Relationship between teaching assistants’ perceptions of student learning challenges and their use of external representations when teaching acid–base titrations in introductory chemistry laboratory courses†
Nicole
Baldwin
 and
MaryKay
Orgill
and
MaryKay
Orgill
 *
*
Department of Chemistry and Biochemistry, University of Nevada, Las Vegas, USA. E-mail: marykay.orgill@unlv.edu
First published on 24th June 2019
Abstract
Practicing chemists use models, diagrams, symbols, and figures to represent phenomena which cannot be detected by the human senses. Although research suggests that these external representations (ERs) can also be used to address the challenges that students have in learning chemistry, it is not clear how instructors' use of ERs aligns with their perceptions of student learning difficulties. In other words, do instructors use ERs to address what they perceive as students' major challenges in learning chemistry, or are they using ERs for other reasons? The answer to this question could have implications for the professional development of chemistry instructors, including both classroom instructors and laboratory facilitators. As a pilot study to guide the development of a larger project focused on the use and interpretation of ERs, we interviewed eleven general chemistry teaching assistants at a major university in the U.S. Southwest about their use of ERs when facilitating acid–base titration laboratory activities. Our data suggest that there is a lack of alignment between teaching assistants’ primary reported use of ERs and the primary challenge that they perceive their students have when learning about acid–base titrations. We discuss potential reasons for this misalignment, as well as implications for teaching assistant training related to the use of ERs in the laboratory learning environment.
Introduction
If you walk into any chemistry classroom, open any chemistry book, or visit any chemistry laboratory, you will see graphs, pictures, symbols, simulations, and animations. These external representations are visual imagery evoked by the models we use in chemistry to explain phenomena (Gilbert, 2005). Representations play an essential role in chemistry. For instance, chemists use representations to organize their data, to portray patterns and relationships in their data, to symbolize the chemical substances they study, to investigate the synthesis of chemical compounds, to verify the structure and composition of materials, and to communicate their findings to the scientific community (Lemke, 1998; Kozma et al., 2000; Wu and Krajcik, 2006; Nyachwaya and Gillaspie, 2016; Shehab and BouJaoude, 2017). Essentially, the use of external representations is a key activity required for membership in the scientific community of chemists.Representations also play an important role in chemistry teaching and learning. Many of the concepts studied in chemistry are abstract and beyond our senses. Therefore, we must use imagination to construct a mental image of these abstract concepts in order to study them (Johnstone, 1991). These mental images can then be transformed into an external representation that can be used by instructors to present abstract concepts to students (Stull et al., 2012; Towns et al., 2012; Wu and Puntambekar, 2012). Thus, external representations can be outward projections of what the instructor is trying to convey and, therefore, hopes the student will understand. Likewise, student-created external representations can demonstrate what the student internally understands about the abstract chemical concepts their instructor is trying to convey (Bodner and Domin, 2000; Sokolowski, 2018).
Representations are also used in the laboratory learning environment (Kozma et al., 2000; Yaman, 2018). For example, an energy diagram can be used to demonstrate Hess's Law conceptually before running the individual reactions that demonstrate Hess's Law experimentally. A balanced equation can be used to demonstrate the principles behind an acid–base titration by connecting the reaction between intangible submicroscopic particles to a macroscopic color change.
There are multiple challenges that students face when learning in the laboratory environment, and representations can be used to address some of them. For example, laboratory activities require students to not only perform unfamiliar tasks, but to simultaneously think about the application of chemistry content to the new physical task, possibly leading to cognitive overload and less efficient learning (Josephsen and Kristensen, 2006). Representations incorporated into laboratory instructions have been shown to facilitate positive student outcomes in the areas of conceptual understanding, affective attitudes, and psychomotor skills (Dechsri et al., 1997). A representation of an experimental set-up may reduce cognitive overload by showing a student how to set up and run an experiment, thus allowing the student to focus more upon the meaning and application of the laboratory results rather than on the mechanics of the experiment. Representations can also be used to summarize conceptual information or to help students visualize the chemical reactions being carried out in a laboratory activity.
We are interested in the specific roles that external representations can play in laboratory teaching and learning in chemistry, as well as in the ways that external representations are currently being used by chemistry laboratory instructors and students. As an initial step, the current pilot study focuses on teaching assistants’ perceptions of the use of representations in the chemistry laboratory in the context of acid–base titration experiments. This context is useful for two reasons: (1) titrations are a common procedure in several laboratory classes, and (2) students have been shown to struggle with concepts related to acid–base chemistry titrations (Sheppard, 2006). It is likely that both general chemistry instructors and students have been exposed to numerous representations of acid–base titration concepts and procedures and will, therefore, be able to comment on their use and usefulness to address some of the challenges that students encounter when trying to learn about and complete acid–base titrations. In the current study, we focus upon teaching assistants’ perspectives because, as laboratory instructors, they typically spend more time discussing titration concepts and procedures—and potentially using external representations of acid–base titrations—with students than do the corresponding lecture instructors. This pilot study will inform the development of a larger investigation examining the use of acid–base titration representations by teaching assistants in the laboratory, the use of acid–base titration representations by instructors in the general chemistry course, and general chemistry students’ understandings of representations of acid–base titrations. We believe that the results of the current study can inform efforts to better equip teaching assistants with skills for effective use of representations in the laboratory learning environment.
Literature review
Our overall focus for the current pilot study is on the use of representations by teaching assistants in the context of the chemistry laboratory learning environment; however, because very little has been published in this specific area, we review the literature about the use of representations in chemistry teaching and learning in general, first elaborating on some of the potential roles and benefits of representations in teaching and learning environments and then focusing on some of the challenges that students meet when learning with representations. Finally, we discuss the literature about how instructors' use of representations affects students' abilities to learn from representations. Each of these areas of literature informs the current study.Use of representations in chemistry teaching and learning
The use of representations in chemistry instruction can be beneficial to the student in a multitude of ways (Stieff et al., 2016). In general, representations scaffold and provide a medium for student learning (Kozma et al., 2000); they help students visualize objects that are too large or too small to see (Treagust et al., 2003; Graulich, 2015); and, because professional chemists use and interpret all manner of external representations—including molecular representations, graphs, and symbols—as a part of their work, the use of representations in the classroom engages students in an authentic practice of science (Roth and McGinn, 1998; Treagust, 2018).In chemistry learning, external representations can be particularly useful because they relate observable concepts and phenomena with their underlying submicroscopic causes, which can lead to more meaningful learning (Treagust et al., 2003). Noh and Scharmann (1997) reported that Korean students who were given pictures and illustrations of concepts such as dissolution improved their understandings of these concepts more effectively than those receiving instruction without representations. Gabel (1993) also demonstrated that instruction at the particulate level with the aid of representations is beneficial to students. When representations in the form of overhead projections and worksheets were provided to students learning chemistry concepts in a high school introductory chemistry class, the students performed better on test items related to these concepts than a class receiving traditional instruction. They also outperformed their peers on test items related to the symbolic and macroscopic levels. Sanger (2000) developed a curriculum to instruct students on the classification of substances at the macroscopic and microscopic levels according to their state of matter and chemical composition. The microscopic level was exemplified by the use of computer-generated visuals. These visuals were initially presented at the macroscopic level and allowed the user to switch over to a microscopic view in specific highlighted areas. First-semester college students receiving the lesson performed better on subsequent quiz items involving the physical composition of matter. This finding suggests that students benefit from the inclusion of representations that demonstrate chemical phenomena at the microscopic level in addition to the macroscopic.
Research also indicates that students who construct their own external representations of chemistry phenomena demonstrate better understanding of the material than those who do not. In a study by Bobek and Tversky (2016), 126 eighth grade students were shown a video lesson on different types of chemical bonds. Students were given a post-test immediately after watching the video. The next day they were divided into two groups. One group was asked to provide a verbal explanation of how atoms bond and the difference between ionic and covalent bonding, and a second group was asked to draw the same explanation using pictures and diagrams only. A delayed post-test was administered to both groups immediately afterwards. Results of the first post-test administered after the lesson were comparable for both groups. However, results of the delayed post-test showed that students generating visual explanations scored significantly higher than those using verbal explanations only. The authors reasoned that the creation of learner-generated representations encourages the students to be more complete in their explanations and therefore have a deeper understanding of the content.
It seems reasonable that the benefits that arise from the use of external representations in the lecture classroom would also extend to students in the laboratory learning environment. For example, ideally, students should be able to connect the macroscopic observations they make in the laboratory with their underlying submicroscopic causes. Representations can support students in (1) visualizing the submicroscopic entities and processes that result in the macroscopic changes and (2) making connections between the macroscopic and submicroscopic worlds.
Challenges students face when learning with representations
Although there are many ways that students could benefit from the use of representations in their chemistry learning, students struggle with understanding and interpreting those representations (Corradi et al., 2012; Ealy, 2018; Allred and Bretz, 2019). Scientific diagrams typically summarize large amounts of information in a concise manner. Often students are unfamiliar with the symbols and discipline-specific conventions that have been used to construct the representations. As a consequence, they cannot use those symbols and conventions to interpret the representation (Lowe, 1989; Minkley et al., 2018).Even when students are familiar with symbols and conventions, they may not know where to focus their attention in the external representations because of their low prior knowledge of chemistry concepts (Johnstone, 1991; Nicoll, 2003). Novices tend to focus mainly upon surface features of representations whereas experts are able to use prior knowledge to construct a more robust understanding of a representation (Novick, 1988; Kozma and Russell, 1997; Taber 2009). As an example, McClary and Talanquer (2011) found that students focused on surface structural features to characterize substances as either acids or bases. Similarly, in a study comparing the responses of professional chemists and undergraduate chemistry students to representations from multiple media sources, Kozma and Russell (1997) found that the novices were more likely to group representations based upon surface features rather than on conceptual information. And finally, Popova and Bretz (2018) used qualitative interviews to determine students’ understandings of reaction coordinate diagrams used in organic chemistry. Their results suggest that students either could not understand the chemistry concepts behind the graphs or they misinterpreted the encoded information that the surface features of the graphs represented.
Moreover, students may not understand why representations are useful in chemistry teaching and learning and, thus, may see them as supplemental—and not essential—information. Cooper et al. (2010) investigated student difficulties when developing representational competence and found that students did not understand why they should learn how to draw Lewis structures. Students who do not understand why or how to use external representations to support their chemistry learning are unlikely to use them effectively. Bowen et al. (1999) discovered that second year university ecology students’ lack of domain-specific experience resulted in their inability to interpret a graph as effectively as a group of experts. It was determined that the students were motivated to provide a correct answer rather than interpreting the graph. As a result, they failed to recognize the graph as a tool for deeper understanding and did not use it effectively.
Finally, because of the large amount of information conveyed in typical external representations, along with the fact that students are often learning how to interpret new types of representations while they are learning about the corresponding chemistry content, the use of external representations can be overwhelming, leading to cognitive overload (Seufert, 2003). Ironically, although representations are meant to simplify and summarize concepts, they can be challenging for students to understand and use.
Instructional challenges when using representations
While many of the difficulties students face in interpreting and using external representations of chemical concepts results from students’ lack of content knowledge and/or students' lack of understanding about the purposes and importance of representations in chemistry, some of the difficulties students experience with representations are caused by instructors (Bodner, 1991) who choose representations that are challenging for students to understand and/or do not present those representations effectively. Most university-level chemistry instructors have not had formal training in teaching strategies (Brownell and Tanner, 2012). Consequently, they may not use or present external representations in effective ways. Here, we briefly provide examples of ways that instructors might contribute to their students’ difficulties in understanding external representations.Instructors choose the representations they present in their classrooms, even though these representations may not be effective for promoting student learning (e.g., they might be overly complicated). During the process of selecting representations to use in their classrooms, instructors can make assumptions about the effectiveness of a representation based upon their own knowledge and perceptions, rather than those of the students (Schönborn and Anderson, 2006; Rigsby and Parker, 2016).
Once a representation is chosen, instructors often assume that the meaning of the representation is so clear that the students will interpret it as the instructor intends and, therefore, will not provide guidance to the students about where to focus their attention and what they should take away from the representation (Erman, 2017). Unfortunately, it is often the case that students will not only interpret a representation differently than will the instructor, but that each individual student will interpret a representation in a unique way. For example, Henderson (1999) provided 45 student teachers with scientific diagrams containing several markings such as circles and ellipses. Then they were given the very simple task of counting the number of shapes contained within the diagram. Their answers varied considerably, suggesting that, if an instructor wishes their students to take away similar understandings from an external representation, they must make those understandings explicit to the students. Likewise, instructors may not be aware of the gap between novice and expert experience levels with symbols and styles used in external representations and, thus, may not explain symbols that seem self-explanatory to an expert in the field. In a study exploring how arrows are used in introductory biology textbook illustrations, Wright et al. (2017) found that undergraduates using these textbooks became confused by the meaning of the arrows in different contexts and when the arrows were illustrated in different styles. The authors concluded that the symbols could be a barrier to understanding for novices since they do not yet possess sufficient experience in their interpretation.
Even instructors who explicitly refer to the representations they use in class might not do so in an effective manner. Instructors, who have a great deal more content knowledge and experience with the conventions of the external representations used in their discipline than do their students, sometimes move between features of the representation quickly and without explanation, leaving the students unable to make connections between those features. In a chemistry context, as an example, a figure might include macroscopic, submicroscopic, and symbolic representations of the same chemical reaction. The instructor points out those different features of the figure to their students, assuming (either consciously or not) that the students will see the connections between the different representations and how the information encoded in the different representations contributes to a more complete understanding of the chemical reaction. The students, however, may not be able to make these connections (Gabel, 1999) and, thus, will see the individual representations as not providing unique information about the chemical reaction but as providing the exact same information about the chemical reaction, decreasing the additive power of seeing multiple representations of the same concept. Rappoport and Ashkenazi (2008) carried out semi-structured interviews with six undergraduate students who were presented with diagrams and equations relating macroscopic phenomena and submicroscopic processes. They found that students tended to provide macroscopic explanations for phenomena and that the students made no meaningful connections between macroscopic phenomena and the microscopic processes that cause them. The researchers caution that instructors must make the connections to submicroscopic processes explicit, or students will continue to rely upon more familiar macroscopic explanations to explain phenomena. Ultimately, when using external representations, it is best to be explicit with students about how they should interpret and what they should learn from the representation.
Overall, what a student is able to learn from a given external representation is influenced not only by their own knowledge of both content and of representational conventions, but also by how these figures, diagrams, equations, and graphs are presented to them by their instructors. Research indicates that external representations are often not presented effectively in lecture classrooms (Orgill and Crippen, 2010). It is likely that, in the laboratory learning environment, students’ attempts to understand and interpret essential external representations are further complicated by the fact that their teaching assistants may have even less experience in selecting and explaining representations than do classroom instructors.
Purpose of the current study
Laboratory activities have long been seen as important components of a chemistry course (Boud et al., 1986; Coe et al., 1999; Bennett, 2000; Johnstone and Al-Shuaili, 2001; Psillos and Niedderer, 2002; Teixeira-Dias et al., 2005; Crawford and Kloepper, 2019). The laboratory environment offers unique opportunities for students to practice “doing” science and to form links between macroscopic phenomena and molecular-level interpretations (Hegarty-Hazel, 1990). Moreover, laboratory activities can stimulate and motivate students to learn more about chemical concepts (Hofstein and Lunetta, 1982, 2004; Deters, 2005).Unfortunately, educational research suggests that the potential for learning in the laboratory is seldom achieved (Hegarty-Hazel, 1990; Hofstein and Lunetta, 2004; Deters, 2005; Reid and Shah, 2007; Holmes et al., 2017). There are numerous challenges associated with learning in the chemistry laboratory learning environment, some of which can be addressed through the use of external representations. For example, representations can be used by both instructors and students to make connections between macroscopic laboratory findings and their underlying submicroscopic causes (Johnstone, 1991; Gabel, 1999).
When completing an acid–base titration activity (the context for the current study), students might encounter multiple representations that could help them overcome specific challenges that impede their ability to complete or understand the laboratory activity. For example, a student who is struggling with physically setting up and carrying out a titration might refer to a picture of the experimental set-up in their lab manual. Alternately, a student who does not understand how the addition of a strong base affects the pH of a buffer system might carry out the titration and then create and interpret a graph that shows how the pH of an acid solution changes as the solution is titrated with base.
Dechsri et al. (1997) measured the effect of including external representations in laboratory instructions on college students’ cognitive, affective and psychomotor outcomes. Students enrolled in a fundamentals of chemistry course were divided into groups that received text-only laboratory instructions and groups that received pictures and diagrams incorporated into the instructions. Each group was tested after 4 laboratories for conceptual understanding, attitude towards the learning quality of the chemistry laboratory, and psychomotor abilities in the laboratory. The experimental group performed higher on some of the concepts from the achievement test than the control group, indicating an advantage to student understanding in cases when representations are incorporated into laboratory instruction. Also improved were some of the items on the attitude survey, indicating that students responded positively to the laboratory as part of their learning experience. Finally, students in the experimental group significantly outperformed the control group on psychomotor skills when observed in the laboratory. These results suggest that the inclusion of representations in the chemistry laboratory manual contribute positively to student understanding of chemistry concepts, their motivation to learn chemistry, and their ability to carry out an experiment.
Because the laboratory learning environment has the potential to have a significant effect on both students’ motivation to study chemistry, as well as their understanding of chemical concepts, and because external representations play a role in laboratory learning, it is important to examine how representations are being used in the laboratory. Although some research has been done about instructors’ use of representations in chemistry classrooms, it is also important to examine how teaching assistants use external representations in the chemistry laboratory learning environment.
Teaching assistants spend approximately three hours per week with students and sometimes more one-on-one time with students than do professors (Sundberg et al., 2005; Mutambuki and Schwartz, 2018). Thus, teaching assistants can potentially have a significant influence on students’ chemistry learning (Reeves et al., 2016; Rivera, 2018). Despite this fact, teaching assistants are marginalized as a group within the context of their teaching roles in the department (Park and Ramos, 2002). A teaching assistant's identity lies at an intersection of student, instructor, and researcher, which can result in a lack of ownership of the laboratory curriculum instead of taking an active role in the teaching process (Park and Ramos, 2002). In addition, the chemistry departments they are working in tend to incentivize research productivity over teaching achievement (Jones, 1993; Brownell and Tanner, 2012). This means that teaching assistants tend to be assigned to teaching positions within the laboratory with little or no prior teaching experience or training (Shannon et al., 1998; Mutambuki and Schwartz, 2018). The training programs that do exist for TAs usually consist of short, pre-semester sessions that do not cover teaching strategies (Goodwin et al., 2018). As a consequence, TAs may not go out of their way to address student learning challenges in effective ways, especially if teaching in more effective ways might require more preparation time on their part (Muzaka, 2009). In the context of the use of external representations in the chemistry laboratory learning environment, then, it becomes important to understand why and how teaching assistants use representations because this information can inform the future training of teaching assistants in the effective use of representations in the laboratory learning environment. The current study takes a first step in this direction by examining teaching students’ perceptions of the purposes for which they use representations to teach concepts related to acid–base titration concepts.
The purpose of the current study is to investigate (1) general chemistry teaching assistants’ perceptions of the challenges their students encounter when learning about and completing acid–base titrations and (2) how the teaching assistants report using external representations to help their students learn about and carry out acid–base titrations. Our ultimate goal is to examine the relationship between these two sets of data in order to determine how teaching assistants’ use of representations in the laboratory classroom aligns with their perceptions of student learning challenges. In other words, are the teaching assistants using external representations to address what they see as major learning challenges or are they using external representations for other purposes? The following research questions guided our pilot inquiry:
(1) What challenges do teaching assistants believe that their students encounter when learning about acid–base titrations in general chemistry?
(2) For which purposes do teaching assistants report using external representations when they are teaching acid–base titration concepts to their students in general chemistry laboratory courses?
Methods
This study was reviewed and approved as exempt by the UNLV Institutional Review Board (Ref. #1026528-1). We interviewed 11 general chemistry teaching assistants about their experiences with teaching acid–base titrations in the laboratory, the challenges they believe their students encounter when learning about acid–base titrations, and about their use of external representations when teaching and facilitating acid–base titration laboratory activities. We chose to study teaching assistants because of the unique role they play as instructors in the laboratory learning environment.Theoretical framework
This study is informed by variation theory. According to this framework, there are many different ways in which a phenomenon can be experienced by a learner. The way in which a given learner experiences or understands a phenomenon is dependent on the features of the phenomenon to which the learner is exposed and the features to which the learner attends (Runesson, 2005: Bussey et al., 2013). For example, two people can view the same piece of classical artwork and take away very different meanings from one another. One viewer may pay attention to the posture of the characters and decide that something outside of the frame influences their current state. The other viewer might examine the shading of the characters to determine what the artist is trying to say about their role in the scene. Both viewers are looking at the same piece of art, but their understandings of the piece are different because they attended to different features of the phenomenon.In an educational context, variation theory refers to the phenomenon to be experienced or the concept to be learned as the “object of learning.” Students’ experience or understanding of the object of learning is affected both by the instructor's intentions for learning and by what is actually presented to the students in a “space of learning.” Thus, in variation theory, the object of learning is examined from three different perspectives: (1) the intended object of learning describes the intentions the instructor has for learning about a particular concept; (2) the enacted object of learning describes what students are exposed to when learning about the concept (instructors’ intentions do not always exactly translate into what occurs in the classroom; thus, it is the enacted object of learning that more directly affects what students can learn about a concept); and (3) the lived object of learning describes what is actually learned about the concept from the students’ perspectives (Runesson, 2005; Bussey et al., 2013). In essence, a study informed by variation theory allows a researcher to examine the relationship between what the instructor intends for students to learn, what the instructor makes possible for students to learn, and what the students actually learn about a given concept (Orgill, 2012; Bussey et al., 2013).
The current pilot study will inform a larger study about the use of representations when teaching acid–base titration concepts. Ultimately, we will examine instructors’ intentions for learning from these representations (both in the lecture and laboratory context), the ways the representations are used in classrooms, and what students understand about these representations. The pilot study described here focuses on the use of representations in the laboratory learning environment. Specifically, we address aspects of the intended object of learning in asking teaching assistants to identify challenges their students face in understanding acid–base titration concepts and performing acid–base titrations (making the assumption that the teaching assistants will identify what students should learn in addressing the challenges the students face; “here is what students should be learning and doing, but they are not”). We also address aspects of the enacted object of learning in asking the teaching assistants to describe how and why they use representations in their laboratory classrooms. Although teaching assistants’ reports of their use of representations in the classroom may not exactly mirror their actual use of representations in the classroom, we believe that providing the teaching assistants with the opportunity to discuss their use of representations and their reasoning for such use provides a richer view into what happens in the classroom than would classroom observations alone, although these are planned as part of the larger examination of the object of learning.
Participants and setting
Participants in the study included eight General Chemistry I teaching assistants and three General Chemistry II teaching assistants teaching at a major southwestern university. Participants ranged in experience in teaching general chemistry laboratory courses at the time of the interview. Three participants were in their first semester as teaching assistants for general chemistry. The other participants ranged from one semester to several years of experience teaching the general chemistry laboratory courses.The responsibilities of the teaching assistants in the general chemistry laboratory are to facilitate the procedure, develop and provide a brief lecture on the chemistry topics related to the experiment, grade laboratory write-ups, give pre-lab quizzes (General Chemistry I only), monitor the safety of the participants, and author problems for two midterm quizzes (General Chemistry I only). A laboratory practicum at the completion of the General Chemistry I lab is overseen by the teaching assistants as well. The General Chemistry II lab is traditionally taught by more senior teaching assistants who receive less structured advice and grading direction than the General Chemistry I teaching assistants. Both the General Chemistry I and II laboratories are considered stand-alone courses that roughly correlate with corresponding lecture courses. Most students are enrolled concurrently in the laboratory and corresponding lecture.
The General Chemistry I acid–base titration laboratory consists of a strong acid/strong base titration which is monitored via an indicator solution. This activity represents the students’ first use of a burette and first titration during the laboratory course. Students add titrant (a strong base) until they see a color change and use the volume of titrant to determine the concentration of the unknown acid through stoichiometric means. The students spend one 3 hour laboratory period completing this activity.
In the General Chemistry II laboratory course, students prepare buffer solutions and titrate them with either strong acid or strong base, using a meter to monitor how the pH changes with the addition of titrant. Students use their data to graphically determine the pKa of the weak acid component of the buffer. Students are expected to recall titration procedures from the previous semester's laboratory class and are not given directions on how to use a burette or how to titrate a solution. Because the use of pH meters is new, students are given instructions on how to use this instrument. Students spend two 3 hour laboratory periods completing this activity. In one week, they titrate a buffer with a strong acid. In the second week, they titrate the same buffer with a strong base.
The training for each teaching assistant consisted of reading through the lab manual and attending a once-per-week meeting. The focus of these lab meetings is not on effective pedagogy or on resources the teaching assistant can utilize, such as representations, but rather on the techniques associated with performing the lab. More senior teaching assistants typically share their knowledge of pitfalls the students are likely to encounter when performing the experiment. An example might be that senior teaching assistants notice that students forget to add indicator solution before beginning a titration. None of the teaching assistants are required to attend formal training.
Interview protocol
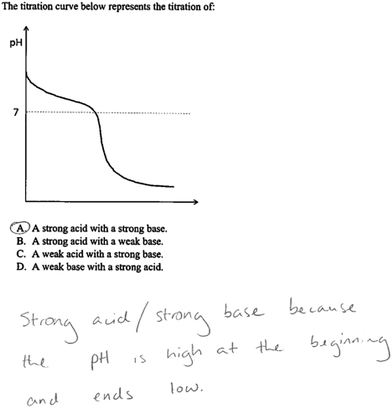
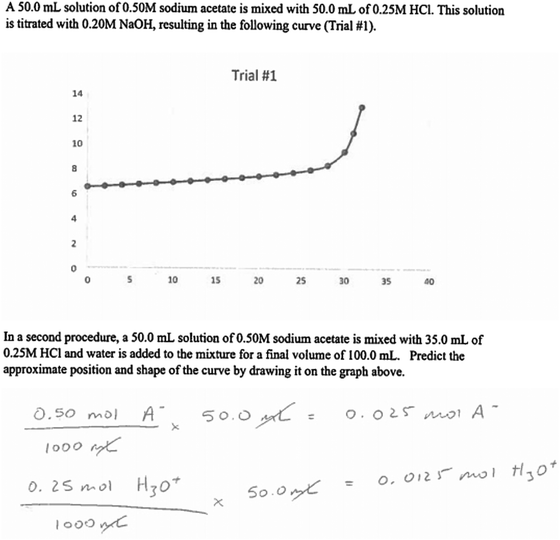
A copy of the interview protocol can be found in Appendix 1. Interviews with the participants were semi-structured and lasted for approximately one hour. We began by asking participants to describe their experiences teaching acid–base titrations in the laboratory environment and to identify the challenges students encounter when learning about this concept. For example, we asked, “What do you think is the most challenging aspect of acid–base titrations for your students to understand?” Then we asked questions about the types of representations the teaching assistants used the laboratory. This included those provided by the laboratory manual, textbook sources as well as any self-constructed representations. This information was gathered to understand the nature of the representations that teaching assistants are using in the laboratory to teach their students: “What types of representations do you believe are the most useful to help with the challenges that students may face when doing acid–base titrations?”Participants were also shown two external representations of acid–base titrations that are typical of those seen in general chemistry textbooks and asked to identify features they felt were important, challenging for students to understand, and useful for teaching. The first representation depicts the steps that are needed to physically carry out a titration (Fig. 4.18 in Brown et al., 2015, p. 152). Four frames are shown in the figure. Each frame includes a photograph of a step in the titration experiment. The first photograph shows a flask containing a colorless liquid, and it has been labelled to indicate that there is an acid in the flask. The second photograph shows the same setup but with a dropper adding indicator solution. The third photograph shows the flask underneath a burette that has been set up on a ring stand. And the final photograph shows the ring stand setup with the titration flask full of a light pink fluid. Text appears in each photograph to describe the observed phenomenon such as “Initial volume reading.” The second representation is a graph that shows how the pH changes when a weak acid is titrated with a strong base (Fig. 17.9 in Brown et al., 2015, p. 741). Certain regions of the graph are labeled, such as the buffer region, equivalence point, and the area of excess base. Underneath the graph are four boxes in which are illustrated particle-level representations of the species that are present at four points on the titration curve. The illustrations in the boxes are connected to points on the graph by lines.
The teaching assistants were then shown two titration problems that included external representations. Both problems were modeled after problems that are part of the laboratory activities that the teaching assistants currently facilitate. The problems were partially completed with common errors that students might make. Participants were asked to describe how they would direct the student to address the errors and complete the problems. Primarily, we were interested in whether or not the teaching assistants would refer to the provided representations (and, if so, how) in response to this task, in order to obtain an initial level of understanding about the teaching assistants’ tendency to use or not use external representations in response to student questions (Fig. 1 and 2).
Interviews were audio recorded and then transcribed verbatim. The transcripts were initially analyzed within the Atlas.ti software program in order to keep the data organized and begin an initial coding process. Based upon an initial reading of the transcripts, several themes were identified that merited further investigation. Two of these themes are relevant to the current discussion: (1) teaching assistants’ perceptions of the challenges students face when learning acid–base titration chemistry, and (2) teaching assistants’ reports of the reasons for which they use representations to teach acid–base titration concepts in the laboratory. Each transcript was coded for categories under each theme, such as specific student challenges identified by teaching assistants (an indication of the teaching assistants’ intentions for learning) and specific mentions of which representations were used in the laboratory, when they were used in the laboratory, and why they were used in the laboratory (an indication of the enacted object of learning). No further themes were identified upon subsequent examinations of the transcripts. The categories were then grouped and analyzed for frequency among participants. All participants were provided with pseudonyms which have been used consistently throughout the study.
Results and discussion
In the sections that follow, we present the challenges that the teaching assistants believe their students encounter when learning about and doing acid–base titrations, as well as the purposes for which the teaching assistants are using representations in their laboratory classrooms. As previously mentioned, we view the learning challenges identified by the teaching assistants as statements about what the teaching assistants believe their students should be learning about acid–base titrations (i.e., “the students should be learning about or doing these things, but they are struggling with them”). The teaching assistants’ reports about why they are using external representations provides initial information about what is happening in their classrooms and why.Because research indicates that external representations can be used to support student learning (Gabel, 1993; Russell et al., 1997; Sanger, 2000; Stieff et al., 2016), we were particularly interested in how the challenges identified by the teaching assistants align with their use of external representations when teaching acid–base titration laboratory classes. In other words, are the teaching assistants using the representations to address the specific learning challenges they believe their students have? While we do not expect teaching assistants, as novice teachers, to recognize all of the learning challenges that their students have (or the sources of those learning challenges), we were optimistic that they would use representations to address those challenges that they believe their students have. Below, we present the results in order from those mentioned by the greatest number of participants to those mentioned by the least number of participants. Arranging the results in this order, although not traditionally part of variation theory, allowed us to compare not only which student learning challenges were addressed with representations, but the relative priorities that this group of teaching assistants assigned to the use of external representations when addressing challenges associated with learning acid–base titration content and procedures. We present the learning challenges and purposes for using representations that were mentioned by most of our participants in detail. Results that were mentioned by fewer teaching assistants will be listed, but not discussed in detail.
Teaching assistants’ beliefs about student challenges
The participants in the study identified several challenges that they believe negatively affect their students’ abilities to understand acid–base titrations and to perform acid–base titrations.Chris, another General Chemistry I teaching assistant, mentioned that he wishes his students would think about what is happening on the submicroscopic scale during a titration and use that to consider their results, noting that students do not tend to think about what is happening at the submicroscopic level even if that information has been explicit in both the lecture class and in the pre-laboratory discussion.
I would like for them to think…to see, like, to see in their minds, like, “oh we've got you know one drop of HCl that I added to a solution of NaOH and so what is that doing, that one drop? And then what's left?” […] There's a significant amount of students who aren't seeing that. You know. They're not thinking about that when they're answering the questions at least. (Chris, General Chemistry I)
Multiple teaching assistants saw the students’ inability to connect their lecture and laboratory learning as a problem that affects what students can learn from many of the laboratory activities, not just the acid–base titration laboratory activities.
I've noticed, and this is not specific to acid–base titrations but I've noticed that within general um, chemical um reactions, they don't seem to, they seem to be missing some of the, the core concepts a lot of times and that's very common […] Um, and I would say that's more than half of the students are making mistakes that, on their labs, that suggest that they don't, that they are misunderstanding some core concept of chemistry. (Chris, General Chemistry I)
Alex noted that, in general, students do not seem to have a good conceptual understanding of why they are doing what they are doing in the laboratory and that this is keeping them from understanding the specific content they should be learning in the lab.
But what's the real concepts behind it? […] that's something that's lost in the […] lab right now […] they don't see that underlying fact at what we see at our level. Of, here's the reason why, and a lot of times it's lost. It's like staring at the forest and not really seeing the trees. (Alex, General Chemistry I)
Another teaching assistant described the students’ tendency to titrate a sample to a dark pink color without realizing that they have overtitrated their sample and, therefore, will calculate an incorrect unknown concentration.
There's issues with the end point…I see a lot of blowing past endpoints…they don't look at a dark pink solution and go OK, if that took 15 mL and I have the same amount of acid in my next sample, then I know that like…probably around 10 mL I want to slow down. (Kaitlin, General Chemistry I)
I remember there being a lot of issues with calculations […] in terms of post lab questions, they had a particularly difficult time because […] they couldn't quite figure out how the math they were doing was related to what they did in lab. (Shea, General Chemistry I)
Steph mentioned that students are unable to determine when an ICE table is required to calculate the pH of a partially titrated buffer in lab, and that sometimes students construct the tables incorrectly. “You can set up an ICE table but if that's not even the right ICE table, you're already starting off on the wrong foot” (Steph, General Chemistry II). Again, TAs noticed that students are experiencing issues applying the calculations they learn about in lecture to the laboratory context.
Teaching assistants’ purposes for using representations
To address our second research question, we asked teaching assistants a series of questions about the representations they use in class when teaching the acid–base titration experiments and their reasons for using those representations. The answers they gave provide some insight into the enacted object of learning, or what is possible to learn based upon the resources the students are exposed to in the laboratory environment. Here we present the teaching assistants’ purposes for using representations when facilitating an acid–base titration laboratory activity.Chris described a representation that he often presents to his students to remind them of the potential safety concerns involved with the experiment.
[I used a representation] which was a cartoon character pouring dangerous chemicals on their face. […] It was meant to warn them about the, about laboratory safety precautions. So, you know, do you have a funnel? Are you wearing a glove? Are you in the sink? Like, these are all details that you could write out with a wall of text, or you could just have a photo [that] encapsulates all those details. (Chris, General Chemistry I)
Claire described a flowchart that she constructed for her General Chemistry I students that she felt made the extensive laboratory instructions easier to understand. “I think it would have been almost easier to visualize and read if I just […] drew the main points out” (Claire, General Chemistry I).
Some of the teaching assistants felt that representations that depicted ions in solution were useful when teaching acid–base titration concepts. For example, some teaching assistants provided chemical symbols to represent the ions present in solution during the titration, and they drew their students’ attention to the charge of each ion as a useful tool for determining their behaviors during a titration. Chris described using the charges of ions in solution during the titration to help students understand the reaction taking place at a level that they cannot see during the experiment.
So, the first thing I would do is try to show the students when they've got an NaOH […] in solution it goes to Na plus and an OH minus and so these two things, when they're solid they're together but when you put them in water they separate and dissolve. (Chris, General Chemistry I)
As another example, Shea demonstrated the behavior of ions in solution by indicating the charge of the ions to her students. She used the symbols of the ions to help her students predict how a double replacement reaction will occur. “So, when we're doing a double replacement reaction, what happens is you need to think about it as a partner swap. So, they're switching partners. The positive guy is now gonna hook up with the other negative guy” (Shea, General Chemistry I).
Claire, for example, directed her General Chemistry I students to start with a balanced equation before attempting the mathematical portions of the post-lab worksheet. “[…] double check on your balanced equation. If you already have one, kind of make sure everything is balanced on both sides” (Claire, General Chemistry I).
Frank provided his General Chemistry I students with a balanced equation on the board to explain how to calculate the concentration of an unknown titrated acid solution such as the students performed in the laboratory. “You know like here's our balanced equation […] this is what we're doing. We're titrating to get this milliliters of this to do that.” (Frank, General Chemistry I).
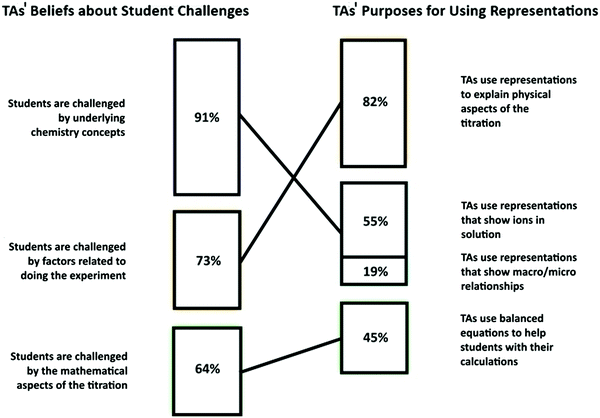
To examine this alignment, we created a graphic containing the information obtained from our data analysis (Fig. 3). In this figure, the challenges teaching assistants believe students have when learning about acid–base titrations are listed on the left and the purposes TAs mentioned for using representations in the laboratory are listed on the right. The specific challenges and purposes are ordered from those mentioned by the greatest number of participants at the top to those mentioned by the least number of participants at the bottom, with the numbers within the bars reflecting the percentage of participants who made at least one mention of that particular category. It is important to note that percentages have been used here as a means to investigate potential correlations between what the TAs believe are significant challenges for their students and the reasons they provide for using representations in the laboratory. It is also important to note that these percentages resulted from interviews with a small number of participants; and, therefore, any conclusions drawn from this data should be considered as tentative. These conclusions, however, do provide a useful foundation for the larger study.
Each box on the left, representing a category of perceived challenges, can be correlated with a box on the right, representing a category of TAs’ purposes for using representations. For example, the TAs perceive that students are challenged by factors related to doing the titration experiment (left-hand side of graphic) and the TAs report using representations to help their students do the titration experiment (right-hand side of graphic). In the graphic, similar categories have been connected by lines. When discussing the challenges they believe their students have in learning about acid–base titrations, the TAs mentioned a lack of multiple different types of basic chemistry content knowledge or a lack of basic chemistry knowledge in general. However, when the TAs reported the purposes for which they use representations in the laboratory environment, they only mentioned two specific pieces of missing content knowledge: (1) a lack of knowledge about how ions interact in solution during an acid–base reaction and (2) a lack of understanding about the connection between particulate-level processes and macroscopic-level observations. Thus, the box on the right-hand side of the graphic is broken into sub-categories relating to the TAs’ use of representations to help students understand these missing pieces of content knowledge. The percentages in these connected boxes on the right-hand side of the graphic are not additive: the same two participants who mentioned using representations of macro/micro phenomena to explain titrations also reported using illustrations of ions in solution.
Although Fig. 3 indicates that, in general, the purposes for which TAs are using representations in the laboratory roughly correlate with the challenges they believe their students have when learning about acid–base titrations, there is an apparent disconnect between the learning challenges mentioned by the greatest number of TAs (understanding underlying chemistry concepts) and the purpose for which the greatest number of TAs mention using representations when teaching acid–base titration concepts (to help students physically do the experiment). Approximately 91% of those interviewed mentioned that they felt their students struggle with underlying concepts when learning about acid–base topics in chemistry, but only about 55% mentioned using representations to address this lack of content knowledge. Moreover, while the TAs recognize many different types of content knowledge that their students seem to lack, they are only using representations to address two specific types of content knowledge: knowledge of ion interactions and knowledge of how to use particulate-level processes to explain macroscopic, observable processes. Instead of addressing the content knowledge the TAs identified as major challenges, 82% of the participants reported using representations to help their students do the experiment.
Conclusions
This study investigated the relationship between the learning challenges that teaching assistants perceive their students have, and purposes for which they report using representations when teaching acid–base chemistry titration concepts. Based on our data, the teaching assistants perceive that students struggle with acid–base titration experiments because they do not have a good understanding of more basic chemistry concepts and how they apply to their work in the laboratory environment. However, the majority use external representations to help students complete the laboratory activity in a timely fashion, analyze their data, and perform the necessary mathematical calculations for the written report. Thus, although the teaching assistants know that it is important that students understand the concepts behind what they are doing in the lab (a cognitive focus for the intended object of learning), the teaching assistants use of external representations focuses more on doing the laboratory activity (a psychomotor focus for the enacted object of learning).There are many potential reasons for this potential disconnect. One possibility is related to the unclear role that the teaching assistant plays as the laboratory instructor. Occupying a level somewhere between student and professor, teaching assistants can become marginalized by their departments, causing a feeling of lack of ownership (Park and Ramos, 2002). A perceived lack of control in the design and implementation of the curriculum may lead some teaching assistants to believe that it is the job of the course instructor to teach the concepts underlying the titration, and it is the job of the teaching assistant to focus on safety and execution of the experiment, particularly when safety and procedures are the typical focus of any training the teaching assistant receives (Shannon et al., 1998; Mutambuki and Schwartz, 2018). This potential cause would be consistent with the teaching assistants’ use of external representations in the current study to demonstrate laboratory procedures.
Studies indicate that teaching assistants are aware of the shift in educational culture towards evidence-based teaching in the classroom and are interested in incorporating more evidence-based teaching into their own curricula (Goodwin et al., 2018). However, several factors—such as lack of departmental incentive, time cost, and the need to self-motivate—are barriers to the implementation of these practices by graduate students. Moreover, many teaching assistants believe that they need only content knowledge to be effective instructors. This may originate from a departmental culture that places research goals above teaching goals, offering little incentive to teaching assistants to develop their own pedagogical skills (Luft et al., 2004). However, content knowledge alone is insufficient for effective laboratory instruction. Herrington and Nakhleh (2003) surveyed both undergraduate introductory chemistry students and their laboratory teaching assistants to determine the most important qualities for laboratory teaching assistants. They found that both students and teaching assistants agreed that the most important factor is teaching assistant knowledge in four main areas: (1) procedures, techniques and safety, (2) concepts related to the laboratory, (3) knowledge of how students learn, and (4) knowledge about teaching in general.
These findings suggest that teaching assistants—and their students—could benefit from training that incorporates issues related to how students learn and effective strategies for supporting students' learning in addition to training about issues related to experimental procedures and safety (Herrington and Nahkleh, 2003). Teaching assistants often operate autonomously within the department, without support, instruction or feedback from faculty and lab coordinators (Luft et al., 2004). When no teacher development or training is offered, the teaching assistants may fall back to instruction in the same style as they themselves were taught. It would stand to reason then, that if teaching assistants receive no explicit training about how representations are useful in both chemistry and in chemistry teaching and learning, or about how to support student learning through the use of representations, they might not see representations as an essential part of learning in chemistry or might not use them effectively. In other words, they may see representations as extra information, useless to students, and not make time to present them in the laboratory. This is unfortunate, as the use of external representations is an authentic practice of professional chemists and, accordingly, a practice in which students should engage.
Implications for teaching assistant professional development
As mentioned in the previous section, teaching assistants need explicit training in order to improve their understandings of the benefits and use of representations in the laboratory. Teaching assistant training programs have been strongly recommended by researchers previously (Shannon et al., 1998; Luft et al., 2004; Muzaka, 2009), but they are rarely required in many academic disciplines (Park and Ramos, 2002). When training programs are offered, they tend to be short, focus upon university policies and procedures instead of professional development, and remain unstructured (Shannon et al., 1998).Teaching assistants are unlikely to have much experience with teaching, let alone with teaching techniques that are known to promote meaningful learning. The current study suggests that teaching assistants need not only general training in safety and laboratory protocol, but in how to facilitate practices that are used by professional chemists, like the use and interpretation of external representations. They need to be given an opportunity to think about and discuss why representations are important in chemistry; the benefits of using representations in chemistry learning; the reasons that students might struggle to use, interpret, or create representations; and the strategies that they can use to help students use external representations.
Based on the results of this pilot study, and knowing that representational competence is important for all scientists (Lemke, 1998; Kozma et al., 2000; Wu and Krajcik, 2006; Nyachwaya and Gillaspie, 2016; Shehab and BouJaoude, 2017), we designed a university workshop intended to (1) introduce graduate students in STEM fields to the use of representations in science, (2) discuss why representations are important to professional scientists, (3) provide some best practice guidelines for the instructional use of external representations, and (4) provide feedback on some of the representations that graduate teaching assistants in STEM fields are already using in their classrooms and laboratories. This training only required 1 hour of the teaching assistants' time, and a training session that follows a similar outline could be delivered as part of a pre-semester chemistry teaching assistant training session or during a weekly teaching assistant meeting with a course supervisor. Because we were interested in offering our workshop to graduate students in all STEM disciplines, we coordinated with the Graduate College on campus and were able to offer our participants credit towards earning a Graduate Teaching Certificate, thus providing additional motivation for attendance. A description of this workshop can be found in Appendix 2.
Limitations and future research
There are several limitations of the current study. As mentioned previously, the findings are based on interviews with a small number of participants and, therefore, cannot be considered conclusive in and of themselves. The site of the study was limited to one chemistry department at one university and with a limited number of teaching assistants. These tentative findings, however, can inform future studies that include a greater number of TAs from a variety of institutional settings.Another limitation of the study is associated with the teaching assistants’ self-reports of their use of representations. We did not observe their actions in the classroom to correlate their reported uses and their actual uses of representations, but this will be important to pursue in the future. In addition to exploring which representations teaching assistants actually use, we must explore when and how teaching assistants use the representations that they present, and why they believe representations are helpful to students.
A third limitation of the study involves the interview protocol itself. Our initial design of the interview protocol focused on the challenges that teaching assistants perceived that their students face when learning about acid–base titrations and the purposes for which they reported using representations in the lab. Only when we started to analyze our data did we recognize that the TAs' perceptions of their roles in the laboratory might have affected their use of representations. For example, although the TAs might complain that students do not understand basic chemistry concepts, they might not believe that it is their responsibility to teach those concepts. As a consequence, they might not use representations for that purpose. We will take this limitation into consideration in the design of the larger project focused on the use of representations of acid–base titrations.
Finally, we have only studied the perspective of teaching assistants and have not yet explored the students’ perspective (what is learned in the lab) or the corresponding lecture instructors’ expectations (what should be learned in the lab). In order to gain a more complete perspective about the use of representations in the laboratory environment, we need to examine these perspectives and how they relate to each other. An examination of these other perspectives is planned as a part of the extension of this pilot project.
Conflicts of interest
There are no conflicts to declare.Appendix 1: interview protocol
(1) Background questions• Can you tell me a little bit about your chemistry background?
■ Tell me a little bit about your college career: where have you studied, what have you studied?
■ What courses do you teach/have you taught at this university?
(2) Questions related to perceived student challenges
• Which concepts do you think students should know about acid–base titrations?
• From these concepts, which are the one or two most important for the students to understand about acid–base titrations?
• Why do you think that these are the most important concepts for the students to understand?
• From these concepts, which do you believe are the most challenging for your students to understand?
(3) Questions related to the types of representations used
• What types of representations have you used in the past when teaching acid–base titration concepts in the laboratory?
(4) Questions related to exemplar images provided to participants for comment.
• Can you describe this image in your own words?
• What information about acid–base titrations do you believe a student should take away from this image?
• Which features of the representation do you believe are the most useful in helping students learn about acid–base titration concepts?
• Which features of the image do you believe are the most difficult to understand for students?
(5) Questions related to the partially solved titration problem and representations
• What concepts about acid–base titrations should the students have learned in order to solve this problem?
• Why do you think that this particular representation has been provided to the student in order to solve this problem?
• Using what they have shown on their paper so far, how would you work through the problem with the student?
Appendix 2: description of representation workshop
The representation workshop presented in Fall 2018 was tailored to graduate students teaching science laboratories, recitations and courses, but was also open to any other graduate students interested in representation use in the classroom. The purpose of the workshop was to inform the graduate students about the benefits of representation use in the classroom, the confusion that students can experience when learning with representations, and some best practices for utilizing representations in the classroom. Attendees were encouraged to bring representations from their own curricula to the workshop so they could obtain feedback about (1) the representation and (2) how to best present the representation to students. The workshop served another purpose less obvious, but no less important: to help teaching assistants realize the power they have over the curriculum, as well as the influence they have on student learning outcomes.Pictures of slides used by the workshop facilitator can be found in the ESI.† The workshop opened with an activity designed to switch the participants’ perspective to that of their students when presented with a complicated science diagram (slide 2 of PowerPoint document). This diagram was presented with little explanation. Attendees were asked to answer the following questions about the diagram: (1) what is the important information that this diagram is trying to get across?; (2) what should you, as the viewer take away from the image?; (3) what are some of the important details that are represented in the diagram?; and (4) where should you focus your attention in the diagram? Participants were encouraged to discuss the representation openly with others and given some time to discuss their answers with the group. The participants were initially eager to determine the meaning of the image when it was shown. The conversation in the room then quieted as the participants wrestled with constructing their own meanings from the dense image. Finally, the participants expressed some understanding during the discussion, but they also mentioned feeling confusion and becoming overwhelmed with the amount of information present on the screen.
After the desired workshop outcomes were presented to the participants (slide 3), the facilitator defined the term “representations” for the participants, providing familiar science examples for different types of representations (slide 4). This was followed by a discussion of the challenges that students often face when using representations in the science classroom, including their inability to interpret implicit information encoded within the diagram, their inability to relate different pieces of information within the representation, and their lack of prior knowledge, which can cause students to misidentify the important portions of the representation (slide 6).
The facilitator then introduced participants to the idea of representational competence: the ability to use, interpret, and create representations (slide 7). The workshop discussion initially focused upon two specific actions that can be taken to help students better understand the representations used in science classes (and, thus, develop the students’ representational competence): (1) teaching students explicitly about the features and conventions of the diagrams used in class and (2) reducing the use of potentially confusing visualizations such as those with overly complicated images or jargon that have not yet been clarified. This initial discussion was followed with additional recommendations for the effective use of representations in science laboratories and classrooms. For example, participants were asked to consider allowing students to work in groups and openly discuss their understandings of representations when they are used in class, to consider the ability level of the students when selecting the appropriate representation to convey an idea, to explain in detail what each image means and what language is encoded within the image, to use several different representations when possible to obtain different perspectives, and to encourage students to generate their own representations when possible (slide 10).
At the end of the discussion, the complex science diagram presented at the beginning of the workshop for group discussion was revisited (slide 11). Participants were asked to critique the image and to suggest what they could do to help students interpret the diagram and to understand the content presented in it. The group had many suggestions that were in line with the suggested best practices that had been discussed earlier in the workshop.
The workshop concluded with the participants working together to provide feedback about the representations they brought to the workshop. A simple worksheet with best practice suggestions was provided to the participants, which they referenced while providing feedback to their peers. A copy of this worksheet can be found in the ESI.† The participants found this feedback to be valuable because it came from peers in different, but closely related, disciplines.
Acknowledgements
We acknowledge the UNLV Top Tier Doctoral Research Graduate Assistanceship Program for providing funding this research. We would like to thank the reviewers for suggestions that have substantially improved the focus of this paper.References
- Allred Z. D. R. and Bretz S. L., (2019), University chemistry students’ interpretations of multiple representations of the helium atom, Chem. Educ. Res. Pract., 20, 358–368.
- Bennett S. W., (2000), University practical work: why do we do it? Educ. Chem., 37, 49–50.
- Bobek E., and Tversky B., (2016), Creating visual explanations improves learning, Cognit. Res.: Princ. Implic., 1(1), 27.
- Bodner G. M., (1991), I have found you an argument: the conceptual knowledge of beginning chemistry graduate students, J. Chem. Educ., 68(5), 385–388.
- Bodner G. M. and Domin D. S., (2000), Mental models: the role of representations in problem solving in chemistry, Univ. Chem. Educ., 4(1), 24–30.
- Boud D., Dunn J. and Hegarty-Hazel E., (1986), Teaching in laboratories, Guildford, Surrey: SRHE and NFER-Nelson.
- Bowen G. M., Roth W. M. and McGinn M. K., (1999), Interpretations of graphs by university biology students and practicing scientists: toward a social practice view of scientific representation practices, J. Res. Sci. Teach., 36(9), 1020–1043.
- Brown T. E., LeMay H. E. H., Bursten B. E., Murphy C., Woodward P. and Stoltzfus M. E., (2015), Chemistry: the central science, Pearson Education.
- Brownell S. E. and Tanner K. D., (2012), Barriers to faculty pedagogical change: lack of training, time, incentives, and… tensions with professional identity? CBE Life Sci. Educ., 11(4), 339–346.
- Bussey T. J., Orgill M. and Crippen K. J., (2013), Variation theory: a theory of learning and a useful theoretical framework for chemical education research, Chem. Educ. Res. Pract., 14(1), 9–22.
- Coe E. M., McDougall A. O. and McKeown N. B., (1999), Is peer-assisted learning of benefit to undergraduate chemists? Univ. Chem. Educ., 3, 72–75.
- Cooper M. M., Grove N., Underwood S. M. and Klymkowsky M. W., (2010), Lost in Lewis structures: an investigation of student difficulties in developing representational competence, J. Chem. Educ., 87(8), 869–874.
- Corradi D., Elen J. and Clarebout G., (2012), Understanding and enhancing the use of multiple external representations in chemistry education, J. Sci. Educ. Tech., 21(6), 780–795.
- Crawford G. L. and Kloepper K. D., (2019), Exit interviews: laboratory assessment incorporating written and oral communication, J. Chem. Educ., 96, 880–887.
- Dechsri P., Jones L. L. and Heikkinen H. W., (1997), Effect of a laboratory manual design incorporating visual information-processing aids on student learning and attitudes, J. Res. Sci. Teach., 34(9), 891–904.
- Deters K. M., (2005), Student opinions regarding inquiry-based labs, J. Chem. Educ., 82, 1178–1180.
- Ealy J. B., (2018), Assessment of students' external representations of mmCIF entries and their biochemical knowledge, Biochem. Mol. Biol. Educ., 46(6), 634–643.
- Erman E., (2017), Factors contributing to students’ misconceptions in learning covalent bonds, J. Res. Sci. Teach., 54(4), 520–537.
- Gabel D. L., (1993), Use of the particle nature of matter in developing conceptual understanding, J. Chem. Educ., 70(3), 193–194.
- Gabel D., (1999), Improving teaching and learning through chemistry education research: a look to the future, J. Chem. Educ., 76(4), 548–554.
- Gilbert J. K., (2005), Visualization: a metacognitive skill in science and science education, in Gilbert J. K. (ed.), Visualization in Science Education, Dordrecht: Springer, pp. 9–27.
- Goodwin E. C., Cao J. N., Fletcher M., Flaiban J. L. and Shortlidge E. E., (2018), Catching the wave: are biology graduate students on board with evidence-based teaching? CBE Life Sci. Educ., 17(3), ar43.
- Graulich N., (2015), The tip of the iceberg in organic chemistry classes: how do students deal with the invisible? Chem. Educ. Res. Pract., 16(1), 9–21.
- Hegarty-Hazel E. (ed.), (1990), The student laboratory and the science curriculum, London: Routledge.
- Herrington D. G. and Nakhleh M. B., (2003), What defines effective chemistry laboratory instruction? Teaching assistant and student perspectives, J. Chem. Educ., 80(10), 1197–1205.
- Henderson G., (1999), Learning with diagrams, Aust. Sci. Teach. J., 45(2), 17–26.
- Hofstein A. and Lunetta V. N., (1982), The role of the laboratory in science teaching: neglected aspects of research, Rev. Educ. Res., 52, 201–217.
- Hofstein A. and Lunetta V. N., (2004), The laboratory in science education: foundations for the twenty-first century, Sci. Educ., 88, 28–54.
- Holmes N. G., Olsen J., Thomas J. L. and Wieman C. E., (2017), Value added or misattributed? A multi-institution study on the educational benefit of labs for reinforcing physics content, Phys. Rev. Phys. Educ. Res., 13(1), 1–12.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assist. Learn., 7(2), 75–83.
- Johnstone A. H. and Al-Shuaili A., (2001), Learning in the laboratory: some thoughts from the literature, Univ. Chem. Educ., 5, 42–51.
- Jones J. L., (1993), TA training: from the TA's point of view, Innov. High. Ed., 18(2), 147–161.
- Josephsen J. and Kristensen A. K., (2006), Simulation of laboratory assignments to support students’ learning of introductory inorganic chemistry, Chem. Educ. Res. Pract., 7(4), 266–279.
- Kozma R. B. and Russell J., (1997), Multimedia and understanding: expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 34(9), 949–968.
- Kozma R., Chin E., Russell J. and Marx N., (2000), The roles of representations and tools in the chemistry laboratory and their implications for chemistry learning, J. Learn. Sci., 9(2), 105–143.
- Lemke J., (1998), Multiplying meaning. Visual and verbal semiotics in scientific text, in Martin J. R. and Veel R. (ed.), Reading science: critical and functional perspectives on discourses of science, Routledge, pp. 87–113.
- Lowe R. K., (1989), Search strategies and inference in the exploration of scientific diagrams, Educ. Psychol., 9(1), 27–44.
- Luft J. A., Kurdziel J. P., Roehrig G. H. and Turner J., (2004), Growing a garden without water: graduate teaching assistants in introductory science laboratories at a doctoral/research university, J. Res. Sci. Teach., 41(3), 211–233.
- McClary L. and Talanquer V., (2011), College chemistry students' mental models of acids and acid strength, J. Res. Sci. Teach., 48(4), 396–413.
- Minkley N., Kärner T., Jojart A., Nobbe L. and Krell M., (2018), Students' mental load, stress, and performance when working with symbolic or symbolic–textual molecular representations, J. Res. Sci. Teach., 55(8), 1162–1187.
- Mutambuki J. M. and Schwartz R., (2018), We don’t get any training: the impact of a professional development model on teaching practices of chemistry and biology graduate teaching assistants, Chem. Educ. Res. Pract., 19(1), 106–121.
- Muzaka V., (2009), The niche of graduate teaching assistants (GTAs): perceptions and reflections, Teach. High. Educ., 14(1), 1–12.
- Nicoll G., (2003), A qualitative investigation of undergraduate chemistry students' macroscopic interpretations of the submicroscopic structures of molecules, J. Chem. Educ., 80(2), 205–213.
- Noh T. and Scharmann L. C., (1997), Instructional influence of a molecular-level pictorial presentation of matter on students' conceptions and problem-solving ability, J. Res. Sci. Teach.34(2), 199–217.
- Novick L. R., (1988), Analogical transfer, problem similarity, and expertise, J. Exp. Psychol. Learn., 14(3), 510–520.
- Nyachwaya J. M. and Gillaspie M., (2016), Features of representations in general chemistry textbooks: a peek through the lens of the cognitive load theory, Chem. Educ. Res. Pract., 17(1), 58–71.
- Orgill M., (2012) Variation theory, in Seel N. M. (ed.), Encyclopedia of the Sciences of Learning, Springer, Boston, MA.
- Orgill M. and Crippen K., (2010), Teaching with external representations: the case of a common energy-level diagram in chemistry, J. Coll. Sci. Teach., 40(1), 78–84.
- Park C. and Ramos M., (2002), The donkey in the department? Insights into the graduate teaching assistant (GTA) experience in the UK, J. Grad. Educ., 3(2), 47–53.
- Popova M. and Bretz S. L., (2018), Organic chemistry students’ interpretations of the surface features of reaction coordinate diagrams, Chem. Educ. Res. Pract., 19(3), 919–931.
- Psillos D. and Niedderer H. (ed.), (2002), Teaching and learning in the science laboratory, Dordrecht, Netherlands: Kluwer Academic Publishers.
- Rappoport L. T. and Ashkenazi G., (2008), Connecting levels of representation: emergent versus submergent perspective, Int. J. Sci. Educ., 30(12), 1585–1603.
- Reeves T. D., Marbach-Ad G., Miller K. R., Ridgway J., Gardner G. E., Schussler E. E. and Wischusen E. W., (2016), A conceptual framework for graduate teaching assistant professional development evaluation and research, CBE Life Sci. Educ., 15(2), es2.
- Reid N. and Shah I., (2007), The role of laboratory work in university chemistry, Chem. Educ., 8, 172–185.
- Rigsby R. E. and Parker A. B., (2016), Using the PyMOL application to reinforce visual understanding of protein structure, Biochem. Mol. Biol. Educ., 44(5), 433–437.
- Rivera S., (2018), A summer institute for STEM graduate teaching assistants: exploring teaching perceptions, J. Coll. Sci. Teach., 48(2), 28–32.
- Roth W. M. and McGinn M. K., (1998), Inscriptions: toward a theory of representing as social practice, Rev. Educ. Res., 68(1), 35–59.
- Runesson U., (2005), Beyond discourse and interaction. Variation: a critical aspect for teaching and learning mathematics, Cambridge J. Educ., 35(1), 69–87.
- Russell J. W., Kozma R. B., Jones T., Wykoff J., Marx N. and Davis J., (1997), Use of simultaneous-synchronized macroscopic, microscopic, and symbolic representations to enhance the teaching and learning of chemical concepts, J. Chem. Educ., 74(3), 330–334.
- Sanger M. J., (2000), Using particulate drawings to determine and improve students' conceptions of pure substances and mixtures, J. Chem. Educ., 77(6), 762–766.
- Schönborn K. J. and Anderson T. R., (2006), The importance of visual literacy in the education of biochemists, Biochem. Mol. Biol. Educ., 34(2), 94–102.
- Seufert T., (2003), Supporting coherence formation in learning from multiple representations, Learn. Instr., 13(2), 227–237.
- Shannon D. M., Twale D. J. and Moore M. S., (1998), TA teaching effectiveness: the impact of training and teaching experience, J. High. Educ., 69(4), 440–466.
- Shehab S. S. and BouJaoude S., (2017), Analysis of the chemical representations in secondary Lebanese chemistry textbooks, Int. J. Sci. Math. Educ., 15(5), 797–816.
- Sheppard K., (2006), High school students’ understanding of titrations and related acid–base phenomena, Chem. Educ. Res. Pract., 7(1), 32–45.
- Sokolowski A., (2018), Teaching and learning representations in STEM, in Scientific inquiry in mathematics-theory and practice, Springer, Cham, pp. 21–28.
- Stieff M., Scopelitis S., Lira M. E. and Desutter D., (2016), Improving representational competence with concrete models, Sci. Educ., 100(2), 344–363.
- Stull A. T., Hegarty M., Dixon B. and Stieff M., (2012), Representational translation with concrete models in organic chemistry, Cogn. Instruct., 30(4), 404–434.
- Sundberg M. D., Armstrong J. E. and Wischusen E. W., (2005), A reappraisal of the status of introductory biology laboratory education in US colleges & universities, Am. Biol. Teach., 67(9), 525–530.
- Taber K. S., (2009), Learning at the symbolic level, in Gilbert J. and Treagust D. (ed.), Multiple representations in chemical education, Springer, Dordrecht, pp. 75–105.
- Teixeira-Dias J. J., de Jesus H. P., de Souza F. N. and Watts M., (2005), Teaching for quality learning in chemistry, Int. J. Sci. Educ., 27, 1123–1137.
- Towns M. H., Raker J. R., Becker N., Harle M. and Sutcliffe J., (2012), The biochemistry tetrahedron and the development of the taxonomy of biochemistry external representations (TOBER), Chem. Educ. Res. Pract., 13(3), 296–306.
- Treagust D. F., (2018), The importance of multiple representations for technology and learning science, in Shelley M. and Kiray A. (ed.), Education research and highlights in mathematics, science and technology, ISRES Publishing, pp. 215–223.
- Treagust D., Chittleborough G. and Mamiala T., (2003), The role of submicroscopic and symbolic representations in chemical explanations, Int. J. Sci. Educ., 25(11), 1353–1368.
- Wright L. K., Cardenas J. J., Liang P. and Newman D. L., (2017), Arrows in biology: lack of clarity and consistency points to confusion for learners, CBE Life Sci. Educ., 17(1), 1–13.
- Wu H. K. and Krajcik J. S., (2006), Inscriptional practices in two inquiry-based classrooms: a case study of seventh graders' use of data tables and graphs, J. Res. Sci. Teach., 43(1), 63–95.
- Wu H. K. and Puntambekar S., (2012), Pedagogical affordances of multiple external representations in scientific processes, J. Sci. Educ. Technol., 21(6), 754–767.
- Yaman F., (2018), Pre-service science teachers’ development and use of multiple levels of representation and written arguments in general chemistry laboratory courses, Res. Sci. Teach., DOI:10.1007/s11165-018-9781-0.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9rp00013e |
| This journal is © The Royal Society of Chemistry 2019 |