Working with mental models to learn and visualize a new reaction mechanism†
Amanda
Bongers
 a,
Georg
Northoff
b and
Alison B.
Flynn
a,
Georg
Northoff
b and
Alison B.
Flynn
 *a
*a
aDepartment of Chemistry and Biomolecular Sciences, University of Ottawa, Ottawa, Ontario, Canada. E-mail: alison.flynn@uOttawa.ca
bInstitute of Mental Health Research, University of Ottawa, Ottawa, Ontario, Canada
First published on 17th April 2019
Abstract
Creating and using models are essential skills in chemistry. Novices and experts alike rely on conceptual models to build their own personal mental models for predicting and explaining molecular processes. There is evidence that chemistry students lack rich mental models of the molecular level; their mental models of reaction mechanisms have often been described as static and not process-oriented. Our goal in this study was to characterize the various mental models students may have when learning a new reaction mechanism and to explore how they use them in different situations. We explored the characteristics of first year organic chemistry students’ (N = 7) mental models of epoxide-opening reaction mechanisms by qualitative analysis of transcripts and written answers following an audio-recorded interview discussion. We discovered that individual learners relied on a combination of both static (with a focus on symbolism and patterns) and dynamic (reactivity as process or as particles in motion) working mental models, and that different working mental models were used depending on the task. Static working mental models were typically used to reason generally about the reaction mechanism and products that the participants provided. Dynamic working mental models were commonly used when participants were prompted to describe how they pictured the reaction happening, and in attempting to describe the structure of a transition state. Implications for research, teaching, and learning from these findings are described herein.
Introduction
Models in science and science education
Models and modelling are foundational tools in science for understanding and predicting factors in our world. Conceptual models represent coherent and scientifically accepted knowledge, and we teach with a variety of these models to communicate scientific concepts to students (Gilbert et al., 1998; Coll, 2006). Learners must develop their own mental models for visualizing and understanding scientific phenomena (Clement, 2000; Greca and Moreira, 2000; Seel, 2003; Ramadas, 2009; Schwarz et al., 2009). Mental models represent our understanding of features of the external world and form the core of human reasoning (Johnson-Laird 1983; Barsalou 1999; Justi and Gilbert 2006).In chemistry, both conceptual and mental models are used to visualize molecules, simulate chemical transformations, and communicate processes using chemistry's symbolic language (Wu and Shah, 2004; Strickland et al., 2010; Akaygun and Jones, 2014; Flynn and Featherstone, 2017; Galloway et al., 2017). A rich mental model of reactivity is one that includes both the symbolic language and dynamic understanding of how molecules behave and interact (Bodner and Domin, 2000; Bhattacharyya, 2013; Caspari et al., 2018b). However, there is evidence that many students lack a rich mental model of processes and reactivity at the molecular level (Tasker and Dalton, 2006; Kelly et al., 2010) and thus see limited meaning and value in the symbolism used by chemists (Bhattacharyya and Bodner, 2005; Cooper et al., 2010, 2012; Caspari et al., 2018b; Popova and Bretz, 2018a). Animations have been designed to better portray the dynamic nature of molecular processes (Tasker and Dalton, 2008), and some studies report that molecular animations help students imagine and describe the sub-microscopic domain (Stieff and Wilensky, 2003; Velázquez-Marcano et al., 2004; Tasker and Dalton, 2006; Kelly and Jones, 2007, 2008; Aldahmash and Abraham, 2009; Ryoo and Linn, 2014; Kelly and Akaygun, 2016). However, learning from chemistry animations can also result in the development or reinforcement of misconceptions in students’ mental models (Tasker and Dalton, 2006), and their design can create challenges for students in moving between multiple representations and in searching for salient features (Kozma and Russell, 1997; Lowe, 2004). Even with advances in model-based teaching and learning in chemistry (Justi and Gilbert, 2002; Coll and Lajium, 2011), developing and using appropriate mental models of reactivity remains challenging for students.
Chemistry's symbolic language
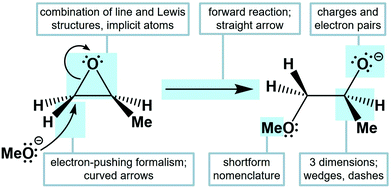
The relationship between reactivity and molecular structure is a key concept in organic chemistry that is represented and taught using abstract models. These models include a system of symbolism—referred to as organic chemistry's language—that chemists use to communicate structure, properties, and reaction mechanisms. Traditionally, these representations combine conventions into an accepted conceptual model (Fig. 1), including Lewis and line structures, which represent molecules in two or three dimensions, and a variety of arrow types to show electron flow, resonance, or reaction progress (Nic et al., 2014). A key aspect of this symbolic system is the electron-pushing formalism (EPF), in which curved arrows are used to represent electron movement. The EPF is an essential tool used by chemists to communicate reactivity and to predict reaction outcomes (Bhattacharyya, 2013).Studies suggest that students’ mental models about reactivity are often not well aligned with conceptual models. Several studies found that many students do not use electron-pushing arrows as a tool for predicting outcomes but rather draw them after deciding the product of a reaction (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008; Grove et al., 2012; Bhattacharyya, 2014). Strickland et al. (2010) reported gaps in graduate students’ mental models of reaction terminology and a lack of process-oriented language. Other research demonstrated how many students “prioritize structure over function” to identify nucleophiles and electrophiles in reaction mechanisms (Anzovino and Bretz, 2015, 2016; Weinrich and Sevian, 2017). While these studies suggested that novices gave little meaning to the EPF and other symbolic formalisms, our prior work has found that students gave meaning to electron-pushing arrows in a curriculum that emphasized general instruction on the symbolic language of mechanisms (Galloway et al., 2017). Our same prior work also showed that students were process-oriented when thinking about a reaction and its mechanism, and consistently attempted to leverage their prior knowledge. While this study found that students described reactions as step-wise processes, we know little about how the students actually pictured the reactions happening. The studies described above mostly characterized students’ mental models of chemistry's symbolic language, while other mental models (e.g., of reaction dynamics, the molecular level, or the transition state) and how they may be used in different contexts, are underexplored.
Research goals and questions
In this work, we used a grounded theory research design to study students’ use of mental models of a reaction mechanism and its transition state (Charmaz, 2011). Our goals were to characterize the mental models students use to describe and reason about a reaction mechanism, to uncover if students have and use more than one mental model of reactivity, and how mental models may be used in different contexts. The long term goals of this work are to better understand how students’ mental models relate to conceptual models, and from there begin to help students learn through building connections. The new reaction mechanism was first presented in a learning activity that contained both traditional representations (with EPF) and simplified Organic Chemware® animations (Nelson, Organic ChemWare, 2018). The learning activity was followed by tasks and an interview discussion, and we used mental models theory as a framework for designing the interview materials and discussion prompts to elicit students' various possible working mental models (Johnson-Laird, 1983; Lesh et al., 2000; Bodner et al., 2005). Our research questions were:1. What are the characteristics of students’ working mental models of a newly learned reaction mechanism and its transition state?
2. What is the influence of the task or interview prompt on the working mental models used by students to describe the reaction mechanism?
Framework
The theoretical framework that guided the design of this research study is mental models theory from cognitive psychology and science education (Johnson-Laird, 1983; Kelly and Lesh, 2000; Bodner et al., 2005). Rapp (2005, p. 46) explained mental models as “internalized, organized knowledge structures that are used to solve problems. They are encoded with respect to the spatial, temporal, and causal relationships of a concept.” Mental models are used for reasoning and reflect an individual's interpretations of causal relationships, with the following characteristics: (1) mental models represent possibilities, (2) are iconic representations, and (3) represent what is true at the expense of what is false (Johnson-Laird, 2010). Johnson-Laird (2010) outlined how these characteristics relate to human reasoning, which depends on having mental models of several possibilities and deriving conclusions from them. For example, the causal assertion “Carbon having three bonds causes it to have a positive charge” involves at least two mental models: (1) carbon has three bonds and is positively charged, (2) carbon does not have three bonds and there is no charge. In this way, mental models are simulations of a situation that are used to reason and make conclusions (Khemlani et al., 2014).Mental models are personal analogies of an external reality, and with new experiences they are always changing and never complete (Greca and Moreira, 2000). In the context of education, the student's mental model of a concept is changed through learning from conceptual models and incorporating new information into prior knowledge (Ausubel, 1968; Novak, 1993; Bretz, 2001). This learning is the result of reorganizing and building of productive connections between ideas (Øyehaug and Holt, 2013), through a processes of adding, integrating, and differentiating (Kelly and Akaygun, 2016). However, the relationship between an individual's mental model and scientifically accepted conceptual models is complex and non-linear (Greca and Moreira, 2000). For this reason, models and modelling is a research intensive area in science education (Clement, 2000; Greca and Moreira, 2002; Adbo and Taber, 2009; Schwarz et al., 2009) and chemistry education (Wang and Barrow, 2011; Lajium, 2013; Akaygun and Jones, 2014; Cheng and Gilbert, 2017; Cooper et al., 2017; Park et al., 2017; Cheng, 2018).
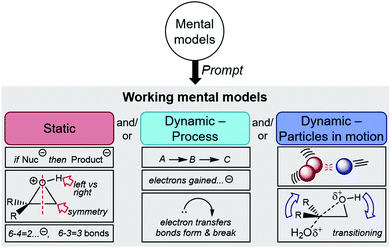
An individual uses a highly contextual mental model to reason in a particular situation by constructing a working mental model with only characteristics considered necessary (Fig. 2) (Johnson-Laird, 2010; Akaygun and Jones, 2014; Kelly, 2014). Mental models theory is closely linked to modern information processing theory, where the working mental model exists in working memory and is constructed from mental models in long-term memory (Mayer, 2012; Schunk, 2012). When learning or problem solving, the learner can use multiple models to construct their working mental model (Greca and Moreira, 2000). Errors can arise when not all necessary models are considered for a situation (Johnson-Laird, 2001). Multiple models are often necessary to explain phenomena in chemistry since the interactions and collisions of molecules can rarely be visualized directly (Jones et al., 2005; Cooper et al., 2017; Cheng, 2018). For example, space-filling models are used to predict steric interactions, while electron-potential maps model electron density to predict reactive sites. A recent study showed that blending of chemistry and mathematics models is key to a deeper understanding and expert-like reasoning about chemical kinetics (Bain et al., 2018), which is likely the case in other areas of science. Blending refers to the interaction of different knowledge structures, and integration of knowledge from different domains (Fauconnier and Turner, 1998; Coulson and Oakley, 2000).
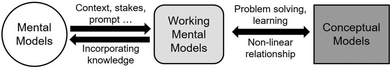 | ||
| Fig. 2 The relationships between mental models, working mental models, and conceptual models in the context of learning and education. | ||
Mental models and modelling activities have been studied in a variety of ways, primarily using qualitative methods, where participants create representative models (Adbo and Taber, 2009; McClary and Talanquer, 2011; Luxford and Bretz, 2013; Kelly, 2014; Cooper et al., 2017), create concept maps or complete survey instruments (Grove et al., 2012; Al-Balushi and Al-Hajri, 2014; Anzovino and Bretz, 2016), or describe their mental model in a think-aloud or interview-about-events format (Harrison and Treagust, 1996; Coll and Treagust, 2001; Strickland et al., 2010; Cheng, 2018). Conceptual models have also been studied this way; Bhattacharyya (2013) surveyed experts to characterize their mental models of the EPF its use in mechanistic reasoning, and the author identified common features to synthesize a conceptual model. In this study used a worksheet prompt and follow-up interview to explore students’ working mental models of a reaction mechanism.
Methods
Participants and setting
Ethics approval was granted by the institution's Research Ethics Board prior to recruiting participants. Organic Chemistry I (OCI) students from a bilingual public research university in Canada (N = 7; 5 female, 2 male)‡ voluntarily participated, gave written informed consent, and were given a $10 gift card as compensation. Pseudonyms are used in this article to protect their identities. The participants came from three equivalent English sections of the course, taught by different instructors, in a patterns of mechanisms curriculum (Flynn and Ogilvie, 2015; Ogilvie et al., 2017). The interviews took place near the beginning of the course when students were learning chemical structures, symbolism, and how to use the EPF, but had not yet learned many specific reaction mechanisms or about transition states. All participants stated that they were unfamiliar with the epoxide-opening reaction mechanism, which was the focus of this study.Instruments and procedure
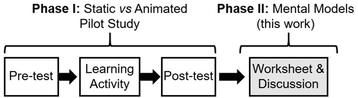
The interview consisted of two phases designed with different research goals (Fig. 3). A pre-test, learning activity, and post-test was completed by participants on a computer (Phase I), followed by a worksheet and interview (Phase II). These instruments were created by the first author and face validity was established by the second and third authors, who are experts in the field. Phase I was the pilot stage of future larger mixed-methods and quantitative studies into learning from traditional vs. animated representations of reaction mechanisms. The learning activity in Phase I showed each participant variations of the epoxide-opening reaction mechanism and included both traditional (EPF) and animated representations (Appendix B). The epoxide-opening reaction was chosen to allow for variety in nucleophiles and substitutions on the epoxide, different regiochemical products depending on the reaction conditions, and because reasoning about the reaction necessitates stereochemical considerations. Phase II was designed to elicit each participant's mental models of the epoxide-opening reaction mechanism, and to explore how these mental models were used in different situations. One goal of Phase II was to create a coding scheme for characterizing students’ working mental models of a reaction mechanism, for use in a follow-up mixed methods study combining aspects of Phase I and Phase II. The data and analyses presented herein are from Phase II of the study.The interview prompts and worksheet questions in Phase II were created using mental models theory, and go beyond those typically used to study reasoning in chemistry which focused on the steps and strategies participants used while problem solving (e.g., connecting-the-dots, mapping, use of EPF as a predictive tool) (meta-analysis: Bhattacharyya, 2014). This design enabled our analysis to explore the mental models underlying reasoning.
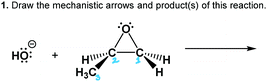
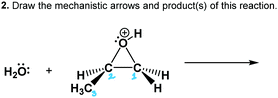
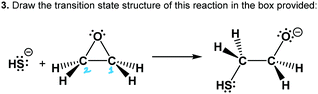
The worksheet had three questions about the epoxide-opening reaction mechanism (Table 1). Questions 1–2 provided starting materials and asked each participant to the draw mechanistic arrows and product(s) of the reaction. We did not include products in an effort to let the participant develop and use their own working mental model of the reaction and its regiochemical and stereochemical implications. Recent work showed that mechanism tasks that include products may mask students’ causal reasoning abilities (DeCocq and Bhattacharyya, 2019). Thus, this activity was used to avoid “connect-the-dots” or “decorating with arrows” approaches observed in prior work (Bhattacharyya and Bodner, 2005; Strickland et al., 2010; Grove et al., 2012). Question 3 asked the participant to draw the transition state of a given reaction, and probed whether the participant's working mental models of the reaction mechanism included transitional features.
Once the worksheet was completed, an audio-recorded semi-structured interview about the participant's worksheet answers followed. The numbers 1, 2, and 3 were drawn in blue ink next to each epoxide carbon atom by the interviewer to aid the discussion. The interview had several prompts (Table 1, A–F) intended to elicit a variety of mental models by evoking discussion of rules, relationships, and results of the models (Lesh et al., 2000). For example, the prompt “How did you approach this question” was chosen to provoke descriptions of the mental model used to solve the problem and understand the mechanism. The prompt “How do you picture this reaction happening?” was aimed to evoke visualization of the process, a key aspect of mental model construction (Bodner and Briggs, 2005; Al-Balushi, 2009).
Data analysis
Audio recordings of the interviews were transcribed verbatim and worksheets were scanned into digital documents. The interview transcripts and worksheets were analyzed using qualitative analysis software by the first author by first coding structurally for which worksheet question was being discussed and for each interview prompt. Then, the transcripts were analyzed using grounded theory and constant comparative method (Charmaz, 2014; Corbin and Strauss, 2015). Initial codes were collapsed into parent codes that were used to synthesize themes and to create a coding scheme (Appendix A). The scanned worksheets were also first coded structurally (e.g., Question 1, 2, 3) using the same codes as for the transcripts, then analyzed within each question to characterize participants’ answers. Trustworthiness of the analysis was established by regular peer-debriefing, searching for disconfirming evidence within the synthesized themes, and providing thick descriptions of the participants’ setting and responses (Cresswell and Miller, 2000). To test inter-rater reliability, one prompted excerpt from Questions 1–2 (prompts A–D) was selected for each participant by the first author (10% of the data). These excerpts were independently analyzed for the parent code and theme (see Appendix A) by the third author. Agreement between the raters was 100%.Results and discussion
Our analysis of participants’ responses to the various interview prompts about their worksheet answers (see Table 1) revealed that participants used one or more working mental models of the reaction mechanism. These working mental models could be categorized as either static models of the symbolic language and patterns, or dynamic models, where the reactivity was described with process-oriented language or as particles in motion (Fig. 4). When a participant used more than one working mental model throughout the interview, the interview prompt or worksheet question had a clear influence on whether the model was static or dynamic. In particular, we uncovered differences between how the students approached solving mechanistic problems and how they viewed the same reactivity in their mind.The following sections will characterize these different working mental models and how they were used by participants to describe the reaction in different ways depending on the worksheet question or interview prompt. The sub-sections “Static models”, “Dynamic models”, and “Influence of the prompt on working mental models” include results and analysis from Worksheet Questions 1 and 2, and the sub-section “Working mental models of the transition state” include results and analyses from Worksheet Question 3.
Static models
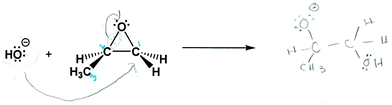
Most participants were found at some point during the interview to use static working mental models, where participants described their worksheet answers or reasoning without reference to electron transfers, the reaction process, or particle dynamics. These examples are similar to those reported by Strickland et al. (2010), where participants were found to rarely make reference to electron movement in a mechanistic task. These static working mental models were also characterized by participants’ descriptions being focused on surface features, such as rules or patterns related to the symbolism or structure, without a link to the dynamic meanings behind the symbols. In these cases we observed an emphasis on Lewis structures, the octet rule, and formal charge. When using static mental models, some participants omitted the chemical meaning of the symbols. For example, when participants used the octet rule to draw the product, they described counting electrons and bonds in terms of simple calculations. Kate explained how she used the octet rule when drawing the mechanistic arrows and product of the acid-catalyzed epoxide opening:“Sure um, in the H 2 O, when you bond it with carbon 1, again oxygen has six lone pairs, and it usually forms two bonds only. But here it is gonna form 3 bonds because you have two Hs and one bond with the carbon, um, so if you subtract 6 minus 3 you get 3 bonds, and you don't really want to have like one lone electron out there, so what I did is I added a positive sign to count this one lone electron.”
Surface-level descriptions of reactivity, where symbols are not given physical meaning, have been observed in other studies of students’ meaning-making and reasoning about reaction mechanisms (Bodner and Domin, 2000; Anderson and Bodner, 2008; Ferguson and Bodner, 2008; Strickland et al., 2010; Galloway et al., 2017), and these parallels suggest that static working mental models are used for the “it gets me to the product” style of reasoning. However, we rarely observed participants providing “connect-the-dots” explanations (Bhattacharyya and Bodner, 2005), likely because the participants also drew the products of the transformation in this study.
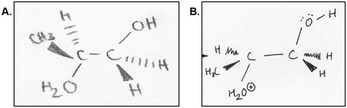
The use of non-chemistry heuristics was evident when the participants were asked about why the reaction occurred at the carbon site that they showed in their product. Four out of the seven participants used a heuristic that the reaction occurred at the site that made the product more symmetrical. This focus on symmetry when drawing Lewis structures was previously reported, the authors found students thought the most stable Lewis structures were symmetrical (Cooper et al., 2010). Amy, who chose the more substituted carbon site for the reaction under both acidic and basic conditions, explained her tendency for symmetry in why she chose her regiochemistry (Fig. 5A):
“I guess like um the, um, the side group, CH 3 group, kinda influenced it because I tried to make it even out. A little bit, but then this came in so like I guess it wasn't that evened out.”
Some participants also noted that they simply used a pattern that they remembered from the learning activity to decide which carbon would react. For example, Jill noticed that in the acid-catalyzed epoxide openings, the nucleophile would always react on the opposite side as where the proton on the epoxide oxygen was pointing (Fig. 5B): “And um, for this one, since there's a hydrogen here and from all the examples I saw with a hydrogen they'd always go like on the opposite side of it. So if the hydrogen was on this side, um, the molecule added was always from that side, so that's why I, um, put the arrow towards this carbon.”
Overall, the working mental models described above were static and focused on symbolic patterns without considering the chemical process or transition that occurs in the reaction mechanism. The participants’ working mental models were also limited by their current knowledge of chemical reactivity and the symbolic language, especially because the learning activity provided no explanations about the reaction mechanisms. However, no participant used a static working mental model exclusively or extensively. More commonly, participants were found to also use some form of dynamic working mental model.
Dynamic models
We found two main types of dynamic working mental models that the participants used during the discussion of their worksheet answers: (1) process and (2) particles in motion. In the first type, participants worked with a mental model in which starting materials change to products in an episodic sequence (i.e., A → B → C) in which bonds in the starting material disappeared and bonds in the product appeared. In this model, symbols have chemical meaning related to the electron transfer process. This model was different than working with a mental model of particles in motion, in which participants imagined (and described) the transition itself as “bonds breaking and forming”, which included the in-between process where bonds were partially formed or broken. This model also included discussions of molecules in motion, sometimes without reference to electron transfers.Participants often used a dynamic working mental model of the reaction as a process throughout the worksheet and prompts. For example, Mary described the mechanism as electron transfers with a focus on formal charge:
“… this bond between carbon 2 and O it has two electrons, so that's gonna go to the O. And then when it gains two electrons it becomes negative; so I notated it with a negative sign there. And then, this negative sign is actually gonna go away as now it's losing electrons from HO, and it's gonna join onto carbon 2, and then I made sure to check that each carbon had four bonds attached to it. And so, um, that's how I approached that one.”
Instead of treating the reaction like a black box, in which starting materials become products by unknown mechanisms, Mary described the process by which the reaction occurred. In prior work in this area, this process-oriented approach to mechanistic problems has been described as rare (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008; Strickland et al., 2010; Grove et al., 2012). However, recent work from our group (Galloway et al., 2017, 2019) and others (Weinrich and Talanquer, 2016; Weinrich and Sevian, 2017; Caspari et al., 2018a; Caspari et al., 2018b) found chemistry students invoking dynamic process in their reasoning about reactivity. In this study, we observed all participants describing reactivity with process-oriented language at some point during the various tasks, and thus using a dynamic mental model of the reaction as a process. This finding suggests that the context plays an important role in which a working mental model is used for mechanism tasks. We were able to elicit these various models by using multiple tasks and prompts.
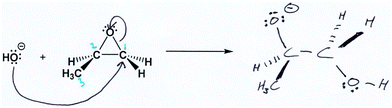
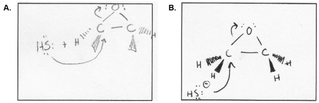
Ben also gave evidence of using a dynamic working mental model of the reaction as a process. In this excerpt, he defined his electron-pushing arrows (Fig. 6) and pointed out where bonds form and break in the reaction process:
“Since the arrow starts, the first arrow, um, it starts at uh… non-bonded electrons, right? And then it ends at carbon 1, it means that there's a bond being formed between HO minus and carbon 1. And the second arrow, that points from the initial bond from, uh, oxygen and carbon 1, that bond is being broken and then—that bond is composed of two electrons, right? Being shared by oxygen and carbon 1 initially, and then since it's being transferred to oxygen, it would gain the electrons, then eventually it would get a negative charge.”
While Mary's process-oriented description was focused on the reactants and products on the page, it lacked details of reaction dynamics for individual steps in the transformation, including what occurs between the states of the process (e.g., at the transition state). Ben, on the other hand, refers to a bond being formed, which indicates that he was also considering the transformation between bonding and non-bonding electrons.
The second type of dynamic working mental model of the reaction we observed was one of particles in motion. This mental model involved describing or visualizing molecules and electrons moving in the reaction, and considering a continuum of states between starting materials and products. When using this type of model, the participants’ explanations typically included action words such as “collision”, “jumping”, and “flow”. For example, Jill described picturing the epoxide-opening reaction mechanism that she drew as “just, gas molecules colliding I guess. And, making new compounds.” Another example came from Mary, who used a dynamic analogy to describe how she pictured the base-catalysed epoxide opening reaction happening:
Mary: “So I imagine it, like, being like a circle, like, as like one is hopping in and the other one is like leaving kind of thing. So it's, it's like a…um, like a… kind of like a ride, something goes in, the other one comes off.
Interviewer: “OK, when you say a ride, can you …”
Mary: “Like a Ferris wheel, like, if someone is coming into a seat, so that will fill in that seat, and then when it comes back down someone will have to come off. So that would be like, the O for instance, that bond is breaking, as that person is leaving, and then that HO that's another person coming in.”
All participants used a dynamic working mental model of particles in motion at one point during the discussion of their worksheet answers, although this was highly dependent on the interview prompt as discussed in the following section. This finding is different than some previous work (Bodner and Domin, 2000; Bhattacharyya and Bodner, 2005; Popova and Bretz, 2018a, 2018b), where it was concluded that “molecules that are dynamic in the minds of experts remain static in the minds of novices” (Bhattacharyya and Bodner, 2005, p. 1406). We instead found that students had dynamic mental models of molecules and reactivity, and visualized molecules and/or electrons in motion. While these mental models may be partly attributed to the animations viewed in Phase I, we cannot make any direct conclusions. Further, above quotes from Mary and Jill showed little resemblance to the Organic Chemware® animations. However, all of the participants said in the interview that the animations helped them follow the flow of electrons or visualize the reaction. These comments suggest that students can be primed to use a dynamic mental model by viewing an animation of the reaction mechanism.
In this study, participants’ working mental models of particles in motion sometimes lacked information about the specific reaction in the question, and were thus difficult for the participants to translate onto the page into the symbolic language. For example, Beth described how she pictured the reaction happening as seeing “electrons jumping” and bonds breaking, and she typically described the reaction as a process:
Interviewer: “And can you tell me about the mechanistic arrows that you drew?”
Beth: “I drew this molecule joined over here [to carbon 1], and then like, um, I'm showing this bond, here, the transfer of the electrons onto the oxygen atom.”
However, Beth had difficulty using this model to draw her mechanistic arrows in Question 1 (Fig. 7). In her answer, she lost track of which bonds were forming and breaking with the electron-pushing arrows that she drew, which showed her difficulties with conveying how she visualized electron flow with the EPF. Beth's difficulty with communicating her mental model may stem from challenges many students face in trying to move between the symbolic and sub-microscopic domains of chemistry (Johnstone, 1993; Taber, 2013). These difficulties also relate to prior work on representational competence (Cooper et al., 2010; Strickland et al., 2010; Grove et al., 2012; Galloway et al., 2017; Bhattacharyya and Harris, 2018). We explore this issue further herein when discussing how participants used their mental models to draw a transition state of the reaction (Worksheet Question 3, Table 1).
Influence of the prompt on working mental models
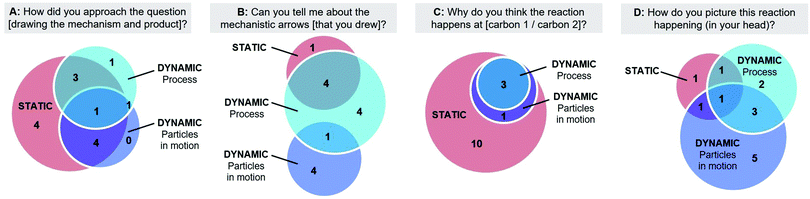
While characterizing the participants’ various working mental models of the reaction mechanism, we observed that for some participants there was a substantial influence of the interview prompt on which working mental model they used (Fig. 8). In responding to prompt A, participants gave their initial explanations of the reaction mechanism and product they provided on the worksheet. We found that participants primarily used static working mental models in response to prompt A, with explanations of how the structures and symbols they drew were related and of the rules and heuristics they used to make decisions. When participants were asked to further explain their mechanistic arrows (prompt B) they became more focused on how the EPF represented a process and were more often coded as using a dynamic working mental model. The participants also often used static working mental models in response to prompt C. Here we observed a few examples of backwards oriented or “connect-the-dots” reasoning for prompt C, where the participants used the product and/or arrows they had drawn to justify the regiochemical outcome of the reaction.Only when prompted to describe how they pictured the reaction happening “in their head” (prompt D) did some participants use their working mental models of reactivity as particles in motion. This question is different from a typical assessment question on an exam (Laverty et al., 2016; Stowe and Cooper, 2017) or prompts used in prior work on students’ mental models of reaction mechanisms which focus on asking students to explain their reasoning after drawing mechanistic arrows (Bodner and Domin, 2000; Bhattacharyya and Bodner, 2005; Strickland et al., 2010; Bhattacharyya, 2014).
The influence of the discussion prompt on working mental models was most pronounced in two of the participants, Jill and Mary, who appeared to have isolated working mental models. Jill and Mary both explained how they visualized the reaction happening in terms of particles in motion. Jill pictured “gas molecules colliding” and Mary described her Ferris wheel analogy (see above, “Dynamic models”). For both participants, their dynamic model helped them visualize the molecules in motion but not visualize, communicate, or predict specific reaction outcomes. When asked to describe the reaction mechanism in other ways, there was no evidence that they used these working mental models. In response to the other prompts, Jill typically used a static working mental model related to the symmetry of the starting material structure, while Mary used a somewhat process-oriented working mental model that focussed on formal charge, as described above.
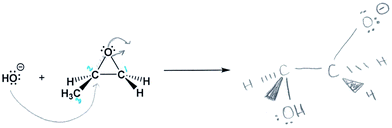
Some participants used one model instead of another after recognizing conflicts, such as when they had difficulty describing the reaction as concerted or using dynamic language. For example, Alex often used a dynamic working mental model; he described picturing the reaction as a flow of electrons from one charged species to another, and noticed that charge “travels through the molecule”. However, when asked to further describe his mechanism and product (Fig. 9) and why the reaction occurred at carbon 2 (prompt C), he resorted to a static mental model that included a pattern in starting material symmetry (which he also he described earlier after prompt A). In the following quote, he switched from describing electron flow to a symmetry “connect-the-dots” answer, and then explained how his models conflicted:
Interviewer: OK, and I think you just touched on this but how did you choose carbon 2 for the …
Alex: For the electron flow?
Interviewer: Yes, for the electron flow
Alex: Uh, I just seen [sic] because it's on the left it must go that way, I think it's just like the imagining of it, um, however it doesn't really match with the way that I, with the way it ends up on this side, because it ends up on carbon 1 [in the product], but the electron flow goes by carbon 2, so I don't know, it was very arbitrary. So there's no… there's really not much logic behind it.
Two participants, Ben and Kate, consistently used a dynamic working mental model to answer the various interview prompts about the reaction mechanism. This working mental model included reaction dynamics, process-oriented descriptions, and the idea of particles in motion. For example, Ben consistently gave a description of the reaction as a process and was keenly focused on how the symbolism represented the process, and there was also evidence that he had a dynamic ‘particles in motion’ mental model of the reaction mechanism, which was most obvious in his worksheet drawing and description of the transition state (Fig. 13A).
Kate consistently described that she was visualizing the reaction as a process and as particles in motion. Although not quite correct, Kate thought that curved arrows represented movement of the molecules and not electron transfers, which gave her a bridge for visualizing the dynamic process from the symbolic representations. When she was asked to describe the mechanistic arrows (prompt B) that she drew for the reaction under basic conditions (Fig. 10), she included many dynamic and process-like components in her answer:
“So the arrows just indicate where, like what, which molecule is going where. So for example the first arrow here, HO, it indicates that it's moving towards carbon 2, and um, you also see some hashes like between carbon 2 and the oxygen bond, it indicated that the bond is breaking, um, and then you also see um, an arrow on the carbon 1 and oxygen bond, to me it indicates that the oxygen is just slightly moving away from carbon 2, because the bond is breaking.”
In summary, the interview responses and answers to the first two questions of the worksheet (Table 1) revealed that the participants had various static and dynamic working mental models of the reaction mechanism. In some cases, there was a clear difference between the working mental models that participants used with different interview prompts. These differences align with mental models theory, where the features of mental models used for reasoning are highly context-dependent (Johnson-Laird, 2010; Akaygun and Jones, 2014; Kelly, 2014). Our finding that specific prompts (B and D) could elicit participants’ dynamic working mental models has implications for future research into mechanistic problem solving and assessment, and it has been shown that certain types of mechanism questions can affect students’ causal reasoning approaches (DeCocq and Bhattacharyya, 2019). In the next section, we explore how participants used their working mental models of the reaction to draw and describe the transition state.
Working mental models of the transition state
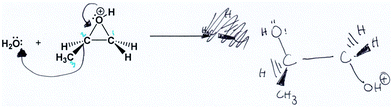
Worksheet Question 3 (see Table 1) required participants to draw the transition state of an epoxide-opening reaction mechanism given the starting materials (including a symmetrical epoxide) and products, but without mechanistic curved arrows. This question probed the participants’ use of a dynamic mental model, which is required for the question, and also their ability to communicate this model both symbolically and verbally. After drawing their structures, participants were asked to describe their approach to the question (prompt E) and describe their transition state structure (prompt F). From these discussions, the participants showed evidence of working with various dynamic mental models of the reaction mechanism while describing their transition state structures and problem-solving approach. Since the concept of a transition state was expected to be new to students at this level, they were also asked to define the term “transition state structure” (prompt G). All participants proposed a plausible definition of the transition state as being a state between starting materials and products. The difficulty and low confidence that the participants expressed when they described their transition state answers showed that they were constructing their own mental models and unable to rely on prior knowledge. Many participants expressed unfamiliarity with the appropriate symbolism to use in this situation, and some overcame the challenge by engaging in creative usage of symbolism and defining their own symbolic meaning.Mary and Jill both used curved arrows to represent change in their transition state (Fig. 11). In the following excerpt, Mary appeared to use a dynamic mental model where bonds were breaking and forming, but was confused about how to convey this process:
Interviewer: “So then uh, for question 3, how did you approach this one?”
Mary: “Um I was confused about the transition state, I was like, is that the arrows or not? Um, as they already had put the product, I guess I was like, okay… let me draw the arrows as that's the thing that's missing and that's what I like, filled in here before. So I saw that they put the HS with carbon 2 and so I said okay then, I'll draw an arrow to… like saying that it's gonna go to carbon 2, and then uh, they added the O and they put it on carbon 1, so I drew an arrow of the bond being broken, and these two electrons, like this bond being intact, and this bond being broken, and the arrow going to the O as those two electrons, is gonna go to that O.”
Beth also showed evidence of using a dynamic working mental model in how she pictured the transition state:
“I see, like the previous ones, you just throw this molecule [the nucleophile] to the carbon atom, and then like, when you like, release these bonds, like the electrons go to the oxygen atom, then I guess the negative charge, and then you use one of the electrons from the sulfur atom to make a bond. And then it comes [sic] like, neutral.”
Beth also explained her difficulty with using her dynamic mental model, and expressed a lack of confidence in her approach for showing transferring electrons in her drawing (Fig. 12A):
Interviewer: “Can you tell me about how you approached it [drawing the transition state] then?”
Beth: […] “It's in between transferring, so like instead of putting like, 3, instead of putting like all 6 electrons I put 5, but like I don't feel like that's right.”
Alex and Amy also showed difficulties with the task of depicting and describing a transition state; both realized conflicts between their dynamic mental models of the reactivity and their understanding of the symbolic language when they were brought together into their working memory. Alex described the transition state in terms of the reaction as a process, as a state between bonds forming and breaking:
Alex: “Uh, it would just be, as the original molecule is preparing itself to form a new bond with whatever incoming substituent group there is. Uh, so, once it broke, the bond, once carbon 2 broke the bond with oxygen, it is, it now has space to make a bond with the new SH.”
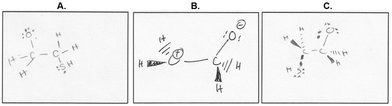
However, Alex's drawing of the transition state appeared more like an intermediate, with no symbols showing partially formed or broken bonds, and was missing the nucleophile (Fig. 12B). Conflation of the transition state with intermediates has been previously reported in a study of students’ interpretations of reaction coordinate diagrams (Popova and Bretz, 2018b).
Amy drew a fairly accurate picture of the transition state—one that looked remarkably similar to the Organic Chemware® animations in the learning activity—and she attributed her answer to the “computer simulations” (Fig. 12C). This resemblance to the animation is not surprising from a participant who was unfamiliar with transition states, since only the animations explicitly showed the transition state of the reaction (see Appendix B). Her description of the transition state also showed evidence that she was using a dynamic working mental model, describing the nucleophile coming in and electrons shifting:
“The computer simulation they showed that, like, as the um, as the HS group comes in, like the, one of the valence pairs of the S bonds to, like, one of the valence pairs becomes the bonded electron pair. So I tried to like show that in my drawing kind of, like these two electrons go and, like, shift so that they're bonded to the C. And then like I also noticed that, like, these two electrons like, bonding the, bonding the second C to the O, they shifted to make a not bonding pair on the O, so I tried to do that in my drawing.”
Amy had some uncertainty about symbolism, purposefully did not include charges in her transition state: “Um, I kind of like left out the like negative and positive because it's transitioning, so like from my guess on what transition state meant, like, I guess like, I probably shouldn't include the negative signs because, like, um, they're changing.”
Ben and Kate, who used consistent working mental models of the reaction in Worksheet Questions 1 and 2, also expressed some uncertainty in how to use symbolism to depict their dynamic transition state on paper. In his description of the transition state (Fig. 13A), Ben invented different symbols to distinguish a bond forming from a bond breaking:
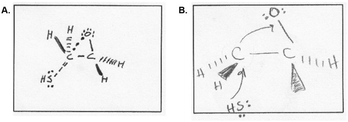 | ||
| Fig. 13 (A) Ben's and (B) Kate's depiction of the transition state. Kate used curved arrows to represent molecular movement, not electron transfer. | ||
“I would break it up into two steps, um, two, well two steps of electrons transfers. So, and then I would also divide the big organic molecule into two halves between carbon 1 and carbon 2. Well, since the two bonded atoms to both carbons are two hydrogens, it's symmetrical, right? So it wouldn't really matter if the HS minus would be bonded to carbon 1 or carbon 2, since it would result in the same molecule. Um, and then when I tried to draw the transition state structure, um, I think I learned it before but I kinda forgot how to draw it, but, I drew these dotted lines as a bond being formed and uh, dotted circle lines I guess [different style of dotted line] that would signify a bond being broken.”
Ben, like Amy, also did not draw charges on his transition state. He said: “Um, well, I wasn't sure if I was supposed to draw the initial or final charge, so I didn't do it at all.” In contrast to Ben's symbolic focus, Kate expressed and drew a dynamic picture of the reaction transition state (Fig. 13B). Since a key part of Kate's mental model was that curved arrows symbolized movement, her transition state included arrows like her mechanism answer and also dynamic changes to how electrons and bonds were represented. She described her answer as follows:
“I thought that maybe I should draw the mechanism arrows, like how is the reaction, like, what is going through the reactants for it to, you know, form those certain products. And that's what I drew. Yeah, I just drew the mechanism arrows like, the bonds moving away and towards to each other as well as the molecules bonding and non-bonding.”
While Kate's understanding of the symbolic language of chemistry included some gaps and alternate conceptions, her ability to visualize the reaction mechanism as a dynamic process likely gave her a better foundation for building her conceptual knowledge.
In summary, we found students using dynamic mental models of the reaction mechanism but having difficulty translating these models into symbolic language on the page. This finding suggests that students who appear to answer mechanistic problems without consideration of reaction dynamics, as observed in prior work (Grove et al., 2012), may actually have and use dynamic mental models but have difficulty portraying these models in their answers. For these students, a key step towards meaningful learning could be to address and resolve these conflicts. Moreover, Caspari et al. (2018a) described how a dynamic approach to change is necessary to reason about a transition state, including how charges and bonds are forming in the process of a reaction. Our work provides a base for future studies into students’ understanding and mental models about the transition state of a reaction, which has so far been underexplored.
Conclusions
This article examined organic chemistry students’ working mental models of the epoxide-opening reaction mechanism and how they were used in different contexts of problem-solving and interview discussion. We collected data about the participants’ working mental models using a worksheet and discussion with various prompts, then analysed the data using the constant comparative method. Our use of a variety of prompts was guided by mental models theory and was successful in eliciting participants’ multiple working mental models of the reaction mechanism (Johnson-Laird, 1983, 2010). We found that the participants used both static (focused on symbolism and simple patterns) and dynamic (either as a process or as particles in motion) working mental models. For some participants, these different models were more isolated and their usage was strongly influenced by the interview prompt.In agreement with prior work (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008; Grove et al., 2012; Bhattacharyya, 2014), the participants used static mental models of reactivity when describing the symbolic images (i.e., reaction schemes) where they explained recognizing and using patterns in the symbols. While pattern recognition is important for learning, we found participants sometimes using these patterns without considering chemical meanings behind the symbols. We also found that participants had and used dynamic mental models of the reaction mechanism, in which they visualized the reaction as particles in motion and/or as a process. These dynamic working mental models were more often used in response to specific prompts: (1) describing how they pictured the reaction happening or (2) when the worksheet question asked them to draw a transition state. This finding of contextual dependence is related to other findings in the mechanistic reasoning literature (Caspari et al., 2018a). In this study, we found that even if students had mental models of reactions as particles in motion, these models were often disconnected from the static mental models (e.g., heuristics) that are typically used in reasoning and were challenging to communicate on paper. Indeed, all of the participants struggled in some way to apply their dynamic working mental models, especially those of particles in motion, to their representations on paper. This struggle was especially clear in participants’ descriptions and depictions of the transition state, which sheds additional light on why moving between the macroscopic, sub-microscopic, and symbolic domains in organic chemistry is difficult for students (Johnstone, 1993; Taber, 2013). This study adds to the growing work into students’ dynamic understanding of reactivity and highlights the need to study students’ mental models and reasoning approaches under a variety of educational contexts (Weinrich and Talanquer, 2016; Caspari et al., 2018a; Caspari et al., 2018b; Talanquer, 2018).
Implications for research
Our results support previous findings that students approach problem-solving about organic reaction mechanisms with a process-oriented view of reactivity, especially when explaining a mechanism on paper (Galloway et al., 2017; Webber and Flynn, 2018). We also found that students’ mental models of reactivity were not limited to their understanding of the symbolism or the concept of process; some participants incorporated prior knowledge about reaction dynamics when picturing the reaction as particles in motion. The variety of working mental models characterized in this study arose from different interview prompts and question types, in particular, specific prompts for the participant to visualize the reaction mechanism resulted in their use of a working mental model of particles in motion. While participants in this study viewed animations while learning about the reaction mechanism in Phase I, we cannot directly attribute the observed dynamic working mental models to this activity: the participants were also building on their prior knowledge and mental models of chemical reactions in general. Explorations into the direct effect of learning from mechanism animations on students’ mental models is underway and will be reported in the near future.This work lends support to the idea that students’ responses can vary widely depending on the task. Thus, when studying students’ mental models in an interview format, the researcher must ask a variety of prompts in order to learn about multiple working mental models that the student can construct. In studies of students understanding of reaction processes, we also recommend not including the reaction products (DeCocq and Bhattacharyya, 2019) in some tasks as well as including the reaction products in other tasks, analogous to situations that researchers regularly face when confronted with unanticipated results. These approaches may allow researchers to characterize a more complete picture of the interviewees’ mental models.
Implications for learning and teaching
Experts have robust mental models of chemical phenomena that include symbolism, structure, process, and reaction dynamics, and can use them in appropriate problem-solving contexts (Grosslight et al., 1991; Williamson and Abraham, 1995; Kozma and Russell, 1997). In contrast, novices may not see the need to use dynamic models when solving a problem and may use limited and static mental models (Bhattacharyya and Bodner, 2005; Bhattacharyya, 2013, 2014; Caspari et al., 2018a) or focus on surface features (Bhattacharyya, 2006; Galloway et al., 2017). The participants in this study most commonly described how they visualized the reaction with a dynamic mental model of reactivity as particles in motion. However, these dynamic visualizations had no resemblance to how they described the reaction mechanism on paper. These participants all attributed their visualization of the reaction mechanism to the animations viewed during the learning activity. This gives evidence that these participants were leveraging prior knowledge of reaction dynamics at the particulate level and that they were cued by dynamic elements of the animation to use this type of model. This finding supports the use of a combination of static and dynamic representations of reaction mechanisms for instruction, and to clearly discuss their connections in order to help students build links between their existing mental models and address conflicts in their understanding. These activities could include constructing and evaluating scientific models used in chemistry (Teichert et al., 2017), or analyzing the purpose, strengths, and limitations of different representational models of a process (Kelly et al., 2017). If the purpose and limitations of conceptual models used in instruction, such as the EPF, are not explicitly taught (Flynn and Ogilvie, 2015), students will struggle to make the necessary connections between various models or use them as tools for prediction (Bhattacharyya, 2006).This work further supports the need for assessment items that cannot be answered sufficiently by using simple pattern recognition (Broman and Parchmann, 2014). The participants in this study described heuristics that were developed from patterns recognized during the learning activity (Phase I, Appendix B), and we found that the use of these heuristics caused a participant's working mental model to be limited to simple symbolic patterns (e.g., symmetry). While it is natural to use heuristics for problem solving, that approach does not help the learner develop their understanding of the mechanism (Talanquer, 2014). However, we can also use this finding to further develop the learning activity to emphasize some types of patterns (e.g., patterns in electron flow) and deemphasize others (e.g., symmetry patterns).
Limitations
The sample size in this study was small (N = 7) and participants were not chosen using purposeful sampling except for their organic chemistry enrollment, so no claims for representation of the entire course or other settings are made (Cresswell and Miller, 2000). Studies into students’ mental models to date have primarily been qualitative, conducted with a wide variety of contexts and prompts—we therefore emphasize the need for additional research before our findings or others can be generalized.Although the interview was designed to elicit various mental models of the participants with different prompts, we do not claim to have characterized the full breadth of participants’ mental models of reactivity. Indeed, we aimed to study only their working mental models at the moment and participants may not have been fully expressing their working mental models. To target our analysis of these working mental models, we limited our instrument to study only one reaction mechanism (a concerted epoxide-opening), although we included various conditions (acidic or basic, with multiple nucleophiles and electrophiles).
Conflicts of interest
There are no conflicts to declare.Appendix A
Table 2| Initial codes | Parent codes | Themes |
|---|---|---|
| Reasoning without process | Static working mental model | |
| • Counting electrons | Describing their answer in terms of symbols and/or structures, without describing the electron-transfer process, and lacking process-oriented language related to reaction dynamics. | Static – reasoning without process: e.g., “When you were adding um, a charged molecule, um, uh, when it created the bond the charge is gone and then that um, this bond that was between the carbon and the oxygen is, like, on the oxygen, so then it has a charge.” |
| • Focusing on only starting material or only product | ||
| • Describing symbols without electron transfer process | ||
| Using symbolic patterns or heuristics | ||
| • Using the octet rule | Using patterns or heuristics based on symbols (e.g., charge signs, substituent positions, symmetry) and not necessarily on chemical concepts. | Static – symbolic pattern: e.g., “But uh if the oxygen is charged then, whatever group we're adding the molecule… we're adding to the group will obtain that charge.” |
| • Using Lewis structure rules | ||
| • Mapping atoms mentally | ||
| • Connecting-the-dots | ||
| • Using patterns in charge symbols | Static – octet rule heuristic: e.g., “In the H2O, when you bond it with carbon 1, again oxygen has six lone pairs, and it usually forms two bonds only. But here it is gonna form 3 bonds because you have two Hs and one bond with the carbon, so if you subtract 6 minus 3 you get 3 bonds…” | |
| • Using patterns in substituents | ||
| • Looking for a pattern in structure symbols | ||
| • Looking for a pattern in explicit lone pairs | ||
| • Using symmetry heuristic | ||
| Describing the reaction as a process | Dynamic process working mental model | |
| • Using arrows to show electron transfer process | Describing and/or visualizing the reaction in terms of an electron-transfer process from A to B, in episodic terms (e.g., a bond breaks here, bond forms there). Using dynamic step-wise terminology when describing electron transfers. | Dynamic Process – describing relationship between electrons and bonds in a mechanism: e.g., “… that bond is composed of two electrons, right? Being shared by oxygen and carbon 1 initially, and then since it's being transferred to oxygen, it would gain the electrons, then eventually it would get a negative charge.” |
| • Looking for patterns in mechanisms or process | ||
| • Describing a step-wise process | ||
| • Reasoning about order of process | ||
| • Describing relationship between electrons and bonds in a mechanism | ||
| • Describing symbols with process | ||
| • Keeping track of electron transfers | ||
| • Imagining that bonds break and form | ||
| Describing particles in motion | Dynamic particles in motion working mental model | |
| • Seeing electrons jumping | Describing and/or visualizing the movement of electrons, atoms, or molecules in transition between A and B (e.g., a bond is breaking here, bond is forming there). Using dynamic transitional terminology like “electron flow” or “collide”. | Dynamic particles in motion – imagining molecules in motion: e.g., “So I imagine it, like, being like a circle, like, as like one is hopping in and the other one is like leaving kind of thing. So it's, it's like a…um, like a… kind of like a ride, something goes in, the other one comes off.” |
| • Describing atoms moving | ||
| • Using arrows to show molecules moving | ||
| • Imagining constant motion | ||
| • Imagining molecules in motion | ||
| • Imagining molecules colliding | ||
| • Imagining bonds or forming and breaking | ||
| • Describing electron flow | ||
| • Picturing bonds moving away and towards each other | Dynamic particles in motion – imagining molecules colliding: e.g., “Just, gas molecules colliding I guess. And making new compounds.” | |
| • Picturing charge travelling through molecule | ||
| • Picturing a quick shift or change | ||
| • Imagining a dynamic transition state | ||
| Difficulty conveying reaction dynamics with symbolism | Translating a dynamic working mental model | |
| • Difficulty with electron transfer arrow placement | Evidence of using a dynamic working mental model, expressing difficulty with using symbols to convey dynamics, such as in the transition state. Sometimes inventing new symbols while feeling uncertain. | Dynamic particles in motion – inventing symbolism for dynamic process while feeling uncertain: e.g., “It's in between transferring, so like instead of putting like, three, instead of putting like all 6 electrons I put 5, but like I don't feel like that's right.” |
| • Inventing symbolism for dynamic process while feeling uncertain | ||
| • Unsure about drawing changing charge in transition state | ||
| • Using arrows in drawing of transition state | Dynamic process – unsure about drawing changing charge: e.g., “I didn't draw the final charges of the resulting substituents in the product, but like, the transition state's basically the molecule between the reactants and the products as the products are being formed, so, I tried to draw that as best as I could.” |
Appendix B
Phase I of this study was as a pilot stage of a future larger quantitative study into how students learn from different representations of reaction mechanisms. Phase I consisted of a series of tasks completed individually by participants on a computer, including a pre-test, interim test, post-test, and a learning activity with traditional (EPF) and animated (Organic Chemware®) representations of the epoxide-opening reaction mechanism (Fig. 14). Data from Phase I are not included in this article.Learning activity, pre-test, and post-test
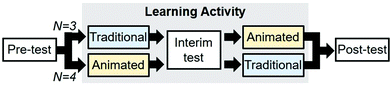
The computer tasks began with a pre-test in which the participants were shown eight variations of the epoxide-opening reaction mechanism with either correct or incorrect products and asked to decide if the product was correct or incorrect (Fig. 15A). In the pre-test, each variation was repeated six times in computer randomized order for a total of 48 questions. The questions were repeated to measure the consistency in participants’ responses, in which inconsistency would indicate guessing. The types of questions contained different combinations of oxygen-, nitrogen-, and sulfur-based nucleophiles, various substituents on the epoxide, and acidic or basic conditions. After the pre-test, there was a learning activity in which participants viewed both traditional and animated representations of the epoxide-opening reaction mechanism (included as electronic supplementary information). Participants were told that they would be viewing correct reaction mechanisms, and that they would repeat the test after the activity. During the activity individual reaction mechanisms were shown on the screen one at a time, for several seconds, with variations in the nucleophile, electrophile, and reaction conditions (Fig. 15B and C). Notably, only the animated representations explicitly showed the transition state of the reaction. As part of the design for a future larger study, the traditional and animated representations were presented in counterbalanced blocks; some participants (N = 3) first viewed traditional then animated representations, and the others (N = 4) first viewed animated, then traditional representations (Fig. 14). Between these two blocks there was an interim test. The learning activity contained no explanations of the reaction mechanisms because of a requirement to keep the future study's protocol as simple as possible. Following the learning activity, the participants completed a post-test on the computer, with the same format and questions as the pre-test.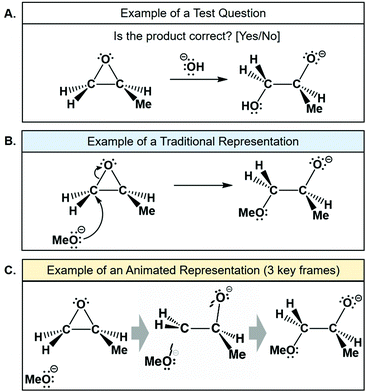 | ||
| Fig. 15 (A) Examples of test questions, (B) one variation in the static learning activity, and (C) three frames from the animation in the animated learning activity. | ||
Acknowledgements
The authors thank the students who volunteered to participate in this study, Kelli Galloway for help with the study, and Prof. Ghislain Deslongchamps for providing animations for the learning activity. Financial support for this research was provided by eCampusOntario and the University of Ottawa (144056).References
- Adbo K. and Taber K. S., (2009), Learners’ Mental Models of the Particle Nature of Matter: A study of 16-year-old Swedish science students, Int. J. Sci. Educ., 31(6), 757–786.
- Akaygun S. and Jones L. L., (2014), Words or Pictures: A comparison of written and pictorial explanations of physical and chemical equilibria, Int. J. Sci. Educ., 36(5), 783–807.
- Al-Balushi S. M., (2009), Factors influencing pre-service science teachers’ imagination at the microscopic level in chemistry, Int. J. Sci. Math. Educ., 7(6), 1089–1110.
- Al-Balushi S. M. and Al-Hajri S. H., (2014), Associating animations with concrete models to enhance students’ comprehension of different visual representations in organic chemistry, Chem. Educ. Res. Pract., 15(1), 47–58.
- Aldahmash A. H. and Abraham M. R., (2009), Kinetic versus static visuals for facilitating college students’ understanding of organic reaction mechanisms in chemistry, J. Chem. Educ., 86(12), 1442–1446.
- Anderson T. L. and Bodner G. M., (2008), What can we do about ‘Parker’? A case study of a good student who didn’t ‘get’ organic chemistry, Chem. Educ. Res. Pract., 9(2), 93–101.
- Anzovino M. E. and Bretz S. L., (2015), Organic chemistry students’ ideas about nucleophiles and electrophiles: the role of charges and mechanisms, Chem. Educ. Res. Pract., 16(4), 797–810.
- Anzovino M. E. and Bretz S. L., (2016), Organic Chemistry Students’ Fragmented Ideas about the Structure and Function of Nucleophiles and Electrophiles: A Concept Map Analysis, Chem. Educ. Res. Pract., 17(2013), 1019–1029.
- Ausubel D. P., (1968), Educational psychology: a cognitive view, New York, NY: Holt, Rinehart and Winston.
- Bain K., Rodriguez J.-M. G., Moon A., and Towns M. H., (2018), The characterization of cognitive processes involved in chemical kinetics using a blended processing framework, Chem. Educ. Res. Pract., 19(2), 617–628.
- Barsalou L. W., (1999), Perceptual symbol systems, Behav. Brain Sci., 22(4), 577–660.
- Bhattacharyya G., (2006), Practitioner development in organic chemistry: how graduate students conceptualize organic acids, Chem. Educ. Res. Pract., 7(4), 240–247.
- Bhattacharyya G., (2013), From Source to Sink: Mechanistic Reasoning Using the Electron-Pushing Formalism, J. Chem. Educ., 90(10), 1282–1289.
- Bhattacharyya G., (2014), Trials and tribulations: student approaches and difficulties with proposing mechanisms using the electron-pushing formalism, Chem. Educ. Res. Pract., 15(4), 594–609.
- Bhattacharyya G. and Bodner G. M., (2005), “It Gets Me to the Product”: How Students Propose Organic Mechanisms, J. Chem. Educ., 82(9), 1402–1407.
- Bhattacharyya G. and Harris M. S., (2018), Compromised Structures: Verbal Descriptions of Mechanism Diagrams, J. Chem. Educ., 95(3), 366–375.
- Bodner G. M. and Briggs M., (2005), A Model of Molecular Visualization, in Science Education, in Gilbert J. K. (ed.), Visualization in Science Education, Dordrecht: Springer, pp. 90–105.
- Bodner G. M. and Domin D. S., (2000), Mental Models: The Role of Representations in Problem Solving in Chemistry, University Chemistry Education, pp. 24–30.
- Bodner G. M., Gardner D. E., and Briggs M. W., (2005), Models and Modelling, in Pienta N., Cooper M. and Greenbowe T. (ed.), Chemists’ Guide to Effective Teaching, Upper Saddle River, NY: Prentice-Hall, pp. 67–76.
- Bretz S. L., (2001), Novak's Theory of Education: Human Constructivism and Meaningful Learning, J. Chem. Educ., 78(8), 1107.
- Broman K. and Parchmann I., (2014), Students’ application of chemical concepts when solving chemistry problems in different contexts, Chem. Educ. Res. Pract., 15(4), 516–529.
- Caspari I., Kranz D., and Graulich N., (2018a), Resolving the complexity of organic chemistry students’ reasoning through the lens of a mechanistic framework, Chem. Educ. Res. Pract., 19(4), 1117–1141.
- Caspari I., Weinrich M. L., Sevian H., and Graulich N., (2018b), This mechanistic step is “ productive”: organic chemistry students’ backward-oriented reasoning, Chem. Educ. Res. Pract., 19(1), 42–59.
- Charmaz K., (2011), Grounded theory methods in social justice research, The Sage handbook of qualitative research, p. 359–380.
- Charmaz K., (2014), Constructing grounded theory, 2nd edn, London, England: Sage.
- Cheng M. M. W., (2018), Students’ visualisation of chemical reactions – insights into the particle model and the atomic model, Chem. Educ. Res. Pract., 19(1), 227–239.
- Cheng M. M. W. and Gilbert J. K., (2017), Modelling students’ visualisation of chemical reaction, Int. J. Sci. Educ., 39(9), 1173–1193.
- Clement J., (2000), Model based learning as a key research area for science education, Int. J. Sci. Educ., 22(9), 1041–1053.
- Coll R. K., (2006), The Role of Models, Mental Models and Analogies in Chemistry Teaching, in Aubusson P. J., Harrison A. G. and Ritchie S. M. (ed.), Metaphor and Analogy in Science Education, Netherlands: Springer, pp. 65–78.
- Coll R. K. and Lajium D., (2011), Modelling and the Future of Science Learning, in Khine M. S. and Saleh I. M. (ed.), Models and Modeling: Cognitive Tools for Scientific Enquiry, Dordrecht: Springer Netherlands, pp. 3–21.
- Coll R. K. and Treagust D. F., (2001), Learners’ Mental Models of Chemical Bonding, Res. Sci. Educ., 31(3), 357–382.
- Cooper M. M., Grove N., Underwood S. M., and Klymkowsky M. W., (2010), Lost in Lewis Structures: An Investigation of Student Difficulties in Developing Representational Competence, J. Chem. Educ., 87(8), 869–874.
- Cooper M. M., Stieff M., and DeSutter D., (2017), Sketching the Invisible to Predict the Visible: From Drawing to Modeling in Chemistry, Top. Cogn. Sci., 9(4), 902–920.
- Cooper M. M., Underwood S. M., and Hilley C. Z., (2012), Development and validation of the implicit information from Lewis structures instrument (IILSI): do students connect structures with properties? Chem. Educ. Res. Pract., 13(3), 195–200.
- Corbin J. and Strauss A., (2015), Basics of qualitative research: Techniques and procedures for developing grounded theory, 4th edn, Thousand Oaks, CA: Sage.
- Coulson S. and Oakley T., (2000), Blending basics, Cogn. Linguist., 11(3), 175–196.
- Cresswell, J. W. and Miller D. L., (2000), Determining validity in qualitative inquiry, Theory Pract., 39(3), 124–130.
- DeCocq V. and Bhattacharyya G., (2019), TMI (Too much information)! Effects of given information on organic chemistry students’ approaches to solving mechanism tasks, Chem. Educ. Res. Pract., 20(1), 213–228.
- Fauconnier G. and Turner M., (1998), Conceptual Integration Networks, Cogn. Sci., 22(2), 133–187.
- Ferguson R. and Bodner G. M., (2008), Making sense of the arrow-pushing formalism among chemistry majors enrolled in organic chemistry, Chem. Educ. Res. Pract., 9(2), 102–113.
- Flynn A. B. and Featherstone R. B., (2017), Language of mechanisms: exam analysis reveals students’ strengths, strategies, and errors when using the electron-pushing formalism (curved arrows) in new reactions, Chem. Educ. Res. Pract., 18(1), 64–77.
- Flynn A. B. and Ogilvie W. W., (2015), Mechanisms before Reactions: A Mechanistic Approach to the Organic Chemistry Curriculum Based on Patterns of Electron Flow, J. Chem. Educ., 92(5), 803–810.
- Galloway K. R., Leung M. W., and Flynn A. B., (2019), Patterns of reactions: a card sort task to investigate students’ organization of organic chemistry reactions, Chem. Educ. Res. Pract., 20(1), 30–52.
- Galloway K. R., Stoyanovich C., and Flynn A. B., (2017), Students’ interpretations of mechanistic language in organic chemistry before learning reactions, Chem. Educ. Res. Pract., 18(2), 353–374.
- Gilbert J. K., Boulter C., and Rutherford M., (1998), Models in explanations, Part 1: Horses for courses? Int. J. Sci. Educ., 20(1), 83–97.
- Greca I. M. and Moreira M. A., (2002), Mental, physical, and mathematical models in the teaching and learning of physics, Sci. Educ., 86(1), 106–121.
- Greca I. M. and Moreira M. A., (2000), Mental models, conceptual models, and modelling, Int. J. Sci. Educ., 22(1), 1–11.
- Grosslight L., Unger C., Jay E., and Smith C. L., (1991), Understanding models and their use in science: Conceptions of middle and high school students and experts, J. Res. Sci. Teach., 28(9), 799–822.
- Grove N. P., Cooper M. M., and Rush K. M., (2012), Decorating with Arrows: Toward the Development of Representational Competence in Organic Chemistry, J. Chem. Educ., 89(7), 844–849.
- Harrison A. G. and Treagust D. F., (1996), Secondary students’ mental models of atoms and molecules: Implications for teaching chemistry, Sci. Educ., 80(5), 509–534.
- Johnson-Laird P. N., (1983), Mental models: towards a cognitive science of language, inference, and consciousness, Harvard University Press.
- Johnson-Laird P. N., (2001), Mental models and deduction, Trends Cogn. Sci., 5(10), 434–442.
- Johnson-Laird P. N., (2010), Mental models and human reasoning, Proc. Natl. Acad. Sci. U. S. A., 107(43), 18243–18250.
- Johnstone A. H., (1993), The development of chemistry teaching: A changing response to changing demand, J. Chem. Educ., 70(9), 701.
- Jones L. L., Jordan K. D., and Stillings N. A., (2005), Molecular visualization in chemistry education: the role of multidisciplinary collaboration, Chem. Educ. Res. Pract., 6(3), 136–149.
- Justi R. and Gilbert J., (2002), Models and Modelling in Chemical Education, Chemical Education: Towards Research-based Practice, Dordrecht: Kluwer Academic Publishers, pp. 47–68.
- Justi R. and Gilbert J., (2006), The Role of Analog Models in the Understanding of the Nature of Models in Chemistry, in Aubusson P. J., Harrison A. G. and Ritchie S. M. (ed.), Metaphor and Analogy in Science Education, Netherlands: Springer, pp. 119–130.
- Kelly A. E. and Lesh R. A. (ed.), (2000), Handbook of research design in mathematics and science education, 1st edn, New York, NY: Routledge.
- Kelly R. M., (2014), Using Variation Theory with Metacognitive Monitoring To Develop Insights into How Students Learn from Molecular Visualizations, J. Chem. Educ., 91(8), 1152–1161.
- Kelly R. M. and Akaygun S., (2016), Insights into How Students Learn the Difference between a Weak Acid and a Strong Acid from Cartoon Tutorials Employing Visualizations, J. Chem. Educ., 93(6), 1010–1019.
- Kelly R. M., Akaygun S., Hansen S. J. R. R., and Villalta-Cerdas A., (2017), The effect that comparing molecular animations of varying accuracy has on students’ submicroscopic explanations, Chem. Educ. Res. Pract., 18(4), 582–600.
- Kelly R. M., Barrera J. H., and Mohamed S. C., (2010), An Analysis of Undergraduate General Chemistry Students’ Misconceptions of the Submicroscopic Level of Precipitation Reactions, J. Chem. Educ., 87(1), 113–118.
- Kelly R. M. and Jones L. L., (2007), Exploring How Different Features of Animations of Sodium Chloride Dissolution Affect Students’ Explanations, J. Sci. Educ. Technol., 16(5), 413–429.
- Kelly R. M. and Jones L. L., (2008), Investigating Students’ Ability To Transfer Ideas Learned from Molecular Animations of the Dissolution Process, J. Chem. Educ., 85(2), 303–309.
- Khemlani S. S., Barbey A. K., and Johnson-Laird P. N., (2014), Causal reasoning with mental models, Front. Hum. Neurosci., 8, 1–15.
- Kozma R. B. and Russell J., (1997), Multimedia and understanding: Expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 34(9), 949–968.
- Lajium D. A. D., (2013), Students’ Mental Models of Chemical Reactions, PhD thesis, University of Waikato, Hamilton, New Zealand, retrieved from https://hdl.handle.net/10289/7839.
- Laverty J. T., Underwood S. M., Matz R. L., Posey L. A., Carmel J. H., Caballero M. M. D., et al., (2016), Characterizing College Science Assessments: The Three-Dimensional Learning Assessment Protocol, PLoS One, 11(9), e0162333.
- Lesh R., Hoover M., Hole B., Kelly A., and Post T. R., (2000), Principles for Developing Thought-Revealing Activities for Students and Teachers, College of Education & Human Development, Lawrence Erlbaum Associates, Inc., pp. 591–646.
- Lowe R., (2004), Interrogation of a dynamic visualization during learning, Learn. Instr., 14, 257–274.
- Luxford C. J. and Bretz S. L., (2013), Moving beyond definitions: what student-generated models reveal about their understanding of covalent bonding and ionic bonding, Chem. Educ. Res. Pract., 14(2), 214–222.
- Mayer R. E., (2012), Information processing, in Harris K. R., Graham S., Urdan T., McCormick C. B., Sinatra G. M. and Sweller J. (ed.), APA educational psychology handbook, col. 1: theories, constructs, and critical issues, Washington: American Psychological Association, pp. 85–99.
- McClary L. and Talanquer V., (2011), College chemistry students’ mental models of acids and acid strength, J. Res. Sci. Teach., 48(4), 396–413.
- Nic M., Jirat J., Kosata B., and Jenkins A., (2014), Chemical reaction equation, IUPAC Compend. Chem. Terminol. XML online corrected version 2.3.3.
- Novak J. D., (1993), Human constructivism: A unification of psychological and epistemological phenomena in meaning making, Int. J. Pers. Constr. Psychol., 6(2), 167–193.
- Ogilvie W. W., Ackroyd N., Browning C. S., Deslongchamps G., Lee F. and Sauer E., (2017), Organic chemistry: Mechanistic Patterns, Nelson Education Limited.
- Organic ChemWare, (2018), http://www.nelson.com/organicchemware/.
- Øyehaug A. B. and Holt A., (2013), Students’ understanding of the nature of matter and chemical reactions – a longitudinal study of conceptual restructuring, Chem. Educ. Res. Pract., 14(4), 450–467.
- Park M., Liu X., Smith E., and Waight N., (2017), The effect of computer models as formative assessment on student understanding of the nature of models, Chem. Educ. Res. Pract., 18(4), 572–581.
- Popova M. and Bretz S. L., (2018a), “It's Only the Major Product That We Care About in Organic Chemistry”: An Analysis of Students’ Annotations of Reaction Coordinate Diagrams, J. Chem. Educ., 95(7), 1086–1093.
- Popova M. and Bretz S. L., (2018b), Organic chemistry students’ challenges with coherence formation between reactions and reaction coordinate diagrams, Chem. Educ. Res. Pract., 19(3), 732–745.
- Ramadas J., (2009), Visual and Spatial Modes in Science Learning, Int. J. Sci. Educ., 31(3), 301–318.
- Rapp D. N., (2005), Mental Models: Theoretical Issues for Visualizations in Science Education, Visualization in Science Education, Dordrecht: Springer Netherlands, pp. 43–60 Search PubMed.
- Ryoo K. and Linn M. C., (2014), Designing guidance for interpreting dynamic visualizations: Generating versus reading explanations, J. Res. Sci. Teach., 51(2), 147–174.
- Schunk D. H., (2012), Learning theories: An Educational Perspective, 6th edn, Boston, MA: Pearson Education Inc.
- Schwarz C. V., Reiser B. J., Davis E. A., Kenyon L., Achér A., Fortus D., et al., (2009), Developing a learning progression for scientific modeling: Making scientific modeling accessible and meaningful for learners, J. Res. Sci. Teach., 46(6), 632–654.
- Seel N., (2003), Model-centered learning and instruction, Tech. Inst., Cogn. Learn., 1, 59–85.
- Stieff M. and Wilensky U., (2003), Connected Chemistry‚ Incorporating Interactive Simulations into the Chemistry Classroom, J. Sci. Educ. Technol., 12(3), 285–302.
- Stowe R. L. and Cooper M. M., (2017), Practicing What We Preach: Assessing “Critical Thinking” in Organic Chemistry, J. Chem. Educ., 94(12), 1852–1859.
- Strickland A. M., Kraft A., and Bhattacharyya G., (2010), What happens when representations fail to represent? Graduate students’ mental models of organic chemistry diagrams, Chem. Educ. Res. Pract., 11(4), 293–301.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14(14), 156–168.
- Talanquer V., (2014), Chemistry Education: Ten Heuristics To Tame, J. Chem. Educ., 91(8), 1091–1097.
- Talanquer V., (2018), Importance of Understanding Fundamental Chemical Mechanisms, J. Chem. Educ., 95(11), 1905–1911.
- Tasker R. and Dalton R., (2006), Research into practice: visualisation of the molecular world using animations, Chem. Educ. Res. Pract., 7(2), 141–159.
- Tasker R. and Dalton R., (2008), Visualizing the Molecular World – Design, Evaluation, and Use of Animations, in Gilbert J. K., Reiner M. and Nakhleh M. (ed.), Visualization Theory and Practice in Science Education, Dordrecht: Springer, pp. 103–131.
- Teichert M. A., Tien L. T., Dysleski L., and Rickey D., (2017), Thinking Processes Associated with Undergraduate Chemistry Students’ Success at Applying a Molecular-Level Model in a New Context, J. Chem. Educ., 94(9), 1195–1208.
- Velázquez-Marcano A., Williamson V. M., Ashkenazi G., Tasker R., and Williamson K. C., (2004), The use of video demonstrations and particulate animation in general chemistry, J. Sci. Educ. Technol., 13(3), 315–323.
- Wang C.-Y. and Barrow L. H., (2011), Characteristics and Levels of Sophistication: An Analysis of Chemistry Students’ Ability to Think with Mental Models, Res. Sci. Educ., 41(4), 561–586.
- Webber D. M. and Flynn A. B., (2018), How Are Students Solving Familiar and Unfamiliar Organic Chemistry Mechanism Questions in a New Curriculum? J. Chem. Educ., 95(9), 1451–1467.
- Weinrich M. L. and Sevian H., (2017), Capturing students’ abstraction while solving organic reaction mechanism problems across a semester, Chem. Educ. Res. Pract., 18(1), 169–190.
- Weinrich M. L. and Talanquer V., (2016), Mapping students’ modes of reasoning when thinking about chemical reactions used to make a desired product, Chem. Educ. Res. Pract., 17(2), 394–406.
- Williamson V. M. and Abraham M. R., (1995), The effects of computer animation on the particulate mental models of college chemistry students, J. Res. Sci. Teach., 32(5), 521–534.
- Wu H.-K. and Shah P., (2004), Exploring visuospatial thinking in chemistry learning, Sci. Educ., 88(3), 465–492.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9rp00060g |
| ‡ The demographic questionnaire asked “What is your gender?” with options “Male”, “Female”, “Prefer not to answer”, and “These options do not apply to me, I identify as: _”. |
| This journal is © The Royal Society of Chemistry 2019 |