009; Chandrasegaran et al., 2011). It is also widely accepted that sub-micro models as such are challenging (Harrison and Treagust, 1996, 2002; Johnson, 1998, 2005, 2012; Taber, 2005), and that students of varying ages have difficulties in visualizing chemical reactions at the sub-micro level (Andersson, 1990; Tasker and Dalton, 2008; Talanquer, 2009), and discerning and understanding the actual meaning of sub-micro models (Kozma and Russell, 1997). The notion that these models may explain the observable is especially demanding (Taber, 2001, 2013). A reason for this may be that traditional chemistry teaching has tended to focus on observations and symbolic representations and has neglected to connect these to events at the sub-microscopic level (Gabel and Bunce, 1994; Smith and Metz, 1996; Gabel, 1999; Chittleborough and Treagust, 2008; Gilbert and Treagust, 2009; Berg et al., 2010), while teachers have taken an inductive instructional approach, resting on the assumption that the phenomenon explains itself in the observation (Säljö and Bergqvist, 1997; Abrahams and Millar, 2008).
Thus, there is a need to develop learning practices in chemistry that deliberately focus on and emphasise representations of the sub-micro level, and the relationship between observations of phenomena and the sub-micro level, i.e., practices that support students’ ability to describe, interpret and explain chemical phenomena (Harrison and Treagust, 2000; Chittleborough and Treagust, 2008; Chandrasegaran et al., 2011).
Tytler et al. (2013) propose student-generated representational work as a basis for learning in science, and argue for a classroom practice that enacts the epistemological practice of the discipline (Ainsworth et al., 2011). In chemistry (education), this means a practice that is characterised by reasoning with and through representational constructions to explain observed phenomena at the sub-microscopic level. A recognition that representations are crucial in learning and knowing chemistry is evident in a growing number of studies on learning through creating representations of the sub-micro level (Kozma and Russell, 2005; Chang et al., 2013; Tytler et al., 2013; Zhang and Linn, 2013).
The aim of this study was to explore which chemistry content was made available when traditional experiments were merged with the representational task of explaining observations at the sub-micro level. We studied primary school teacher students as they collaboratively conducted experiments in electrochemistry, and created explanatory stop-motion animations.
Background
The chemistry triplet
One model for describing chemistry and the relationship between observable phenomena and explanations at the atomic level was put forward by Johnstone (1991), who presented the notion of chemistry as involving three different levels of knowledge: a descriptive level (the macro level), a symbolic level, and an explanatory level (the sub-micro level). This model has been widely adopted in chemistry education research and used in curriculum projects. It is now widely accepted that learning chemistry involves learning to identify and understand the meaning of each of these levels, as well as their interrelations; i.e., to represent and translate chemical problems between the levels of the chemistry triplet (Johnstone, 1991, 1993; Kozma and Russell, 1997; Harrison and Treagust, 2000; Kozma, 2003; Gilbert and Treagust, 2009).Until recently, the basic assumptions of Johnstone's (1991) triplet model have gone unchallenged. However, Taber (2013) argues that the three levels are not as distinct from each other as suggested by the model. First, the macro level can refer to both the chemical phenomena studied in chemistry and the concepts used to formalise knowledge about those phenomena. Second, the symbolic level is not distinct from either the macro or the sub-micro levels (Taber, 2013). In order to address these two problems, Taber (2013) elaborates upon Johnstone's model and, instead of the symbolic level, introduces an experiential level, allowing the symbolic level to instead form a bridge between the macroscopic and sub-microscopic conceptualisations of chemical phenomena (Fig. 1).
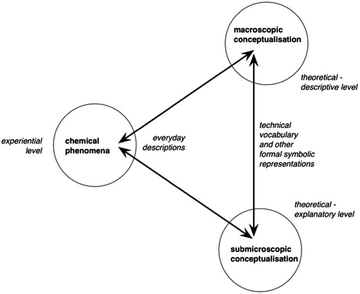 | ||
| Fig. 1 Taber's (2013) reconceptualised model of the chemistry triplet. | ||
The explanatory basis of chemistry concerns the sub-micro level. This includes theoretical models of abstract particles: the properties and interactions of atoms, ions, molecules and electrons. As mentioned earlier, it is widely accepted that these models are challenging for chemistry learners. However, in light of Taber's work (2013), learning about the observable aspects of phenomena is also challenging, since it involves relating the experienced phenomenon in terms of observational descriptions using everyday language (i.e., a white substance, a colour change, bubbles; the experiential level) to abstract concepts such as substance, compound, chemical reaction and so forth. Hence, learning chemistry involves learning to coordinate understanding at two levels: to “see” something as something specific in relation to a macroscopic framework of theoretical concepts, and as events at the sub-microscopic level explained by theoretical models. In the present work, we are interested in exploring whether the content constituted by students who engage in a learning practice that involves producing a phenomenon and creating an animation to explain the observations concerns conceptualisation of the experiential level at the macroscopic and sub-microscopic level, and coordination of these levels. Hence, we are using Taber's revised model (2013) in our analysis of the students’ reasoning (see below).
Visualisation – a way to promote integrated chemistry understanding
Phillips et al. (2010) point to the fact that “visualization objects assist in explaining, developing, and learning concepts in the field of science” and that research on the role of visualisations in science education has been increasing over the past few decades. This is especially true for chemistry since the subject relies heavily on theoretical models of the invisible world of atoms (Kozma and Russell, 1997; Kozma, 2003; Tasker and Dalton, 2008; Phillips et al., 2010). Static visualisations help students to develop meaning at the sub-microscopic level (Barnea and Dori, 1996; Dori and Barak, 2001; Venkataraman, 2009). For example, Wu and Shah (2004) concluded from their literature review that it is critical for students to manipulate concrete models in order to develop the ability to represent concepts at the sub-micro level.A drawback of static representations is that they do not directly visualise the motion of molecules or how chemical systems change over time (Williamson and Abraham, 1995; Ardac and Akaygun, 2004, 2005; Suits and Sanger, 2013; McElhaney et al., 2015). Accordingly, animations have become particularly valuable to represent these aspects (Russell et al., 1997; Sanger and Greenbowe, 2000; Yang et al., 2004; Tasker and Dalton, 2006; Gregorius et al., 2010a, 2010b; Jones, 2013; Levy, 2013; McElhaney et al., 2015). Importantly, they can support students in connecting the macro and sub-micro levels (Williamson and Abraham, 1995; Dori et al., 2003; Kelly et al., 2004; Kelly and Jones, 2008; Barak and Hussein-Farraj, 2013).
A common approach to helping students integrate the different levels of a chemical concept, and to overcome the notion that a model is an image of the real thing, is to present students with multiple representations of the target (Harrison and Treagust, 2000; Chandrasegaran et al., 2011). Ainsworth (2006) suggests that multiple representations can support learning by constraining or complementing one another, or be used to construct a deeper understanding of the target.
Student-generated representations
Today, a growing number of studies suggest that letting students create their own representations promotes learning in science, increases engagement and improves representational skills (Davidowitz et al., 2010; Hoban and Nielsen, 2010; Ainsworth et al., 2011; Zhang and Linn, 2011; Prain and Tytler, 2012; Waldrip and Prain, 2012; Chang et al., 2013; Tytler et al., 2013). Engaging students in creating visualisations can also provide complementary information alongside their verbal and written representations and hence be used to evaluate student understanding (Harrison and Treagust, 2000; Cheng and Gilbert, 2009). But, despite the centrality of visual representations in science, it is rare for students to be encouraged to create their own representations – rather, they are put in the position of interpreting those of others (i.e., experts) (Ainsworth et al., 2011).In chemistry, several studies have shown that generating drawings of chemical processes at the sub-microscopic level can help students to interpret visualisations, make connections with prior knowledge, and promote understanding and model-based reasoning (i.e., Ainsworth et al., 2011; Zhang and Linn, 2011, 2013; Prain and Tytler, 2012; Akaygun and Jones, 2014; Cooper et al., 2017). Regarding physical models, Nicoll (2003) reported successful results using play-doh rather than traditional ball-and-stick kits to model molecules, and suggests that model-kits limit students in showing aspects like bond length or lone pairs. Also software is available as a tool for student-generated visualisations. Kozma's (2000, 2003) study of university students focused on an experimental setup and the physical characteristics of the compound they were synthesising (macro level). The modelling software (Spartan) allowed these students to engage at the sub-micro level as they were building and then explain the molecular structure of the synthesised compound. However, and importantly, the students did not connect the molecular models with the substances that they had synthesised, i.e., the sub-micro with the macro level.
Another way to promote student learning and engagement in chemistry is through generating animations using software tools. Studies of students using such software to create animations point out three major gains: first, it provides a tool for engaging with the dynamic features of chemical reactions (e.g., Akaygun, 2016). Second, it can help students to develop their descriptions of the particulate nature of matter (substance, mixture, phase changes) (e.g., Chang et al., 2010). Schank and Kozma (2002) had students generating drawings and animations cooperatively and videotaped the sessions so that they could analyse the student interactions. From their analysis of the videotaped sessions, the authors concluded that using the animation tool required the students to consider sub-micro-level aspects in a way that they would not normally do (e.g., the number of molecules involved, or the sequence of steps in a reaction).
Third, there are indications that animation may encourage students to link different levels of explanation of chemical phenomena. In a study by Chang et al. (2013), students (7th grade) were encouraged to create either animations or static visualisations of chemical reactions at the sub-micro level to compare the effect on conceptual understanding. The results showed that only eight of the 30 students were able to connect their molecular visualisation with the phenomenon. Interestingly enough, students who had chosen to generate dynamic visualisations outperformed those who had chosen static ones on linking to the macro level. The authors concluded that this indicates that the lack of a dynamic view on sub-micro processes may affect the ability to make connections between the sub-micro visualisation and the experienced phenomena.
However, regarding the third gain, animations do not necessarily lead to students being able to make connections between the different levels of chemical phenomena. Albert (2012) examined senior high school students’ conceptual learning while creating animations of phase changes using software programs. The findings indicate that creating animations supported students’ understanding of the dynamic and compositional aspects of sub-micro particles. However, the results also showed that students tended to focus on either the macro- or sub-micro level, but rarely used both representations in a single animation, despite being encouraged to consider this. These results are in line with those of Chang et al. (2013), and corroborate students’ difficulties with relating observed phenomena to theory.
Another thing that has been stressed in relation to representational work in chemistry is the value of peer collaboration (e.g., Schank and Kozma, 2002). Yaseen (2018) and Yaseen and Aubusson (2018) argue that peer interactions contribute to learning about states of matter at the sub-micro level through cooperatively generated animations. Possible explanations for this are given in a study by Michalchik et al. (2008). They explored how the task of cooperatively creating animations using ChemSense supported laboratory practice, focusing on the dissolving of NaCl in water. The qualitative analysis found that students used their self-generated representations as rhetorical artefacts in discussions, and that the students’ conversation became more “chemical” as the creation process evolved. In collaborative tasks, students are encouraged to use representations to communicate chemistry, which seems to help them conceptualise the chemical phenomena under study.
Animation work often involves making a storyboard. Schank and Kozma (2002) found in their study that making a storyboard for the animation forced students to reason about the reactions in a more detailed way. In their study, Williamson et al. (2013) investigated the effect of students generating animations using Chemsense versus generating storyboards (pencil and paper). Post tests on students’ mental rotation ability and equilibrium content knowledge revealed significant gains regardless of method, and the authors call for further research on the effect of generating storyboards and animations with other concepts in chemistry.
Student-generated stop-motion animations
Student-generated stop-motion animation has also been proposed as a way of creating animations in learning chemistry. Although stop-motion – the technique used in the present study – is an old technique for creating animations, its simplicity and applicability to classroom teaching have improved with the introduction of digital resources in schools and common access among students to mobile phones and tablets. Hoban (2007) emphasised that digital cameras and free stop-motion software make it possible for anybody to become an animator. Unlike animation software tools, stop-motion requires no subject-specific software, thus enabling learners to create animations of any concepts or phenomena and “possibly provide a new way to represent their science knowledge” (Hoban et al., 2011, p. 5).There are several studies on students generating narrated stop-motion animations for learning science concepts (Hoban, 2007; Hoban et al., 2009; Hoban et al., 2011; Hoban and Nielsen, 2013; Kamp and Deaton, 2013; Deaton et al., 2014; Nielsen and Hoban, 2015). The science content in these studies is mainly in biology and astronomy at a macro (e.g., lifecycles of insects) or cellular (e.g., mitosis) level. The results of these studies suggest that the approach has potential to support meaning making, motivation and attitudes towards science. Constructing a stop-motion animation involves designing and creating a sequence of representations in different modes such as spoken, written, drawn, and physical models. It is suggested that this process of representational work helps students to develop an understanding of a concept because they need to reflect upon it in multiple ways (i.e., Hoban and Nielsen, 2010).
In chemistry education, Wishart (2017) studied collaborative student-generated stop-motion animations of chemical processes. The results showed that the opportunities for peer discussion that arose during the animation process were valued as the most important learning activity by the students. Content analysis of the students’ discussions showed that the predominant topic was how to make the animation itself (i.e., debating the best way to represent the science concept being modelled), followed by content referring to the science behind the concept. The author concluded that the animation task forced students to think through the concept from these two perspectives, and as such prompted discussion. However, Wishart (2017) did not analyse or discuss whether the task led to learning at the sub-micro level and/or its relation to the observation of phenomena (no experiments were conducted in the study).
Integrating student-generated animations in learning practices
To conclude, previous research suggests that generating animations supports students’ chemistry learning, especially when it comes to the understanding and conceptualisation of the sub-micro level, as well as affording the use of scientific language. Also, generating animations seems useful in helping students to understand the dynamic aspects of the sub-micro level. Studies on students who cooperatively generate animations indicate that student interaction contributes to learning chemistry in an important way. In terms of using the stop-motion technique, the single study by Wishart (2017) confirms this picture. The challenge seems to be how to integrate the generation of animations to a learning practice that can better address the challenge of supporting students to discern and connect the macro- and sub-micro levels. In this, collaborative tasks that combine experimental work in the laboratory with the construction of an explanatory animation seem to be a way forward to facilitate students’ integrated understanding of chemical phenomena.Research questions
In the present work, we designed a laboratory learning practice that includes the task of collaboratively producing, observing, documenting and then explaining a phenomenon. The task involves generating a narrated stop-motion animation to explain the observed phenomenon as documented in a video, at the sub-micro level, and to merge a video of the phenomenon (macro level) with the animation (sub-micro level). During the task, the students have to re-represent their understanding of the phenomenon during different representational activities, such as making physical models of sub-micro particles and writing a narration for the animation.Our interest concerns the characteristics of the learning practices followed by the students as they solve the task, and hence which chemistry knowledge is possible to develop through participation in these practices. Our research questions are:
What specific aspects of the content of the phenomenon to be explained – the experiential, macroscopic and sub-microscopic level, and the relations between these levels – is constituted during different representational activities?
What cultural tools do the students use during their interactions to represent and negotiate meaning, and how do these tools afford meaning-making?
In what specific ways do the different representational activities afford the constitution of specific content aspects of the phenomenon?
We regard chemistry content as consisting of both the phenomenon to be explained, and how to represent it. In addition, we consider the observed phenomenon as experienced by the senses – “the experiential level” (Taber, 2013) – to be separate from macroscopic conceptual constructs – the macro level.
Theoretical and analytical perspectives
Social interaction and the framework of Representational Construction Affordances
To be able to follow the students’ ways of making meaning at the different levels of chemistry (Taber, 2013) throughout the learning activity, our analysis focused on the interactions between students, including their use of cultural tools (material and symbolic tools). The analysis was guided by the framework of Representational Construction Affordances (RCA) formulated by Prain and Tytler (2012).At its foundation, the RCA framework rests on the ideas of social constructivism (Wertsch, 1998; Roth, 2003; Mercer, 2004; Scott et al., 2006; Säljö, 2011). In order to learn, learners need to participate in a social practice (Wertsch, 1998) that supports the appropriation of the competences, values, communicative patterns and ways to solve problems that characterise the studied practice (Säljö, 2011; Tytler et al., 2013). In other words, the content, or knowledge, is embedded in the practice. The goal of instruction is hence to help students to engage with the forms of thinking and doing that distinguish the practice to which they are being introduced, i.e., to engage in the activities, ways of talking, and using cultural tools that, for example, chemists do (Scott et al., 2006; Säljö, 2011). The distinctiveness of the RCA framework, as compared to other socio-semiotic perspectives in general, is the focus on open-ended, exploratory student representational constructions, and the identification of particular affordances that support meaning making (Prain and Tytler, 2012).
Affordances (seen as enabling constraints from perceptual interaction with the environment) within this framework also include learnt behaviours and strategies for reasoning and arguing (Prain and Tytler, 2012). Prain and Tytler (2012, p. 2758) argue that representational construction is afforded by “its purpose, context and the various physical and conventional resources available for any particular type of representation”. Representing an explanation of a dynamic process using pen and paper offers different constraints from generating, for example, a verbal explanation or an animation. Not only are the material features of representations and the social contexts they are used in crucial for how a representation can enable meaning making, but the background knowledge and experience an individual has with the particular representation also affects what sense and use that individual can make of it (Kozma, 2003). To emphasise affordances in this way means stepping away from an interest in the mental processes of an individual towards how the physical and social context in which the person is situated can both enable and constrain the actions that person can take to achieve his or her goals (Tytler et al., 2013, pp. 70–71). From such conjectures, an analysis of students’ interactions with each other and their physical tools reveals what it is possible to learn through participating in a studied practice (Wertsch, 1998).
Prain and Tytler (2012); see also Ainsworth et al. (2011) state that a considerable number of studies on knowledge production in the history of science (e.g., case studies of Faraday and Maxwell) confirm the central role of self-generated representations in creating, integrating and justifying ideas (Gooding, 2006), i.e., in framing thinking and contributing to knowledge production. Supporting this claim, Kozma et al. (2000) showed that chemists use multiple representations to support their thinking and doing in the laboratory as well as for social interaction (Kozma, 2003). Prain and Tytler (2012) emphasise the understanding of science as a specific set of knowledge production practices around representation, and argue that, when students are encouraged to construct their own explanatory representations in various modes, they enact the epistemic practices of science inquiry.
Within the RCA framework, all models are viewed as representations, but, as accentuated by Tytler et al. (2013), not all representations are viewed as models but rather as a range of tools for supporting reasoning processes. Representations like students’ exploratory talk, gestures, drawings, manipulation of artefacts etc. are sometimes highly situated and short-lived. This fluid-like characteristic of a representation is distinct from the more deliberate and resolved models, which are developed explicitly to explain or interpret an aspect of the world. Students can construct and interpret models through representations, or the representations as such can be models (Tytler et al., 2013). In the current study, we take the RCA framework perspective on representation and model. In relation to Taber's model, the technical vocabulary and other symbolic representations connecting the submicroscopic and macroscopic conceptualisations are the result of this epistemic work by generations of chemists.
Within the RCA framework, meaning-making viewed as an epistemological activity concerns the knowledge-building process of reasoning with and through representational construction (Prain and Tytler, 2012). Tytler et al. (2013) argue that language and representation frame thinking in that they jointly generate the context out of which they emerge. In other words, they contend that a representational challenge demands and affords reasoning through productively constraining it in particular ways. This includes spatial, temporal, topological, causal and mathematical constraints on the representation. Each of these constraints channels attention and forces the representation-makers to make choices in specific ways, thus enabling particular forms of reasoning processes and a particular explanatory end. Hence, the reasoning opened up by representation construction involves the “refinement of a mix of relations between aspects of the phenomena being interpreted and aspects of the representation” (Tytler et al., 2013, p. 106). Since a representation involves analysis, selection and choice of abstraction, Tytler et al. (2013) argue that each representation can be seen as a reasoned claim. Importantly, claims and warrants do not constitute formal linguistic reasoning, but are distributed across the representation in terms of the deliberate choices of selection and synthesis of aspects made by the students through reasoning.
The design of the task in the present study, in terms of foregrounding representational generation, coordination and transformation, focusing on both observational and theoretical aspects, acknowledges the critical role of representational work in science-building activities as it contributes to chemical reasoning. Of special interest in this study is the fact that students repeatedly have to re-represent the chemistry content (Hoban et al., 2009) and that makes this task different from many previous studies, in which students used different digital tools to generate animations. Constructing a stop-motion animation involves designing and making a sequence of representations in different modes such as spoken, written, drawn, and physical models, not just copying and pasting ready-made representations. We consider generating and evaluating representations in pursuit of the construction of a theoretical explanation for the observations made during an experiment to be an activity that lies at the heart of scientific practice (Ainsworth et al., 2011). From that perspective, the RCA framework (Prain and Tytler, 2012) is suitable for framing our analysis of the students’ interactions in the present study.
Method
The design of the teaching sequence
Two of the researchers lectured to a limited extent during the preceding semester within the teacher-training program, and the students were hence familiar with them. These two researchers conducted all the teaching throughout the teaching sequence, and collected all the data. The teaching design of the programme depended to varying degrees on tasks to be worked with cooperatively, and for this purpose the students were divided into six work-groups (Groups A–F). These were mainly left intact during this study. However, two groups with eight students each (Groups A and B) decided to split into two smaller subgroups (Groups A1 and A2, B1 and B2) during this particular part of the course. Consequently, we ended up with a total of eight groups. There were four students each in Groups A1, A2, B1, B2 and F, five students in Group C, seven in Group D and four in Group E. In the result section, the students are numbered (S1–Sn) in each group, where n equals the number of students in the group.
The main task for the students was to create an instructional and explanatory video of an experiment, including episodes of: (a) video clips showing how to perform the experiment, (b) video clips showing the observable phenomena at the macro level, and (c) a multimodal animation explaining the observations at a sub-micro level. The intended audience for the video was primary teachers.
The rationale behind this task design, having students creating animations of the processes at the sub-micro level, was to facilitate the development of a relational understanding of chemical reactions at different levels. In doing so, we allowed students to work together to solve the problem of how to visually explain the experiments.
| Teaching activity | (1a) Lecture 1–2 | (1b) Lecture 3–4 | (2) Experiments | (3) Storyboard workshop | (4) Animation workshop | (5) Seminar |
| Was divided into three different representational activities | Presentations | |||||
| Discussions | ||||||
| Representational activities | RA1: Documenting and performing experiments | RA2: Making a storyboard for the final video, including the animation | RA3: Making physical models | Not analysed | ||
| RA4: Photographic animation work | ||||||
| RA5: Editing work – narration | ||||||
| Time | Week 1 | Week 2 | Week 2 | Week 3 | Week 3 | Week 4 |
Lectures. The four-week teaching sequence began with four ninety-minute lectures distributed over a period of eight days designed to cover the background knowledge of chemistry that was needed to understand and explain the experiments the students were to perform. The first lecture laid the foundations for a representative meta-perspective and focused on the question of how to represent something that is invisible, stressing the relation between model and reality and that a model can come in many shapes, sizes and styles. One aim was to illustrate that a representation focuses on certain aspects of a phenomenon while other aspects are overlooked. The subsequent lectures covered basic theoretical aspects of electrochemistry. We especially emphasised the macro/sub-micro relation, as well as visualisation of the sub-micro level in terms of drawings and animations.
The lectures were followed by four student activities (see Table 1): experimental practical work in the laboratory, a storyboard workshop, an animation workshop and a seminar in which the students presented their own animations along with their analysis of another group's animation.
The experiments. With the ambition of creating a variety of contexts for a restricted number of electrochemical phenomena, instructions for about 20 experiments were gathered into a booklet, which was handed out to the students at the start of the teaching sequence. The selection of experiments was made based on the criterion of being possible to perform in an ordinary classroom in primary school. In addition to being uncomplicated and safe to perform (not requiring, e.g., a hood), the experiments should require as few “laboratory chemicals” and laboratory tools as possible and should instead use artefacts from everyday life (coins, nails, fruit etc.). All experiments focused on electrochemical phenomena of different kinds, ranging from corrosion experiments, simple redox reactions and making fruit batteries to plating experiments. The written instructions were step-by-step explanations of how to produce the phenomena, although some explicitly encouraged further enquiry.
The groups were instructed to prepare for the practical work by: (a) choosing three to four experiments from the booklet and studying the instructions, and (b) reasoning about how the experiment connected to theoretical concepts covered during the lectures, and searching for answers to questions. The students were instructed to film and/or take photographs of their experimental setup and the phenomenon as such. The instruction to document the experiment and the phenomenon aimed to reinforce and preserve the experience (Roth and Lawless, 2002a). Finally, the groups were told to choose two of their experiments for the task of explaining them at the sub-micro level through generating an animation. During this activity, we as instructors took a step back, figuratively speaking. We did not interfere with the students’ actions unless they had problems of a theoretical or practical nature and explicitly asked for help. When this happened, our approach was firstly to try to guide them to find the answer in the resources provided, and secondly to inform them.
Workshops. To support the students in their task, they were firstly introduced to: (a) the idea of constructing and using a storyboard, and (b) the animation software program (IStopmotion for iPad). The students were then instructed to create a storyboard for their final (verbally or textually) narrated video, which should include the expository animation integrated with the video clips/photos from the experiment. They were instructed to use the animation to explain the experiment at the sub-micro level. However, we gave no instructions about whether they should include the experiential/macro level in the animation or not. During the introduction to the animation workshop, the students were instructed to create 2D or 3D models to be used in the animation, and then continue to the work of taking photos for the animation. They were offered a variety of construction materials to choose from, ranging from clay to coloured paper and aluminium foil. For both the workshops, we gathered several school textbooks for the students to use as complements to their course textbooks and the internet when searching for background information. During the workshops, the students gathered background information that was typically related to questions regarding the characteristic features of the sub-micro particles.
Of the total of 180 minutes in the workshop, approximately 20 minutes included an introduction and information about copyright issues and issues of a practical nature. The teachers/researchers played the same role as in the practical (laboratory) work, helping and guiding the students to find their own solutions to any arising problems by using guiding questions.
The final seminar. Following Prain and Tytler (2012) and the RCA framework, and studies such as Chang and Quintana (2006), Chang et al. (2010) and Yaseen and Aubusson (2018), the final seminar was intended to open up further reasoning and learning opportunities by having the students discuss the final video and animation. The qualitative analysis of the final seminar is beyond the scope of this study and is not presented here.
Data collection
In order to analyse the five different representational activities, video recordings of the associated teaching activities (experiment, making the storyboard and creating the animation) were made. Videos were recorded to capture students’ interactions, both verbal and non-verbal, with other students, instructors and artefacts during each representational activity. We also collected empirical data in the form of the storyboards and videos created by the students (including the animation).Due to the restricted number of available video cameras, only four groups could be recorded during each teaching activity. As we could not know beforehand which groups were to be most interesting, and as this could vary between the different teaching activities, a random selection of four out of the eight student groups was made during each teaching activity. As a consequence of this, no single group was followed through all teaching activities. This was not seen as a problem since our focus was affordances directly related to the corresponding representational activities, and not their dependence on the teaching history of a certain group.
Analysis
| Experiential level | Macroscopic level | Relation experiential level – macroscopic level | Sub-microscopic level | Relation experiential/macroscopic level – sub-microscopic level | |
|---|---|---|---|---|---|
| Chemistry content in students’ interactions | Content: perceptual descriptions of the phenomena | Content: the phenomena at the macroscopic level | Content: macroscopic conceptualisation – relating perceptions of the phenomena to concepts at the macroscopic level. | Content: the phenomena at the sub-microscopic level. | Content: relating perceptions, or macroscopic conceptualisations, to the submicroscopic level. |
| Example: “We show the picture [of the shiny and clean silver spoon].” | Example: “That is if it's heat and oxygen that causes some reaction.” | Example: “Bubbles….it must be a reaction.” | Example: “We move the electrons like this [trembling gesture].” | Example: “It goes from atomic ion to element. That's why it [the silver spoon] becomes shiny.” |
Guided by the RCA framework, we analysed the selected episodes, focusing on the characteristics of the students’ meaning-making by using the following two questions:
• What cultural tools do the students use to represent the meaning of a phenomenon during the knowledge-building process of reasoning with and through representational construction?
• How do these tools afford meaning-making?
Secondly, the analysis focused on the characteristics of each representational activity in terms of demanding and enabling reasoning, because of the specific way in which they channel attention and direct choices made by the students:
• What productive constraints does each representational activity offer to enable reasoning about chemistry content?
The results of these analyses, and the selected episodes, are presented in the next section. The episodes were translated to English by the authors, and validated by a person who is a native English speaker with a high level of fluency in Swedish, and who also possess knowledge of the research context. Validation was made using back translation, and a few inconsistencies were identified and corrected. We wish to emphasize that nuances of the original may have been be lost in translation. The original transcripts in Swedish are available in Appendix 1.
Results and discussion
The aim of this study was to explore what chemistry knowledge is possible to develop through participation in a learning practice that involves producing and documenting a phenomenon and generating an explanatory stop-motion animation at the sub-micro level. The main finding is that the students are provided with opportunities to develop an integrated understanding of the phenomenon, i.e., an ability to conceptualise the experience of the phenomenon at both the macro and sub-micro levels, and to link these levels to each other. This means that the chemistry content constituted in the students’ learning practice included aspects of the phenomenon at all three levels of the Taber (2013) chemistry triplet, i.e., all five categories in the coding scheme (Table 2).The results also show that the students used a variety of cultural tools to represent and negotiate meaning (see Table 3). One important finding is that the making of the physical models in particular afforded meaning-making at the sub-micro level as well as the relation between the macro and sub-micro levels. As illustrated in Table 3, each representational activity afforded a specific pattern of (1) aspects of content constituted in the students’ interactions, and (2) cultural tools used by the students. Notably, the laboratory work did not afford reasoning about the sub-micro level.
| Representational activity | Aspects of constituted content of the phenomenon | Cultural tools used by the students to represent and negotiate meaning | |
|---|---|---|---|
| Level | Relations between levels | ||
| RA1: Experiments – practical work in the laboratory | Experiential | Laboratory equipment, photographs. | |
| Macro | |||
| RA2: Storyboard workshop | Experiential | Experiential – sub-micro | Drawings, physical objects at hand, gestures, texts from the internet, zooming-in approach. |
| Macro | Experiential – macro | ||
| Sub-micro | |||
| RA3: Animation workshop | Macro | Macro – sub-micro | Physical models of particles and macro objects, drawings, reinforcing adjectives. |
| Sub-micro | |||
| RA4: Animation workshop | Experiential | Experiential – sub-micro | Physical models of particles and macro objects, drawings, the evolving animation. |
| Sub-micro | |||
| RA5: Animation workshop and follow-up | Sub-micro | Macro – sub-micro | The animation, photographs, chemical concepts. |
In the following section, the results of the analysis are presented in terms of five headlines corresponding to each of the five representational activities (RA1–5; see Table 1). Under each heading, associated examples of episodes are presented, categorised according to the coding scheme in Table 2. We present the representational activities in sequence, in order to mirror the development of the learning practice.
Summary of results
Table 3 presents a summary of the results concerning which aspects of content of the phenomenon to be explained that was constituted, and which tools the students used, during each representational activity.Representational activity: practical work in the laboratory (RA1)
During the practical work in the laboratory, the students performed experiments. In parallel with this, they documented the experimental material and the observed chemical phenomena (photographs and video), to later merge with the animations into a final video. During this activity, the students’ reasoning focused only on the experiential and macro levels.It is important to note that none of the groups made any attempt to conceptualise their observations at the sub-micro level, which indicates that the work of producing, observing and documenting phenomena does not support such “mental gymnastics” (Johnstone, 1991).
Experiential level
S1: Now it's bubbling in [inaudible]. Look.
S2: Mm.
S1: Interesting, interesting.
S1: Now bubb… now it must be like…
S2: It's some reaction.
S1: Mm.
[—]
S1: Now it smells very strong here. (Episode 1)
This discourse focuses on descriptions of the phenomenon in terms of what the students perceive with their senses – the forming of bubbles and a strong smell, the experiential level of chemical phenomena. The students also conceptualise the description at the macro level – “it has to be/—/some reaction” – but do not elaborate upon this observation.
For example, group B2 performed an experiment in which, among other things, they put a piece of steel wool into a beaker containing copper sulphate solution. During the experiment, they first made observations of the beaker and what happens during the experiment. Then they also start to compare the photos taken during the experiment with the beaker containing the steel wool and the beaker containing the concentrated copper solution (see Fig. 2). As a result, the descriptions of the colour and colour changes becomes more refined. During this process, they verbally re-represent their observations several times, comparing them with each other and assessing the result. However, they remain at the experiential level.
 | ||
| Fig. 2 Students in group B2 are taking pictures of the beaker containing steel wool and copper sulphate solution. | ||
To conclude, for some of the groups, the representational task of documenting the phenomenon afford a more detailed discernment of the perceivable as compared to the initial experiential descriptions.
Macroscopic level
The making of the storyboard (RA2)
The activity of creating the storyboard for the final video, in which photos/video of the experiential level and an animation of the sub-micro level are to be merged, channelled the students towards the relation between the macro and sub-micro levels. In addition, it prompted questions about how the observable may be explained and what it means to create a visualisation of the sub-micro level. This was very demanding for the students, and explicit reasoning at the sub-micro level was sparse during the activity. The episodes presented here illustrate the students’ struggle with the task, and their actions to deal with the problems.The relation between macro and sub-micro levels
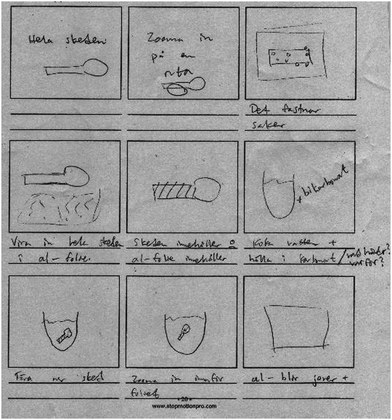
S3: I figure that one has… say that one has [inaudible] this picture [starts to make a sketch of a spoon in a beaker with liquid] [—] and so one has this picture [puts his hand on the sketch of the spoon in the beaker] and then zzz [makes a buzzing sound] one zooms in one more time. Here one sees [draws a circle around a small part of the spoon]. [—] and then one circles this one [draws another circle on top of the first one] […] and so we zoom in, and then it becomes, and then we have aluminium [makes a new sketch below the first one, drawing two parallel lines] and … [is interrupted by S4 questioning his approach]. (Episode 2)
S3 implicitly states that he sees the explanation, in terms of the sub-micro level, as something that may be reached by magnifying – zooming in on – the experiential level. However, S3's sketch does not visualise the sub-micro level, and apparently he has difficulties in illustrating it “in drawing”, or lacks access to language (and/or imagery) for reasoning about it. Accordingly, S4 objects, seemingly missing references to something abstract in S3's drawing: “but one should make icons, use icons or symbols for explanation”. Student S5 then also points out that “maybe we must do something, so, so we also see it like this [—] we have clay and maybe make a dot and then show that this is silver.” Presumably, “silver” stands for a silver atom and, hence, S5 here proposes a way to model and visualise a sub-micro particle as a “dot” of clay. Altogether, the students’ way of expressing themselves indicates that they are groping for an image of what the sub-micro level really amounts to, and how it may be visualised.
Later during the storyboard workshop, S3 and S1 in Group C continue the discussion of how to explain the experiment. In parallel with their talk, S4 starts to make drawings and write short notes in the frames of a storyboard template (Fig. 3). S4 here follows the zooming-in approach suggested by S3 (above), but the sub-micro level is not visualised in S4's drawings. The excerpt below illustrates, however, that the students approximate the sub-micro level as their talk revolves around the zooming-in approach:
S3: The spoon is there [points at the sketch in frame 1 of the storyboard].
S1: Mm.
S3: Zoom in on the surface [points at frame 2]. The surface, here things get stuck [points at frame 3].
S1: Mm. And then we have to show that this [points at frame 3] lies in a beaker [inaudible].
S3: But then we still have… should we take the entire [inaudible] we take the entire. And then we zoom again. Can’t we do it like that?
[—]
S3: In this one [points at frame 5], should we once again zoom in, and show the spoon contains this?
S1: Yes, exactly. (Episode 3)
From S3's second utterance, it becomes clear that zooming in on the spoon, visualised in frame 3, also takes place at the macro level: it aims to show that the surface of the silver spoon is stained. However, S4's utterance in relation to frame 5 – “show that the spoon contains this” – and his notes on frame 5 – “the spoon contains” and “the aluminium contains” – implies that his proposal to zoom in aims to show something within the spoon. When zooming in on the spoon (and the aluminium foil), its contents will be exposed. Hence, they are here aiming to make the jump from the macro/experiential to the sub-micro level. What the zooming in will show, a representation of “the contents”, is, however, not specified. Nevertheless, their representational strategy of zooming in seems to work as a thinking tool that helps them to stepwise undertake the journey from the experiential to, at least, the steep path down to the sub-micro level.
The macro level
S3: I was supposed to explain, if one imagine something grey that is like the silver spoon [puts a hand on the notepad representing the silver spoon], then one has, then one makes the coating, this one here [puts pencils along the edge of the notepad, representing “the coating” on the spoon].
S4: The silver sulphide.
S3: Yes exactly, in pieces. So that one can/…/one has, well, blue paper that is sort of in pieces, so that one like removes it [removes the pencils from the notepad] as it continues, are you following me? (Episode 4)
By using resources at hand to represent the macro level in terms of the silver spoon (notepad) and its silver sulphide coating (pencils), S3 makes a representation of a dynamic process as he takes pieces of coating away from the spoon. However, representing the disappearance of silver sulphide from the spoon as “pieces” of the substance continuously being removed, implies that he doesn't understand the process as a reaction where silver sulphide is reduced to silver. It seems that S3 has difficulties representing something that goes beyond the observable. It is important to note that S3 here abandons sketching as a way to represent his understanding. Using pencil and notepad as representational tools affords a visualisation of a dynamic process.
The sub-micro level
S3: Then a plus goes from the aluminium ion, or the aluminium, [makes his left hand into a fist and puts his right hand on his left], a plus goes [moves his right hand away from the left], leaves a minus [—] The plus wanders over to [moves his right hand to the right]… which makes… [S4 goes quiet, shakes his head and makes a resigned gesture, also with his hands]. (Episode 5)
This episode shows that the students have severe difficulties in interpreting and visualising the textual and symbolic representations at the sub-micro level. It also shows how S3's representational action helps him to reveal the gaps in his own understanding. It is important to note that S3, again, needs objects as tools to visualise the dynamic process. This implies that sketching does not afford the students to communicate all aspects of their understanding.
Making the physical models (RA3)
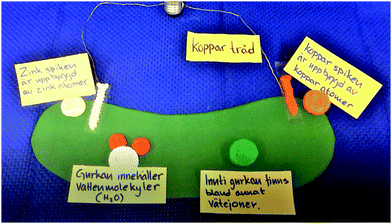
The making of the physical models for the animation forced the students to consider the size aspects of the relation between the macro and sub-micro levels, as well as the size relation between electrons and atoms and atoms and molecules. It also prompted discussions about what a model really is, as well as discernment of the relation between matter and atoms. During the making of physical models of sub-micro particles, the students were faced with representational choices, such as the number of electron models to make. In turn, these choices afforded reasoning about the organisation and nature of the particles, the systems they are parts of and their role in the chemical process.The relation between macroscopic/experiential and sub-microscopic levels
S2: We cut it out and make it look like nails sitting [on the cucumber] [S2 points at the green paper].
[…]
S1: We’re not supposed to see the nails at the atomic level [since we will zoom in].
S2: No, but you still need it, and then you must go in, and then you will [inaudible]. You need to show how the cucumber itself looks. Or?
S1: Or we can make a small cucumber with two nails in it. Because then you’re supposed to only see like … [begins to place small LEGO bricks in a row on the table]. If we imagine the nail looks like …
S1 draws a straight line in the air and concludes that “you see that there's a straight line sort of but it's built of atoms.” S3 disagrees with the suggestion of using a small cucumber model and emphasises that they should use a bigger model where they zoom in on the nails. However, S1 objects:
S1: I think it won’t be realistic in size. I think. Because the atom is so tiny, tiny, tiny, tiny, tiny compared to the whole cucumber. Do you know what I mean?
S3: But it's a model.
S1: Yes.
S3: It's a model, models aren’t realistic.
S1: But you can make rather realistic ones. (Episode 6)
Although it is difficult to follow all the turns in the dialogue, it seems that S1 has problems with the representational approach advocated by S2 and S3 – focusing on the two nails and the cucumber (macro level) – since an explanation of the phenomenon needs to also consider the sub-micro level: it will not be “realistic”. The students are here negotiating what a model really is. They both stress important representational aspects – what features of a phenomenon a representation should and should not account for. However, it seems that they have difficulties in discerning which aspects are important and which are not.
II is important to note that, although none of the three students explicitly uses the terms ‘macro’ or ‘sub-micro level’, they communicate their competence and show their awareness of the different levels using other tools – gestures, physical models and reinforcing adjectives.
In relation to Roth and Lawless (2002a), the tentative cucumber model makes up a (semi)perceptual version of the experimental setup. This, in turn, works as common ground against which the students enact metaphorical gestures of the abstract (electrons, atoms). The common ground and the gestures hence complement verbal utterances as a representational function. However, whereas the students in Roth and Lawless's research (2002a) use the actual, material setup as common ground, the students in our study re-construct a “semi-perceptual” replica of the same, which allows them to use it at the moment when they need it. This implies that the students need to revisit (the model of) the material setup again and again during their representational work.
S1: And how big are the atoms then?
S3: But you draw them as big as you want [irritated] [S3 makes a circle with her fingers and repeatedly puts it in different places on the paper].
S1: And then the electrons are supposed to be smaller?
S3: Yes.
S1: I think the electrons will be so small that you won’t be able to see them in the movie. (Episode 7)
Here S1 also focuses on the size-relation nail–atom–electron, thus highlighting that there are two levels within the sub-micro level. She argues that, if this difference in scale is also to be considered, the representational approach of synthesising the experiential and sub-micro levels becomes even more problematic. S1 here draws attention to the fact that there are also differences in size within the sub-micro level to account for.
Following this, S3 uses the storyboard to describe how the animation can be divided into two scenes to solve the problem: the experimental setup as such, and “what's happening”, respectively. She communicates “what's happening” through gestures, making repeated jumps with her hand on the green paper from one nail to the other, which may be interpreted as a number of small moves of electrons (i.e., electrons moving in the circuit). The static representation, the storyboard, does not convey the dynamics, so gestures have to be added. S3 obviously views the electron transport as a critical event.
This mind-change illustrates how difficult it is for the students to find a way to deal with the task of merging the invisible with the visible in a reasonable way. In their final animation, they do not use a zooming in approach, but show a cucumber with two nails and electrons being transported (see Fig. 6). This mixing of levels was common in the final animations. It seems that the experiential level becomes important for the students to frame sub-microscopic aspects of the phenomenon. It is interesting to note that these questions of different levels of organisation were not an issue during the storyboard work. It was not until the students were put in the position of actually making the physical models that the complexity of the choice of scale was brought to light.
To conclude, the episodes presented under the relation between the macroscopic/experiential and sub-microscopic levels show that the students’ choice to merge macro/experiential and sub-micro into one representation afforded reasoning about several aspects critical to understanding chemistry: the meaning of a model, the matter–atom relation and scale differences at the sub-micro level.
Sub-microscopic level
S2: How many yellow ones do we need, the yellow ones?
S4: Electrons.
S2: Electrons.
S1: As much as you like.
S2: I don’t really know what they are. Haha.
S3 How many of them are supposed to disappear then?
S2: There are supposed to be two left, let's say. Or? Is that what you say? Two left? [Places two electron models beside the iron wool model].
S3: We were supposed to have electrons here on those [points at the iron atoms in the iron wool model], but then all of them must stay, be on that one, there must be four on that one.
S2: So let's say four. Then some are supposed to go to the oxygen.
[They continue to discuss how many electrons that are needed]
S1: And it's these that go away, then there will be none left in the outermost shell. [makes circles in the air with a finger]
S2: Is this the idea then? That it should be like this [places more yellow electron models onto the larger green iron wool model] that there is like two in each, or?
S1: Well yes, these electrons like to [make a circle with one hand above the models on the table] wander.
S2: So they just have to be around [the iron atoms]?
S1: They're free like Sven [one of the physics teachers] said.
S3: Aha, because I thought, aha, ok, there is none stuck on the atoms themselves.
S2: They're inside the atoms, or…
S1: Yes. [Nods]
S2: But we won’t be able to show that.
S3: But these are the atoms? [points at the green clay models on the table].
[…]
S2: The electrons [holds an electron model] are free.
[…]
S1: They are free within the piece of iron. Right? That is why they…
S3: They can go away, yes. (Episode 8)
S2's “how many do we need” question initiates several new questions, and it becomes obvious that S2, S3 and S4 have not yet been able to discern some critical aspects of what it is they are constructing (models of what), how the process is shaped, or how these aspects relate to theoretical models at the sub-micro level. The content of the discussion stretches from process-oriented questions – how many electrons are supposed to “go away” and “these electrons wander in the iron” – to structure-oriented questions. The latter leads to reasoning that clarifies both the number of valence electrons and that these “go away”, while the electrons “inside” the atom do not, as well as the properties of the valence electrons – they are “free”. The excerpt shows that the discussion also involves clarifying statements in relation to what is what in their model – “but these are the atoms”.
Another example from Group F shows how the creation and subsequent observation of student-made models prompted insights into the sub-micro level. The students have made clay models of all the particles in the cucumber solution – water molecules, hydrogen ions and zinc ions – and placed them on the table next to each other (see photo-frame final animation in Fig. 9). Now, the students can simultaneously observe the whole system of particles. After some discussion among the group members about the models, S3 looks at the model of the cucumber solution and its particles with a troubled expression:
S3: But now really the question is whether these should actually be green [points at the red hydrogen atom in the water molecule model]. Because this [points at the hydrogen atom in the water molecule] is the same as that [points at the green hydrogen ion model]. Except that this is an ion and that is an atom. (Episode 9)
The simultaneous observation of the two kinds of hydrogen particles obviously enables S3 to discern the relation between the two different hydrogen particles. Consequently, she questions the inconsistency in the colours of the two models – one is green and the other red. It is important to note that this relation was not discerned during the making of the models. Although the students talked about and symbolised it as “H plus” (carved in the green clay model), they had obviously not gained the insight that it was a hydrogen ion. Choosing to give it a green colour implies that they rather saw it as a “cucumber particle”. However, when the models are finished and observed simultaneously, the students can discern their relation and hence see the hydrogen ion in the cucumber solution as an ion of a hydrogen atom.
Taking photographs to produce the animations (RA4)
When the students were instructed to create animations using stop-motion, their attention was channelled towards the dynamic features of the modelled sub-micro process, such as in what way, how far and in which direction different particles move, and where they end up. The stop-motion work also afforded the discernment of new aspects of the modelled process as it came to life in the “scene”. During the photographic activity, the students were also forced to consider the overall task: explain observations of the phenomenon at the sub-micro level. This is illustrated in the first episode below.The relation between experiential and sub-microscopic levels
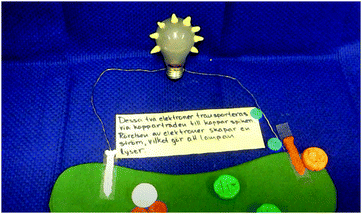
S1: But, don't we have to show [in the animation] that it [the silver spoon] becomes shiny and clean [inaudible]?
S2: Yes, but that's what we’re saying at the same time as they [the electrons] wander. First we say the electrons wander. Then maybe we make a pause there [in the animation] and then show the picture [of the silver spoon] once again; now when these electrons have got stuck on a silver atom each, it becomes … it becomes, it goes from atomic ion to element. That's why it becomes shiny. (Episode 10)
In the example, the students discuss how the observational change – “becoming shiny” – relates to interactions at the sub-micro level. They also discuss different modes of representation (verbal, visual). In doing this, S2 suggests how the link between observation and the sub-micro level may be represented in the final video: “we make a pause [in the animation of the sub-micro level] and then show the picture [photograph of the silver spoon – experiential level] again.”
The second example comes from Group C, and is about relating an observable non-event at the experiential level to the sub-micro level. Their experiment (identical to that of group A1, see above) concerned the rusting of steel wool in a closed beaker containing water (case 1), and in a closed beaker containing water in which the air lacks oxygen (case 2). Here, the group has finished animating the formation of iron hydroxide at the sub-micro level (case 1), and has verbally linked it to the observation of a rusty piece of steel wool. When they are about to animate the process in the oxygen-free bottle (case 2), they are thus faced with relating a non-event (no observation of rust formation) to the sub-micro level. Students S1 and S3 wonder what it is that their animation of the sub-micro level can actually explain since no chemical reaction takes place. S1 then suggests that they move the electrons in the steel wool. Making a trembling gesture with his hand to represent this movement, S1 highlights that, although they have experienced the experiential level as static in their observations, the electrons are nonetheless in motion.
In both examples, it is obvious that the task of creating an animation that explains the observational changes at the sub-micro level also triggers questions about how to coordinate this animation with photographs and videos from the experiment into a coherent explanatory story. This coordination had been a focus since the storyboard workshop, but it is not until the animation work, and the channelling of attention towards the dynamic aspects, that the students begin to reason in more detail about the experiential/sub-micro relation.
Sub-microscopic level
 | ||
| Fig. 7 Group A1 rehearses the story they are about to animate. This productively constrains the students’ attention towards aspects of the chemical process at the sub-micro level. | ||
S1: Then these iron atoms, which are eager to give away their valence electrons, comes along.
S2: Mm
S1: So, one iron atom goes away together with water [pick up a model of an iron atom from the model on the table].
S2: With the water?
S1: Yes. Dissolves in the water. [pick up a model of a water molecule]
S2: Oh, yes.
S1: And they leave their valence electrons [pick up small yellow electron models from the tabletop] behind.
S2: But hey, is this one supposed to take a water and just go away [pick up a green model of an iron atom together with a model of a water molecule], or what?
S4: He he.
S1: Ehh.
S2: Should it fly away with it [move the iron and water models up in the air in a big motion]? There will still be something left, won’t it? (Episode 11)
It seems that S1's initial description, together with moving the models, makes it possible for S2 to really discern that iron ions actually leave the iron piece. However, S2 asks for a more precise description – the hydrated ion can’t just “fly away”. S2 then highlights a critical aspect: how far away from the iron piece should the hydrated iron ion be moved, or might it be that it “stays on top” of the iron piece? S3, seemingly trying to find a reasonable compromise, suggests that it “sticks around but it doesn’t remain in the piece”. It seems that the students are trying to synthesise their observation of the rusted steel wool with the “sub-micro story” initially outlined by S1; they observed rust formation on the surface of the steel wool, yet the iron ions actually leave the steel wool surface. S2 then suggests how this outline may best be represented in terms of making these aspects of the process discernible to a prospective observer: moving the hydrated ion “just a bit [away from the iron piece]”.
This example shows how the photographic animation work (R4) forces the students to consider and reason about the process in detailed dynamic and mechanism-like terms. During this process, they re-represent the chemical process several times and elucidate critical dynamic aspects of the model, one of which was that no particles just disappear. This illustrates the benefit of having a physical manifestation of the particles; they have to go somewhere. As long as the particles only existed in the students’ minds, they could disappear when they stopped thinking about them. The example also shows that reasoning about the dynamics prompted representational choices – what dynamic aspects of the process should the model account for, and how should it be represented? Hence, the photographic animation activity evolved in the same way as during the making of the models (R3) – chemical and representational reasoning were tightly intertwined and prompted each other.
S2: There needs to be more green ones [refers copper ion models] Otherwise …
S1: No but I think it's enough.
S2: Okay.
S1: Let's try this [modelling approach].
S2: Otherwise all the green ones disappear, so it…
S1: Yes at the end they disappear. But where do the yellow ones [refers sulphate ion models]
disappear?
S2: That's why I say that there needs to be more green ones.
S1: Yes, but where do the yellow ones go?
S1 [to S3 who just entered the room]: Where do the yellow ones go? (Episode 12)
Initially, S1 does not see the disappearing “green ones” as a problem. However, she then focuses on the sulphate ions on the scene – “but where do the yellow ones go?” S2 replies “that's why I say that there must be more green ones”, implying that she understands that the number of copper and sulphate ions in the solution ought to be equal. S1 repeats the question while looking at their representation, seemingly confused by what she sees. However, her confusion deals with the surplus of sulphate ions rather than the deficit of copper ions.
Neither S1 nor S2 has realised that the reduction of copper ions to copper atoms on the surface of the key is balanced by an equal number of dissolving copper ions from the copper plate (the anode); in other words, that the number of negative sulphate ions and positive copper ions is equal until the copper plate is consumed (and the reaction comes to an end).
Before the start of the photographic animation work, they talk about what is to happen: The sulphate and copper ions are to “move” towards the copper plate and the key respectively. The copper ions are to be reduced to copper atoms, and copper will precipitate onto the key. Hence, they are seemingly on the right track. However, what they “miss” at this point is that every copper ion that is reduced to a copper atom on the surface of the key is replaced by a copper ion from the copper plate. It is not until they start the photographic animation work that they face the imbalance in ion concentration and consider it a problem. Although they do not settle on a solution to the problem of imbalance, the photographic animation work seems to help them notice that there is a problem in their modelling of the chemical processes involved.
Adding a narration (RA5)
The work of adding the narration had the potential to channel the students’ attention towards the question of how to represent the physical models, and their dynamic interactions, in scientific language. Representational choices regarding language afforded reasoning at the sub-micro level and about the macro/sub-micro relation.The relation between the macro and sub-microscopic levels
S2: When water meets the iron atoms … should one say the iron atoms come loose or let go. Or what does one say?
S3: They dissolve. Right?
S2: But yet, some stay in … there were only some … there are only some atoms that go away, the others don’t go away.
S1: Yes.
S2: When it dissolves, everything disappears, I figure.
S1: Yes, some iron atoms dissolve in the water and drop their valence electrons in the iron.
S2: Should we write some of the atoms or a few of the atoms, or something?
S1: Yes.
S2: Because it's not all of them.
S1: No it's not.
S2 [writes and talks]: The atoms [stops the writing, looks up] so, dissolve …
S3: Dissolves away in the water.
S2 [writes and talks]: Dissolves away … in the water. Dissolves away sounds strange. Dissolves.
S1: Are dissolved.
S2: Yes, exactly. Are dissolved [writes] … in the water … and then leave behind … or? (Episode 13)
The students discuss the wording when describing the interaction between a water molecule and an iron atom in their multiple-particle model of a piece of steel wool. Initially, S3 suggests the word “dissolve”, but student S2 disagrees. She refers to the experiential/macro level and argues that when something dissolves “everything disappears”. Although her conceptual understanding of dissolving is impaired, her statement indicates that she relates their observations at the experiential/macro level – the steel wool had not disappeared – to their model at the sub-micro level – only a few atoms “let go”. The students’ choice of wording, “atoms dissolve”, presumably mirrors a lack of ability to represent their (correct) sub-micro model in scientifically correct language, to discriminate between concepts for macro-level processes and descriptions of how particles move and interact at the sub-micro level. At the same time, the work of writing a narration forces the students to discuss in detail what is actually happening at the macro and sub-microscopic levels, and in this way they start to challenge whether they are using scientific concepts in the correct way.
S2: What should one say … those atoms leave behind their valence electrons in the iron piece? Or in the steel wool? [Turns to S1] Or just leave behind, maybe enough, leave their valence electrons behind.
[…]
S2: With the other atoms that are in the iron?
S1: Can’t you say …
S3: It's actually the iron piece.
S1: The iron piece.
S3: It's in the steel wool.
S2: It feels like …
S1: But it feels a bit muddled to always talk at the atomic level and then talk about …
S2: Yes. Maybe we shouldn’t use steel wool but only iron, iron atoms really. (Episode 14)
S2 reflects upon the verbal representation of the valence electrons being left behind by the iron ions; are they left in the piece of iron (macro level) or with “the other atoms that are in the iron” (mix of sub-micro and macro level)? S3 says that “it's really the iron piece/—/the steel wool”. S1 disagrees and expresses a concern about keeping the verbal representation at the sub-micro level only. S2 agrees and says that “maybe we should use/—/[the wording] iron atoms”. This example shows how difficult, or even unfeasible, it may be to conceptually approach multiple-particle models when describing events at the sub-micro level. These models lie in a no-man's-land between the object (macro level) and its single-atom building blocks, and one may question whether it is a “piece of iron” or an assembly of iron atoms. However, the students place particular emphasis on trying to correctly designate the model and navigate between seemingly unproblematic and scientifically correct macro-level representations (“iron piece”) and their corresponding sub-micro-level representation (“iron atoms”). This emphasis includes an awareness of the (didactic) risk of mixing macro and sub-micro concepts in the same representation – “it feels a bit muddled” (S1's last utterance).
In summary, the process of writing a manuscript for the narration during R5 calls for a very thoughtful selection and use of words in order to create a representation that makes sense of the phenomenon. The verbal presentational “tryouts” afford students to consider the meaning of concepts as they try to fit them with their model of the process.
Concluding discussion
The study at hand is built upon previous research indicating that: (1) students have difficulties in making connections and transformations across the experiential/macroscopic and sub-microscopic levels (Albert, 2012; Chang et al., 2013), which hinder their ability to develop an integrated understanding of chemistry (Kozma, 2003; Gilbert and Treagust, 2009; Taber, 2013); (2) traditional experimental laboratory work does not do enough to support students in developing scientific reasoning (Abrahams and Millar, 2008); (3) representational activities may encourage chemical reasoning (Davidowitz et al., 2010; Yaseen and Aubusson, 2018). Based on these premises, a teaching sequence was designed in which traditional practical laboratory work was extended with representational activities that aimed to force the students to constantly re-represent their experiments in order to explain the observable at a sub-micro level. Below is a summary of how the constituted chemistry content developed through the representational activities, followed by a discussion of our main findings.Animated laboratory work as a practice that develops chemical reasoning with and through re-representation
During the practical work in the laboratory (RA1), only the experiential and macroscopic levels were present in the students’ reasoning. The results of this study are hence consistent with the findings of other studies concerning the learning gains of practical work. Observations need respect and careful attention in order to support the discernment of something more than the perceptual (Schank and Kozma, 2002; Kozma, 2003; Abrahams and Millar, 2008). However, we saw that taking photographs during the experiments afforded observational attention (experiential level), and that the photos and the experimental materials afforded tentative macroscopic conceptualisations.It was during the making of the storyboard (RA2) that the students began to address the relation between the macro and sub-micro levels. The idea of zooming in from the experiential to the sub-micro level became a tool for approaching (an explanation at) the sub-micro level. However, the behaviours of the sub-micro particles were not discussed, whether because they were taken for granted or, more likely, too ambiguous to deal with. It appears that the step from visible reality (e.g., a dirty spoon becomes shiny) to the chemical models of an unseen sub-microscopic level (e.g., silver ions are reduced to silver atoms) was not made without effort. We could also see that the students’ use of everyday objects and gestures facilitated their communication about the macro- and sub-micro-level processes, something that may be interpreted as a sign of them having difficulties in conceptually addressing the sub-micro level. In addition, these tools afforded inroads to representations of the dynamic aspects of the macro and sub-micro levels.
The sub-micro level, and the relation between it and the macro level, became more prevalent in the students’ reasoning when they made the physical models (RA3). The representational challenges of relating the experiential level of the various phenomena with the sub-microscopic level of a chemical explanation were mainly dealt with by merging these levels into one representation, a so-called hybrid model (Harrison and Treagust, 2002). We observed that this approach prompted questions about what a model is, and channelled the students’ attention towards organisational issues, e.g., the nail–atom–electron relation. Working with clay, paper, Lego and other materials to generate physical models, the students engaged in reasoning about the size, number, electron distribution, surfaces, particle systems (atom–electron, ion–atom) and other properties of the modelled sub-micro particles. The results also showed that observing and comparing the models constructed by other students in the group, or by other groups, led the students to discern and address more sub-microscopic aspects then they would have if working alone. In addition, it made salient the fact that different physical models can highlight different features of a theoretical model. We believe that many of these issues would not have been raised if only ready-made models had been used. We believe that the students’ different representations of the same referent may be regarded as forming a system of multiple representations, thus constraining the phenomenon in multiple ways and making its boundaries more defined (Prain and Tytler, 2012). Here, we believe, lay one important value of working together with others.
It was not until the activity of taking photographs of the models (RA4) that the dynamic aspects of the sub-micro world the students were trying to conceptualise became salient and were really handled in the groups. When given the form of physical models “in motion”, the sub-microscopic particles became real material objects. As such, the students had to give them physical properties such as size, speed, number and consistency; that is, aspects that forced careful consideration of their models of the sub-micro processes.
Finally, adding a narration (RA5) afforded reasoning about the meaning of concepts and how to use them. In addition, it afforded discernment of the complexity of conceptualising multi-particle models, when relating the macro and sub-microscopic levels. RA5 hence involved fitting verbal and visual sub-micro and macro-level representations together – a complex process that demands reflection about the meaning of concepts (Hoban and Nielsen, 2013).
Student-made physical models as tools for chemical reasoning
The communication during RA1–4 was afforded by the students’ use of different cultural tools. During RA1 and 2, these tools were experimental materials (real or photos) or artefacts at hand, such as a pencil and a pad. These tools, together with accompanying gestures, enabled communication about the discernible aspects, and descriptions at the experiential and macro levels. During RA3 and 4, the physical models afforded reasoning about the sub-micro level, as well as the relation between the experiential and sub-micro levels.As shown in the results, the students used the physical models during RA3 and 4 as tools for speculative thinking and reasoning, such as trying out how sub-micro particles might interact and move (e.g., episode 11), and for generating ideas about how they relate to macro-level objects (e.g., S1 in group F builds a nail from Lego bricks and says that “there are lots and lots of atoms that are stuck together and form zinc and copper”). We claim that, during these verbal “try-outs” and manipulations of the physical models, the students engage with the kinds of thinking and doing that distinguish the practice of chemists in terms of reasoning with and through representational construction. Hence, we view these knowledge-building processes as a productive feature of the application of the RCA approach to student learning. Our results illustrate how the physical models may function as a practical enabler of chemical reasoning.
There is, to our knowledge, no prior study of student-generated animations of chemical processes focusing on the role of physical models. Previous studies have used animation software programs such as Chemation (e.g., Chang et al., 2013) and Chemsense (e.g., Schank and Kozma, 2002), which provide students with digital representations depicting atoms and chemical bonds, or programs like K-sketch in which students make digital drawings of atoms/molecules (e.g.Yaseen, 2018). However, none of these studies report findings that resemble ours, such as the students manipulating the digital models and using them as tools for thinking and spontaneous tryouts. Hence, it seems as though software programs do not afford this kind of thinking and doing, something we believe has to do with the models being “tied to the screen” and not readily available for instant manipulation. Such a hypothesis of instant availability also fits into our results from the storyboard workshop, and may explain why the students abandon “reasoning with the pen” in favour of gestures and physical tools. To conclude, the results of this study are novel in terms of bringing to light the learning potential of having students manipulate physical models of sub-micro particles with the aim of representing chemical processes.
The generation of physical models during RA3 and 4 also forced choices and prompted reasoning about the number of atoms and their spatial arrangement in a molecule. The studies by Schank and Kozma (2002) and Chang et al. (2013) report similar findings. However, the results of our study show that the students also faced choices regarding other aspects, such as the relative size of different particles: atom–electron and atom–atom (the latter not included in the results), how to represent the atom: as a sphere/circle/other, or including electrons (valence only or the entire e-configuration), colour (related to the macro level/conventions/other or using colour changes as an indicator of the reduction of an atom). As such choices are (to our knowledge) limited in software programs, it seems that this possibility enabled the students to engage in more nuanced reasoning about aspects of the sub-micro particles.
Representational challenges that can afford chemical reasoning
A specific challenge in the context of chemistry is to represent the relation between the experiential and sub-microscopic levels in a meaningful way. When, as in this study, the students were encouraged to create a representation encapsulating this relation, they approached it using one of two strategies. The first strategy resulted in a final video consisting of video clips/photos from the experiment (experiential level) and an animation that focused on the sub-micro level only. This strategy was rare – only one animations used it. In the second strategy, the students also included video clips from the experiment, but when creating the animation they chose to merge the sub-micro level with the macro level in terms of representing discrete particles together with objects (e.g., a cucumber or a key, see Fig. 5 and 8) or representing an object as an assembly of atoms arranged into the shape of the object (e.g., a nail or a steel wool sample, see Fig. 5 and 9a). As illustrated by the students' struggle in Episode 6, the problem with the first strategy is that the relation to the visible the experimental setup (the cucumber and the two nails) is lost, while a representation using the second strategy does not look “realistic”, but also raises question about how to refer to merged representation (e.g., episode 14 – “iron or iron atoms”). Hybrid representation is often painted as problematic in research on students’ interpretations of representations (Harrison and Treagust, 2002). However, when the students generated hybrid representations themselves, our results indicate that the challenges they confronted made them reflect on limitations of both the representational strategies. This included reflections on what a model is, and on the (size) relation between matter and atoms. Based on this, we disagree with Michalchik et al. (2008), who argue that only focusing on the sub-micro level when creating an animation is something to strive for. In this study, almost all the groups generated hybrid models, indicating a reluctance to leave the macro level; the students seem to need the physical models of the experimental set-up to help them think and communicate (e.g., episode 6) (compare with Roth and Lawless, 2002a).Generating an animation afford chemical reasoning about the dynamic aspects of chemical reactions
During the storyboard activity (RA2) we found, in line with Chang et al. (2013), that static representations on paper make it possible for learners to ignore the dynamic events hidden behind the arrow in the reaction formula. Consequently, the pictures on the storyboards only showed a “before and after”. Storyboarding only allows visualisation of the dynamics to a limited extent; consequently, we observed many examples where the students exchanged paper and pen for gestures and physical objects as representational tools, to try to add this dimension to their meaning-making.The activity of moving the models and taking the photographs (RA4) provided the necessary tools to explore and visualise the dynamics of the processes in detail. Hence, our study confirms what Akaygun (2016) suggests: generating animations afford discernment of motion more than storyboarding. In line with Chang et al. (2013), Yaseen and Aubusson (2018) and, Michalchik et al. (2008), our results also show that the making of the animation (RA4) forced the students to reason about the chemical reactions in terms of bond breaking, bond formation and atom rearrangement. However, our students went beyond a mere focus on the reaction mechanism. The incremental work of moving and photographing the particle models (RA4) also raised questions about how the particles move, and where the “new” particles (e.g., iron hydroxide) should move. The how question is presumably a result of the absence of constraints on movement using the stop-motion technique, while the where-to question is prompted by the macro context within which the sub-micro process is animated (the hybrid-model approach as used by most of the student groups); animating the formation of new particles is not enough, the macro environment demands choices about final position. The reasoning following episode 11 illustrates how the students turned to their observations (rust precipitated at the surface of the steel wool) to guide them in their modelling (they move the iron hydroxide particle to sit on top of the steel wool model).
To conclude, our results are new in the sense that they suggest that using open formats for animation (e.g., stop-motion technique) afford: (1) more reasoning about the characteristics of the particle motion, and (2) afford hybrid representations, which in turn prompts more questions about the direction and final destination of new particles, and hence the relation between observation and the sub-micro level, compared to software programs. Noteworthy is that Akaygun (2016) identified specific affordances linked to higher levels of freedom for representing motion in software programs. When students generated animations of the oxygen atom using three different software programs, it was found that the software with the greatest flexibility also to a greater degree afforded students to represent what they intended in terms of particle motion, and to show more qualified atomic models.
Writing a narration afford the use of, and reflections on, scientific concepts
Our results imply that the task of creating the narration (RA5) enabled the students to engage with scientific language in a more precise manner than during RA1–4. This meant moving away from the context-specific everyday terms and “muddled” talk displayed during the previous activities (RA1–4), and trying to form a more general and precise scientific terminology (Roth and Lawless, 2002b). This change has not been noticed in earlier studies on students adding a narration to their animations (see Hoban and Nielsen, 2013; Hoban and Nielsen, 2014; Nielsen and Hoban, 2015). One reason for this, we believe, is the more concrete and everyday learning objects used in those studies, such as the ladybird lifecycle (Hoban and Nielsen, 2013), or the phases of the moon (Hoban and Nielsen, 2014; Nielsen and Hoban, 2015). In contrast, the students in our study were given the task of animating abstract concepts. Thus, narration activities may prove especially fruitful in relation to sub-microscopic phenomena.Our results point to some challenges in dealing with the abstract sub-micro level. The students sometimes lacked sub-micro language and adopted macro-level language to describe sub-micro processes (e.g., atoms dissolve), even though their animation of the sub-micro process was legitimate. We interpreted this as a lack of awareness of verbs that are reserved for representing sub-micro events. We concur with the point made by Taber (2001) about the importance of teachers not using the same verbal label for both macro and sub-micro events – an otherwise common habit among teachers – since it hampers the students’ ability to discern each specific level and the relationships between them.
Concluding remarks
The results of this study illustrate that the task of producing, observing, documenting and explaining a phenomenon in an animation afforded students to engage in reasoning at all three levels of the chemical triplet (Taber, 2013) and enabled them to co-construct an integrated explanation of the phenomenon. Importantly, the macro and experiential levels were ubiquitous during the entire construction process, and formed a background against which the sub-micro aspects were described.Prior studies on student-generated animations focusing the relation between observation and submicro level show mixed results. Albert (2012) andChang et al. (2013) found that most students were not able to discern this relation through creating animations, while the Michalchik et al. (2008) study more strongly indicates development of this ability. The students in the Michalchik study, as well as in our study, worked cooperatively and produced the phenomena to be explained. Considering the promising results of both studies, we suggest that these features are critical for (integrated) chemistry learning. Moreover, we conclude that our results illustrate the productive features of the application of the RCA approach to student learning in various ways (Prain and Tytler, 2012; Tytler et al., 2013). Firstly, the task of cooperatively making a storyboard, physical models and a stop-motion animation to explain them afforded exploratory representational constructions. Secondly, each representational activity afforded meaning-making through productively constraining it in particular ways. However, the results imply that storyboarding is too constrained by paper and pen tools; to better support exploratory visualisations of the particle dynamics during the storyboard work, we suggest also providing the students with temporary particle models (e.g., LEGO bricks). Thirdly, the results illustrate how the constituted learning practice engaged students in “reasoning with and through representational construction” (Prain and Tytler, 2012, p. 2761). Fourthly, a profound characteristic of the students’ chemical reasoning during RA2–5 was how representational issues prompted questions about the various phenomena, and vice versa. Hence, we identify this reasoning, as opened up by representation construction, to involve the “refinement of a mix of relations between aspects of the phenomena being interpreted and aspects of the representation” (Tytler et al., 2013, p. 106). Particularly in the case of chemistry, with its wide range of different representations in both research and education, this approach seems to have a promising potential as a framework to further explore student understanding and learning.
Implications for practice
It is worthwhile noting that the students spent a considerable amount of time, and put a lot of effort, into the tasks they were set to solve. In the course evaluation, 60 percent of the respondents answered that they would definitely use this task in their own teaching. Despite their interest and engagement, the task is time-consuming for both students and teachers. We are thus motivated to ask whether just making the storyboard, and/or using ready-made models or simulation software may be a time-efficient yet worthwhile alternative. However, our results confirm those of Hoban and Nielsen (2010); each representational activity (RA1–5) afforded the discernment of specific critical aspects of the phenomena, as provided by its particular mode. Hence, we conclude that critical learning opportunities diminish if one chooses to sidestep one or more of the activities. In this regard, we wish to emphasise that the actual experience, and especially the documentation of, the observed phenomenon supported the task of explaining it. The documentation, as well as the experimental objects, became crucial resources for the observational focus and conceptualisations of the observable. In addition, the design of the task forced the students to repeatedly return to the observational experience – it served as a “core” around which the animation work revolved. To conclude, we believe that merging experiments with the representational task of explaining observations at the sub-micro level enables students to develop an integrated understanding of chemistry. Hence, we suggests that this is preferable to having students, for an equivalent length of time, do more traditional work in the laboratory and write superficial reports.Conflicts of interest
There are no conflicts to declare.Appendix 1. Transcripts of episodes in Swedish and English
Episode 1S1: Nu bubblar det i [ohörbart]. Titta!
S2: Mm.
S1: Interesting, interesting.
S1: Nu bubb… nu måste det vara liksom att
S2: Det är någon reaktion.
S1: Mm.
[—]
S1: Nu luktar det väldigt starkt här.
S1: Now it's bubbling in [inaudible]. Look!
S2: Mm.
S1: Interesting, interesting.
S1: Now it bubb… now it must be like…
S2: It's some reaction.
S1: Mm.
[—]
S1: Now it smells very strong here.
Episode 2
S3: Jag tänker att man har, säg att man har [ohörbart] den här bilden [börjar skissa en sked i en bägare med en vätska] […] och så har man den bilden [placerar handen på skissen med skeden i bägaren] och sen så [gör ett surrande ljud] zoomar man in en gång till. Här ser man [ritar en cirkel runt en liten del av skeden] [—] och sen så ringar man in den här [ritar ännu en cirkel ovanpå den första] och så zoomar vi in, och då blir det liksom […] och då har vi aluminium [ritar ännu en cirkel ovanpå den första]. och… … [blir avbruten av S3 som ifrågasätter hans tillvägagångssätt, se nedan].
S3: I figure that one has… say that one has [inaudible] this picture [starts to make a sketch of a spoon in a beaker with liquid]. [—] and so one has this picture [puts his hand on the sketch of the spoon in the beaker] and then zzz [makes a buzzing sound] one zooms in one more time. Here one sees [draws a circle around a small part of the spoon]. [—] and then one circles this one [draws another circle on top of the first one]. […] and so we zoom in, and then it becomes, and then we have aluminium [makes a new sketch below the first one, drawing two parallel lines] and … [is interrupted by S3 questioning his approach, see below].
Episode 3
S3: Där är skeden [pekar på skeden I ruta 2 I storyboarden].
S1: Mm
S3: Zooma in på ytan [pekar på ruta 2] ytan, här fastnar det saker [pekar på ruta 3].
S1: Mm. och sen ska vi visa at det här [pekar på ruta 3] liksom ligger i en bägare.
S3: Men då måste vi fortfarande… ska vi ta hela [ohörbart] vi tar hela. och sen zoomar vi ner oss, kan vi inte göra så
[…]
S3: I den här [pekar på ruta 5] ska vi ännu en gång zooma in, och visa; skeden innehåller detta.
S1: Ja just det.
S3: The spoon is there [points at the sketch in frame 1 of the storyboard].
S1: Mm.
S3: Zoom in on the surface [points at frame 2]. The surface, here things get stuck [points at frame 3].
S1: Mm. And then we have to show that this [points at frame 3] lies in a beaker [inaudible].
S3: But then we still have… should we take the entire [inaudible] we take the entire. And then we zoom again. Can’t we do it like that?
[—]
S3: In this one [points at frame 5], should we once again zoom in, and show the spoon contains this?
S1: Yes, exactly.
Episode 4
S3: Jag skulle förklara, om man tänker sig någonting grått då som är då silverskeden. sen har man, då gör man beläggningen alltså den här…
S4: Silversulfiden.
S3: Ja precis i bitar. så att man kan […] man har, ja, blått papper som är liksom i bitar så att man liksom plockar bort det (flyttar bort pennorna från blockets kant) allteftersom. Är du med?
S3: I should have explained, if one figures something grey that is the silver spoon [puts his hand on his notepad representing the silver spoon], then one has, then one makes the coating, thus this one here [puts pencils along the edge of the notepad, representing “the coating” on the spoon].
S4: The silver sulphide.
S3: Yes exactly, in pieces. So that one can […] one has, well, blue paper that is sort of in pieces, so that you sort of remove it [removes the pencils from the notepad] as it continues. Do you get it?
Episode 5
S3: Sen går ett plus från aluminiumjonen, eller aluminiumet [knyter vänstrahanden och lägger högra handen på den vänstra] ett plus går [flyttar högra handen bort från den vänstra] lämnar ett minus […] pluset vandrar över till [ rör högra handen åt höger]… vilket gör att…[tystnar, skakar på huvudet och gör en uppgiven gest med armar/händer].
S3: Then a plus goes from the aluminium ion, or the aluminium, [ties his left hand and puts his right hand on his left], a plus goes [moves his right hand away from the left], leaves a minus/--/The plus wanders over to [moves his right hand to the right]…. which makes… [S4 gets quiet, shakes his head and makes a resigned gesture also with his hands].
Episode 6
S2: Vi klipper ut och gör så att så det ser ut som spikar sitter (pekar på gröna pappret)
[…]
S1: Vi ska ju inte se spikarna på atomnivån
S2: Nä men du måste ju ändå ha det, och sen ska du ju gå in, och sen så får du ju xxx vi måste ju visa hur själva gurkan ser ut också. Eller?
S1: Eller så kan vi göra en liten gurka [mindre än den som S3 föreslår] med två spikar i. För sen så ska du ju typ bara se… [börjar placera små Lego bitar i en rad på bordet] Om vi tänker oss att spiken ser ut som… […] och så är det massa, massa atomer, som sitter ihop som bildar zink och som bildar koppar.
S1 drar ett rakt streck i luften och säger”du ser att det är ett rakt streck liksom men det är byggt av atomer”. S3 disagrees with the suggestion of using a small cucumber model and emphasises that they should use a bigger model where they zoom in on the nails. However, S1 objects:
S1: Jo men jag tänker det blir inte realistiskt stort tänker jag, för att atomen är ju så pytte, pytte, pytte, pytte, pytte jämfört med hela gurkan. Förstår ni hur jag tänker?
S3: Men det är en modell.
S1: Ja.
S3: Det är en modell, modeller är inte realistiska.
S1: Fast man kan ju göra ganska realistiska
S2: We cut it out and make it look like nails sitting [on the cucumber] [S2 points at the green paper].
[…]
S1: We are not supposed to see the nails at the atomic level [since we will zoom in].
S2: No, but you need it, and then you have to go in, and then you will [inaudible] we need to show how the cucumber itself looks like. Or?
S1: Or we can make a small cucumber with two nails in it. Because then you're supposed to only see like …. If we figure the nail looks like … [begins to place small Lego bricks in a row on the table] and then there are lots and lots of atoms that stick together and form zinc, and form copper.
S1 draws a straight line in the air and concludes that “you see that there is a straight line sort of but it's built of atoms.” S3 disagrees with the suggestion of using a small cucumber model and emphasises that they should use a bigger model where they zoom in on the nails. However, S1 objects:
S1: I think it won’t be realistic in size. I think. Because the atom is so tiny, tiny, tiny, tiny, tiny compared to the whole cucumber. Do you know what I mean?
S3: But it's a model.
S1: Yes.
S3: It's a model, models aren't realistic.
S1: But one can make rather realistic ones.
Episode 7
S1: Och hur stora är atomerna då?
S3 [med irritabel ton]: Ja men du ritar ju dem hur stora du vill att de ska vara [ritar cirklar med fingret på olika ställen på det gröna pappret]
S1: Och sen ska elektronerna vara mindre?
S3: Ja.
S1: Jag tänker att elektronerna blir så små så man kan inte se dem på filmen.
S1: And how big are the atoms then?
S3: But you draw them as big as you want them to be [S3 makes a circle with her finger and repeatedly puts it in different places on the green paper].
S1: And then are the electrons supposed to be smaller?
S3: Yes.
S1: I think the electrons will be so small so you won’t be able to see them in the movie.
Episode 8
S2: Hur många gula behöver vi? De gula.
S1: Hur mycket du vill.
S4: Elektroner.
S2: Elektroner.
S2: Jag vet inte riktigt vad de är. Haha.
S3: Hur många är det som ska försvinna då?
S2: Det är två som ska vara kvar säger vi då. Eller? Är det det ni säger? Två kvar? [placerar två elektronmodeller bredvid stålullsmodellen.]
S3: Vi skulle ju ha två elektroner här på de där [pekar på järn atomerna i stålullsmodellen] men då måste ju alla stanna kvar, sitta på den där, det måste vara fyra som sitter på den där.
S2: fyra säger vi, sen ska ju några iväg till syret.
[de fortsätter att diskutera hur många elektroner som behövs]
S1: Och dom här som ska gå iväg, så då kommer det inte finnas nått kvar I yttersta skalet [gör cirklar med fingret i luften]
S2: Är det det som är tanken då? Att det ska vara så här då [placerar gula elektronmodeller på den större gröna stålullsmodellen] Att det liksom är två i varje, eller?
S1: Asså, ja. de här elektronerna vandrar [gör svepande cirkel med handen över modellen på bordet] gärna.
S2: Så de behöver bara vara runtomkring?
S1: De är fria som Sven sagt.
S3: Jaha för jag trodde, jaha ok. Det är ingen som sitter fast på själva atomerna
S2: De är inuti atomerna, eller
S1: Ja, [Nickar]
S2: Men det kan vi ju inte visa liksom.
S3: Men det här är atomerna? [pekar på den gröna lermodellen på bordet]
[…]
S2: Elektronerna är fria.
[…]
S1: De är fria I järnbiten. Eller hur? Det är därför de…
S3: De kan gå iväg ja.
S2: How many yellow ones do we need, the yellow ones?
S1: As much as you like.
S4: Electrons.
S2: Electrons.
S2: I don’t really know what they are. Haha.
S3 How many of them are supposed to disappear then?
S2: There are supposed to be two left, let's say. Or? Is that what you say? Two left? [places two electron models beside the iron wool model].
S3: We were supposed to have electrons here on those [points at the iron atoms in the iron wool model], but then all of them must stay, be on that one, there must be four on that one.
S2: So let's say four. Then some are supposed to go to the oxygen.
[they continue to discuss how many electrons are needed]
S1: And it's these that go away, then there will be none left in the outermost shell. [makes circles in the air with a finger]
S2: Is this the idea then? That it should be like this [places more yellow electron models onto the larger green iron wool model] that there is like two in each, or?
S1: Well yes, these electrons like to [make a circle with one hand above the models on the table] wander.
S2: So they just have to be around [the iron atoms]?
S1: They are free like Sven [one of the physics teachers] said.
S3: Aha, because I thougth, aha, ok, there is none stuck on the atoms themselves.
S2: They are inside the atoms, or …
S1: Yes. [Nods]
S2: But we won’t be able to show that.
S3: But these are the atoms? [points at the green clay models on the table].
[…]
S2: The electrons are free.
[…]
S1: They are free within the piece of iron. Right? That's why they…
S3: They can go away, yes.
Episode 9
S3: Men nu är frågan om de här ska va gröna egentligen [pekar på den gröna vätejonmodellen] för det här [pekar på den röda väteatomen i vattenmolekylmodellen] är samma som det där [pekar på den gröna vätejonmodellen] förutom att det här är en jon och det där är en atom.
S3: But now really the question is whether these should actually be green [points at green hydrogen ion model]. Because this [points at the red hydrogen atom in the water molecule model] is the same as that [points at the green hydrogen ion model]. Except that this is an ion and that is an atom.
Episode 10
S1: Men måste vi inte visa [i animationen] att den [silverskeden] blir skinande och ren [ohörbart].
S2: Ja men det är det vi säger samtidigt som de [elektronerna] vandrar. Först säger vi att elektronerna vandrar, sen kanske vi gör en paus där [in the animation] och sen visar fotot [av silverskeden] igen. Nu när de här elektronerna har fastnat på varsin silveratom blir den… blir den, går den från atomjon till grundämne. Det är därför den blir skinande.
S1: But, don't we have to show [in the animation] that it [the silver spoon] becomes shiny and clean [inaudible].
S2: Yes but that's what we are saying at the same time as they [the electrons] wander. First we say the electrons wander. Then maybe we make a pause there [in the animation] and then show the picture [of the silver spoon] once again; now when these electrons have got stuck on a silver atom each, it becomes … it becomes, it goes from atomic ion to element. That's why it becomes shiny.
Episode 11
S1: Sen kommer de här järnatomerna som vill jättegärna ge bort sina valenselektroner.
S2: Mm
S1: Så en järnatom går iväg med vattnet [plockar bort en järnatomsmodell från modellen på bordet]
S2: Med vattnet?
S1: Ja, löser sig i vattnet [plockar upp en vattenmolekylsmodell]
S2: Jaha
S1: och de lämnar kvar sina [plockar upp små gula eletronmodeller] valenselektroner.
S2: Men vadå? Ska den här ta ett vatten och åka iväg [plockar upp grön järnatomsmodell och en vattenmolekylsmodell] eller vadå?
S4: He, he.
S1: Ehh
S2: Ska den flyga iväg med den? [flyttar modellerna upp i luften i en stor rörelse] De blir väl ändå någonting kvar?
S1: Then these iron atoms, which are eager to give away their valence electrons, comes along.
S2: Mm
S1: So, one iron atom goes away together with water [pick up a model of an iron atom from the model on the table].
S2: With the water?
S1: Yes. Dissolves in the water. [pick up a model of a water molecule]
S2: Oh, yes.
S1: And they leave their valence electrons [pick up small yellow electron models] behind.
S2: But hey, is this one supposed to take a water and just go away [pick up a green model of an iron atom together with a model of a water molecule], or what?
S4: He he.
S1: Ehh.
S2: Should it fly away with it? [move the models up in the air in a big motion] There will still be something left, won’t it?
Episode 12
S2: Det måste finnas mer gröna… annars så…
S1: Jag tror det räcker så.
S2: Ok.
S1: Vi provar så.
S2: Annars försvinner alla.
S1 [till lotta som nu kommer in i rummet]: Vart tar de gula vägen?
S2: There needs to be more green ones [green refers copper ion models]. Otherwise …
S1: No but I think it's enough.
S2: Okay.
S1: Let's try this [modelling approach].
S2: Otherwise all the green ones disappear, so it…
S1: Yes at the end they disappear. But where do the yellow ones [sulphate ion models] gp?
S2: That's why I say that there needs to be more green ones.
S1: Yes, but where do the yellow ones go?
S1 [to S3 who just entered the room]: Where do the yellow ones go?
Episode 13
S2: När vattnet kommer i kontakt med järnatomerna… ska man säga järnatomerna släpper. eller vad säger man?
S3: De löses ju upp väl. var det inte så?
S2: Men samtidigt stannar vissa kvar i… det är bara vissa, det är bara vissa atomer som far iväg. de andra far ju
inte iväg.
S1: yes
S2: när det löses upp försvinner allt tänker jag.
S1: Ja, vissa atomer löser sig i vattnet och släpper sina valenselektroner i järnet.
S2: ska jag skriva vissa av atomerna eller några av atomerna, eller nåt.
S1: ja.
S2: det är inte alla
S1: nej det är det inte.
S2 [skriver och pratar]: atomerna [slutar att skriva och tittar upp] alltså, löser…[tittar på S1]
S3: löser sig upp i vattnet
S2 [skriver och pratar]: löser upp sig… i vattnet. Löser upp sig låter konstigt, löser sig.
S1: Löses upp.
S2: ja precis löses upp… i vattnet [skriver] och lämnar då kvar… eller?
S2: When water meets the iron atoms … should one say the iron atoms come loose or let go. Or what does
one say?
S3: They dissolve. Right?
S2: But yet, some stay in … there were only some … there are only some atoms that go away, the others don’t
go away.
S1: Yes.
S2: When it dissolves, everything disappears, I figure.
S1: Yes, some iron atoms dissolve in the water and drop their valence electrons in the iron.
S2: Should we write some of the atoms or a few of the atoms, or something.
S1: Yes.
S2: Because it's not all of them.
S1: No it's not.
S2 [writes and talks]: The atoms [stops the writing, looks up] so, dissolve …
S3: Dissolves away in the water.
S2: [writes and talks]: Dissolves away … in the water. Dissolves away sounds strange. Dissolves.
S1: Are dissolved.
S2: Yes, exactly. Are dissolved [writes] … in the water … and then leaves behind … or?
Episode 14
S2: Vad ska man säga… de atomerna lämnar då kvar sina valenselektroner i järnbiten? Eller i stålullen? [vänder sig till S1] eller bara lämnar kvar, kanske räcker, lämnar då kvar sina valenselektroner.
[…]
S2: Hos de andra atomerna som är i järn?
S1: Kan man inte säga…
S3: Det är egentligen järnbiten.
S1: Järnbiten.
S3: Det är ju i stålullet da.
S2: Det känns som…
R: Men det känns lite rörigt hela tiden att prata om atomnivå och sen prata om …
S2: Ja vi kanske inte ska använda stålull utan bara järn, järnatomer egentligen.
S2: What should one say … those atoms leave behind their valence electrons in the iron piece? Or in the steel wool? [Turns to S1], or just leave behind, maybe enough, leave their valence electrons behind.
[…]
S2: With the other atoms that are in the iron?
S1: Can’t you say …
S3: It's actually the iron piece.
S1: The iron piece.
S3: It's in the steel wool…
S2: It feels like …
S1: But it feels a bit muddled to always talk at the atomic level and the talk about …
S2: Yes. Maybe we shouldn't use steel wool but only iron, iron atoms really.
References
- Abrahams I. and Millar R., (2008), Does practical work really work. A study of the effectiveness of practical work as a teaching and learning method in school science, Int. J. Sci. Educ., 30(14).
- Ainsworth S., (2006), A conceptual framework for considering learning with multiple representations, Learn. Instruct., 16(3), 183–198.
- Ainsworth S., Prain V. and Tytler R., (2011), Drawing to learn in science, Science, 333(6046), 1096–1097.
- Akaygun S., (2016), Is the oxygen atom static or dynamic? The effect of generating animations on students’ mental models of atomic structure, Chem. Educ. Res. Pract., 17, 788.
- Akaygun S. and Jones L. L., (2014), Words or pictures: a comparison of written and pictorial explanations of physical and chemical equilibria, Int. J. Sci. Educ., 36(5), 783–807.
- Albert J. L., (2012), Using Student-Generated Animations about Water Boiling to Impact Student Understanding of the Particulate Nature of Matter, Diss., North Carolina State University.
- Andersson B., (1990), Pupils' conceptions of matter and its transformations (Age 12–16), in Relating macroscopic phenomena to microscopic particles, Lijnse P. L., Licht P., De Vos W. and Waarlo A. J. (ed.), Utrecht: CM-Press, pp. 12–35.
- Ardac D. and Akaygun S., (2004), Effectiveness of multimedia-based instruction that emphasizes molecular representations on students' understanding of chemical change, J. Res. Sci. Teach., 41(4), 317–414.
- Ardac D. and Akaygun S., (2005), Using Static and Dynamic Visuals to Represent Chemical Change at Molecular Level, Int. J. Sci. Educ., 27(11), 1269–1298.
- Barak M. and Hussein-Farraj R., (2013), Integrating Model-Based Learning and Animations for Enhancing Students’ Understanding of Proteins Structure and Function, Res. Sci. Educ., 43(2), 619–636.
- Barnea N., and Dori Y. J., (1996), Computerized molecular modeling as a tool to improve chemistry teaching, J. Chem. Inform. Comput. Sci., 36, 629–636.
- Berg A., Löfgren R. and Eriksson I., (2010), Observationer i kemiklassrummet – att lära sig se kemiska reaktioner, in Innehållet i fokus – kemiundervisning i finlandssvenska klassrum, Eriksson I. (ed.), Stockholm: Stockholms Universitets Förlag, pp. 37–69.
- Chandrasegaran A., Treagust D. F. and Mocerino M., (2011), Facilitating High School Students' Use of Multiple Representations to Describe and Explain Simple Chemical Reactions, Teach. Sci., 57(4), 13–20.
- Chang H.-Y. and Quintana C., (2006), Student-Generated Animations: Supporting Middle School Students’ Visualization, Interpretation and Reasoning of Chemical Phenomena, in Proceedings of the 7th international Conference on Learning Sciences, pp. 71–77.
- Chang H.-Y., Quintana C. and Krajcik J. S., (2010), The impact of designing and evaluating molecular animations on how well middle school students understand the particulate nature of matter, Sci. Educ., 94(1), 73–94.
- Chang H. Y., Quintana C. and Krajcik J., (2013), Using Drawing Technology to Assess Students’ Visualizations of Chemical Reaction Processes, J. Sci. Educ. Technol., 1–15.
- Cheng M. and Gilbert J. K., (2009), Towards a Better Utilization of Diagrams in Research into the Use of Representative Levels in Chemical Education, in Multiple Representations in Chemical Education. Models and Modeling in Science Education, Gilbert J. K. and Treagust D. (ed.), Dordrecht: Springer, vol. 4.
- Chittleborough G. and Treagust D., (2008), Correct interpretation of chemical diagrams requires transforming from one level of representation to another, Res. Sci. Educ., 38(4), 463–482.
- Cooper M. M., Stieff M. and DeSutter D., (2017), Sketching the Invisible to Predict the Visible: From Drawing to Modeling in Chemistry, Top. Cognit. Sci., 3–21.
- Davidowitz B., Chittleborough G. and Murray E., (2010), Student-generated submicro diagrams: a useful tool for teaching and learning chemical equations and stoichiometry, Chem. Educ. Res. Pract., 11(3), 154–164.
- Deaton C. M., Deaton B. E., Ivankovic D. and Norris F. A., (2014), Creating stop-motion videos with iPads to support students’ understanding of cell processes, J. Digit. Learn. Teach. Educ., 30, 67–73.
- Dori Y. J. and Barak M., (2001), Virtual and physical molecular modeling: fostering model perception and spatial understanding, Educ. Technol. Soc., 4(1), 61–74.
- Dori Y. J., Barak M. and Adir N., (2003), Web-Based Chemistry Course as a Means To Foster Freshmen Learning, J. Chem. Educ., 80(9), 1084.
- Gabel D. L., (1999), Improving Teaching and Learning through Chemistry Education Research: A Look to the Future, J. Chem. Educ., 76(4), 548.
- Gabel D. L. and Bunce D. M., (1994), Research on problem solving: Chemistry, in Handbook of research on science teaching and learning, vol. 11, pp. 301–332.
- Gee J. P. and Green J. L., (1998), Discourse analysis, learning, and social practice: a methodological study, Rev. Res. Educ., 23, 119–169.
- Gilbert J. K. and Treagust D. F., (2009), Introduction: macro, submicro and symbolic representations and the relationship between them: key models in chemical education. Multiple representations in chemical education, in Multiple Representations in Chemical Education, Models and Modeling in Science Education, Gilbert J. K. and Treagust D. F. (ed.), Dordrecht: Springer, 4th edn, pp. 1–8.
- Gooding D. C., (2006), From phenomenology to field theory: Faraday's visual reasoning, Perspect. Sci., 14(1), 40–65.
- Gregorius R. M., Santos R., Dano J. B. and Guitierrez J. J., (2010a), Can Animations Effectively Substitute for Traditional Teaching Methods? Part I: Preparation and Testing of Materials, Chem. Educ. Res. Pract., 11, 253–261.
- Gregorius R. M., Santos R., Dano J. B. and Guitierrez J. J., (2010b), Can Animations Effectively Substitute for Traditional Teaching Methods? Part II: Potential for Differentiated Learning, Chem. Educ. Res. Pract., 11, 262–266.
- Harrison A. G. and Treagust D. F., (1996), Secondary students' mental models of atoms and molecules: implications for teaching chemistry, Sci. Educ., 80(5), 509–534.
- Harrison A. G and Treagust D. F., (2000), A typology of school science models, Int. J. Sci. Educ., 22(9), 1011–1026.
- Harrison A. G. and Treagust D. F., (2002), The particulate nature of matter: challenges in understanding the submicroscopic world, in Chemical education: towards research-based practice, Gilbert J. K., Jong O. D., Justi R., Treagust D. F. and Van Driel J. H. (ed.), Dordrecht, Netherlands: Kluwer Academic Publishers.
- Hoban G., (2007), Using slowmation to engage presevice elementary teachers in understanding science content knowlege, Contemp. Issues Tech. Teach. Educ., 7(2), 75–91.
- Hoban G. and Nielsen W., (2010), The 5 Rs: a new teaching approach to encourage slowmations (studentgenerated animations) of science concepts, Teach. Sci., 56(3), 33–38.
- Hoban G. and Nielsen W., (2013), Learning science through creating a “slowmation”. A case study of preservice primary teachers, Int. J. Sci. Educ., 35(1), 119–146.
- Hoban G. and Nielsen W., (2014), Creating a narrated stop-motion animation to explain science: the affordances of “Slowmation” for generating discussion’, Teach. Teach. Educ., 42, 68–78.
- Hoban G. F., Macdonald D. C. and Ferry B., (2009), Improving preservice teachers' science knowledge by creating, reviewing and publishing slowmations to TeacherTube, Paper presented at the Proceedings Society for Information Technology & Teacher Education International Conference, Chesapeake, USA: Association for the Advancement of Computing in Education, pp. 3133–3140.
- Hoban G., Loughran J. and Nielsen W., (2011), Slowmation: Preservice Elementary Teachers Representing Science Knowledge Through Creating Multimodal Digital Animations, J. Res. Sci. Teach., 48(9), 985–1009.
- Johnson P. M., (1998), Progression in children's understanding of a ‘basic’ particle theory: a longitudinal study, Int. J. Sci. Educ., 20(4), 393–412.
- Johnson P. M., (2005), The development of children's concept of a substance: a longitudinal study of interaction between curriculum and learning, Res. Sci. Educ., 35(1), 41–61.
- Johnson P. M., (2012), Introducing Particle Theory, in Teaching Secondary Chemistry, K. S. Taber (ed.), Association for Science Education/John Murray, 2nd edn, pp. 49–73.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assist. Learn., 7, 75–83.
- Johnstone A. H., (1993), The development of chemistry teaching: a changing response to changing demand, J. Chem. Educ., 70(9), 701–705.
- Jones L. L., (2013), How multimedia-based learning and molecular visualizations change the landscape of chemical education research, J. Chem. Educ., 90 (12), 1571–1576.
- Kamp B. and Deaton C., (2013), Move, Stop, Learn: Illustrating Mitosis through Stop-Motion Animation, Sci. Activit., 50, 46–153.
- Kelly R. M. and Jones L., (2008), Investigating Students’ Ability To Transfer Ideas Learned from Molecular Animations of the Dissolution Process, J. Chem. Educ., 85, 303–309.
- Kelly R., Phelps A. and Sanger M., (2004), The effects of a computer animation on students’ conceptual understanding of a cancrushing demonstration at the macroscopic, microscopic, and symbolic levels, Chem. Educ., 9(3), 184–189.
- Kozma R., (2000), Students collaborating with computer models and physical experiments, in Roschelle J. and Hoadley C. (ed.), Proceedings of the Conference on Computer Supported Collaborative Learning 1999, Mahwah, NJ: Erlbaum.
- Kozma R., (2003), The material features of multiple representations and their cognitive and social affordances for science understanding, Learn. Instruct., 13(2), 205–226.
- Kozma R. and Russell J., (1997), Multimedia and Understanding: Expert and Novice Responses to Different Representations of Chemical Phenomena, J. Res. Sci. Teach., 34(9), 949–968.
- Kozma R. and Russell J., (2005), Students Becoming Chemists: Developing Representation Competence, in Visualization in Science Education, Gilbert J. (ed.), London: Kluwer Academic Publishers, pp. 121–146.
- Kozma R., Chin E., Russell J. and Marx N., (2000), The roles of representations and tools in the chemistry laboratory and their implications for chemistry learning, J. Learn. Sci., 9(2), 105–143.
- Levy D., (2013), How dynamic visualization technology can support molecular reasoning, J. Sci. Educ. Technol., 22(5), 702–717.
- Lijnse P. L., Licht P., de Vos W. and Waarlo A. J. (ed.), (1990), Relating macroscopic phenomena to microscopic particles, Utrecht: CDBèta Press.
- Louw S., Todd R. W. and Jimarkon T., (2014), Picking the ripe cherry: extract selection in qualitative research, in Proceedings of the International Conference: DRAL 2/ILA 2014.
- McElhaney K. W., Chang H.-Y., Chiu J. L. and Linn M. C., (2015), Evidence for effective uses of dynamic visualisations in science curriculum materials, Stud. Sci. Educ., 51(1), 49–85.
- Michalchik V., Rosenquist A., Kozma R., Kreikemeier P. and Schank P., (2008), Representational Resources for Constructing Shared Understandings in the High School Chemistry Classroom, in Vizualisation: Theory and practice in Science education, Gilbert J. K., Reiner M. and Nakleh M. (ed.), Springer.
- Mercer N., (2004), Sociocultural discourse analysis: analysing classroom talk as a social mode of thinking, J. Appl. Linguist., 1(2), 137–168.
- Nicoll G., (2003), A Qualitative Investigation of Undergraduate Chemistry Students' Macroscopic Interpretations of the Submicroscopic Structures of Molecules, J. Chem. Educ., 80(2), 205.
- Nielsen W. and Hoban G., (2015), Designing a digital teaching resource to explain phases of the moon: a case study of preservice elementary teachers making a slowmation, J. Res. Sci. Teach., 52, 207–233.
- Phillips L. M., Norris S. P. and Macnab J. S., (2010), Visualizations and Science, in Visualization in Mathematics, Reading and Science Education, Series: Models and Modeling in Science Education, Phillips L. M., Norris S. P. and Macnab J. S. (ed.), Dordrecht: Springer, vol. 5, pp. 103–132.
- Prain V. and Tytler R., (2012), Learning Through Constructing Representations in Science: a framework of representational construction affordances, Int. J. Sci. Educ., 34(17), 2751–2773.
- Roth W.-M., (2003), Scientific literacy as an emergent feature of collective human praxis, J. Curric. Stud., 35(1), 9–23.
- Roth W.-M. and Lawless D., (2002a), Scientific investigations, metaphorical gestures, and the emergence of abstract scientific concepts, Learn. Instruct., 12, 285–304.
- Roth W.-M. and Lawless D., (2002b), Science, Culture and the emergence of language, Sci. Educ., 86(3), 368–385.
- Russell J., Kozma R., Jones T., Wykoff J., Marx N. and Davis J., (1997), Use of simultaneous, synchronized macroscopic, microscopic and symbolic representations to enhance the teaching and learning of chemical concepts, J. Chem. Educ., 74(3), 330–334.
- Säljö R., (2011), Learning in a sociocultural perspective, in Learning and Cognition in Education, Aukrust V. G. (ed.), Oxford: Elsevier, pp. 59–63.
- Säljö R. and Bergqvist K., (1997), Seeing the light: discourse and practice in the optics lab, in Reznick R., Säljö R. and Pontecorvo C. (ed.), Discourse, tools, and reasoning: Essays on situated cognition, Berlin: Springer Verlag, pp. 385–405.
- Sanger M. and Greenbowe T., (2000), Addressing student misconceptions concerning electron flow in aqueous solutions with instruction including computer animations and conceptual change strategies, Int. J. Sci. Educ., 22(5), 521–537.
- Schank P. and Kozma R., (2002), Learning Chemistry Through the Use of a Representation-Based Knowledge Building Environment, J. Comput. Math. Sci. Teach., 21(3), 253–279.
- Scott P. H., Mortimer E. F. and Aguiar O. G., (2006), The tension between authoritative and dialogic discourse: a fundamental characteristic of meaning making interactions in high school science lessons, Sci. Educ., 90(4), 605–631.
- Smith K. J. and Metz P. A., (1996), Evaluating student understanding of solution chemistry through microscopic representations, J. Chem. Educ., 73(3), 233.
- Suits J. P. and Sanger M. J., (2013), Dynamic Visualizations in Chemistry Courses, in Pedagogic Roles of Animations and Simulations in Chemistry Courses, ACS Symposium Series 1142, Suits J. P. and Sanger M. J. (ed.), Washington, DC: American Chemical Society, pp. 1–13.
- Taber K. S., (2001), Building the structural concepts of chemistry: some considerations from educational research, Chem. Educ.: Res. Pract., 2(2), 123–158.
- Taber K. S., (2005), Learning quanta: barriers to stimulating transitions in student understanding of orbital ideas, Sci. Educ., 89(1), 94–116.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14(2), 156–168.
- Talanquer V., (2009), On cognitive constraints and learning progressions: the case of ‘structure of matter’, Int. J. Sci. Educ., 31, 2123–2136.
- Tasker R. and Dalton R., (2006), Research into practice: visualisation of the molecular world using animations, Chem. Educ. Res. Pract., 7, 141–159.
- Tasker R. and Dalton R., (2008), Visualising the Molecular World: The Design, Evaluation, and Use of Animations, in Visualisation: Theory and Practice in Science Education, Series: Models and Modeling in Science Education, Gilbert J., Reiner M. and Nakhleh M. (ed.), Springer, vol. 3, pp. 103–132.
- The Swedish Research Council, (2017), https://www.vr.se/download/18.2412c5311624176023d25b05/1529480532631/God-forskningssed_VR_2017.pdf.
- Tytler R., Prain V., Hubber P. and Waldrip B., (2013), Constructing representations to learn in science, Springer Science & Business Media.
- Venkataraman B., (2009), Visualization and interactivity in the teaching of chemistry to science and non-science students, Chem. Educ. Res. Pract., 10, 62–69.
- Waldrip B. and Prain V., (2012), Learning from and through representations in science, in Second International Handbook of Science Education, Fraser B. J., Tobin K. and McRobbie J. (ed.), Dordrecht: Springer, pp. 145–155.
- Wertsch J., (1998), Mind in action, New Yourk: Oxford University Press.
- Williamson V. M. and Abraham M. R., (1995), The effects of computer animation on the particulate mental models of college chemistry students, J. Res. Sci. Teach., 32(5), 521–534.
- Williamson V. M., Watkins J. T. and Williamson K. C., (2013), The effect of student-constructed animations versus storyboards on students’ mental rotation ability, equilibrium content knowledge and attitudes, in Pedagogic Roles of Animations and Simulations in Chemistry Courses, ACS Symposium Series 1142, Suits J. P. and Sanger M. J. (ed.), Washington, DC: American Chemical Society, pp. 293–311.
- Wishart J., (2017), Exploring How Creating Stop-Motion Animations Supports Student Teachers in Learning to Teach Science, J. Res. Technol. Educ., 49(1–2), 88–101.
- Wu H. K. and Shah P., (2004), Exploring visuospatial thinking in chemistry learning, Sci. Educ., 88(3), 465–492.
- Yang E., Greenbowe T. and Andre T., (2004), The effective use of an interactive software program to reduce students' misconceptions about batteries, J. Chem. Educ., 81(4), 587–595.
- Yaseen Z., (2018), Using student-generated animations: the challenge of dynamic chemical models in states of matter and the invisibility of the particles, Chem. Educ. Res. Pract., 19, 1166–1185.
- Yaseen Z. and Aubusson P., (2018), Exploring Student-Generated Animations, Combined with a Representational Pedagogy, as a Tool for Learning in Chemistry, Res. Sci. Educ., 1–20, DOI:10.1007/s11165-018-9700-4.
- Zhang Z. H. and Linn M. C., (2011), Can Generating Representations Enhance Learning With Dynamic Visualizations? J. Res. Sci. Teach., 48(10), 1177–1198.
- Zhang Z. H. and Linn M. C., (2013), Learning from Chemical Visualizations: comparing generation and selection, Int. J. Sci. Educ., 35(13), 2174–2197.
| This journal is © The Royal Society of Chemistry 2019 |