University chemistry students’ interpretations of multiple representations of the helium atom
Zahilyn D.
Roche Allred
 and
Stacey Lowery
Bretz
and
Stacey Lowery
Bretz
 *
*
Miami University, Department of Chemistry & Biochemistry, Oxford, OH 45056, USA. E-mail: bretzsl@miamioh.edu
First published on 28th January 2019
Abstract
Multiple chemistry education research studies at the secondary level have characterized students’ difficulties regarding a conceptual understanding of the quantum model of the atom. This research explores undergraduate students’ interpretations of multiple representations of the atom. Semi-structured interviews were conducted with first-year university chemistry students (n = 26) and second-semester physical chemistry students (n = 8) after they were taught and tested on the quantum model of the atom in their respective courses. During the interview, students were asked to interpret four representations of the atom (an electron cloud model, a probability representation, a boundary surface representation, and the Bohr model) and to rank each of the representations from most preferred to the least preferred. Nearly two-thirds of the students ranked the electron cloud and Bohr-model as their two most preferred representations. Students invoked ideas from classical mechanics to interpret the electron cloud model and used probabilistic language to describe the Bohr model of the atom.
Introduction and background
An understanding of the electronic structure of the atom is fundamental knowledge to be learned in a student's first year of university chemistry. However, research has shown that students do not readily adopt the quantum model of the atom, but rather prefer more concrete representations of the electronic structure of the atom (Griffiths and Preston, 1992; Harrison and Treagust, 1996, 2000; Taber, 2002; Cokelez and Dumon, 2005; McKagan et al., 2008; Adbo and Taber, 2009; Park and Light, 2009; Stefani and Tsaparlis, 2009; Ünlü, 2010; Akaygun, 2016; Papageorgiou et al., 2016; Zarkadis et al., 2017; Muniz et al., 2018). According to Novak's theory of meaningful learning (Novak, 1977, 1993; Novak and Gowin, 1984; Bretz, 2001) in order for students to learn about the electronic structure of the atom, they seek to make connections between what they already know and what they need to know. If students misinterpret representations used in textbooks based upon their prior knowledge, then their understanding of the electronic structure of the atom may be flawed. Many previous studies explored students’ ideas of the electronic structure of the atom by asking students to generate their own representations. The research reported herein investigated students’ interpretations of multiple representations of the atom typically used in textbooks. The research presented here is part of a larger study that investigated students’ to ideas about the electronic structure of the atom with regards to probability and energy quantization.Previous research
One of the anchoring concepts articulated by the American Chemical Society Examinations Institute is that “matter consists of atoms that have internal structures that dictate their chemical and physical behavior” (Holme and Murphy, 2015). The model of the atom as understood by students affects how they reason about other concepts in chemistry. For example, Adbo and Taber (2009) found that students’ mental models of the atom affected how they thought about phase changes. The students in this study who held a Bohr-model reported that while electrons move around the nucleus, the atoms themselves do not move and when heat is added to atoms, this causes the bonds within molecules to “fall apart.” Other studies have also explored how students’ preferences for concrete models such as the Bohr-model or the boundary surface model affect their thinking about molecules and chemical bonds and lead to alternative conceptions about the structure of the atom (Harrison and Treagust, 1996, 2000; Chittleborough and Treagust, 2007). Harrison and Treagust (1996) documented high school students’ preference for the “orbit” model often presented in popular television shows or media where electrons are shown in elliptic orbits rather than the quantum mechanics orbital model. Multiple studies conducted with different groups of students who were asked to depict the atom found that students did so by drawing Bohr-like models of the atom (Taber, 2002; Park and Light, 2009; Stefani and Tsaparlis, 2009; Ünlü, 2010; Akaygun, 2016; Muniz et al., 2018) or describing the atom as a sphere or a two-dimensional circle (Griffiths and Preston, 1992; Cokelez and Dumon, 2005). Students’ illustrations of the atom as a sphere or a circle have been suggested to be influenced by images from scanning tunneling microscopes (STM) because students do not realize these images are not actual photographs of atoms, but rather computer-generated images of decoded raw data (Harrison and Treagust, 1996; Budde et al., 2002b; Taber, 2002; Ünlü, 2010).More recent studies have examined how students’ depictions of the atom depends upon the task given. Papageorgiou et al. (2016) and Zarkadis et al. (2017) investigated which type of atomic representations students reason from when asked to engage in different types of tasks. In their study, students were given three tasks where they were asked (1) to describe in detail how they imagine the atom if they could observe it through a “powerful microscope,” (2) to represent atoms based on the motion of the electrons as particles, and (3) illustrate an atom where electrons were part of an electron cloud. Their results revealed that the nature of the task appeared to have a substantial effect on the type of representations that students created and that students could switch from one model to another depending on the task at hand. Zarkadis and colleagues (2017) also described inconsistencies between and within students’ illustrations of representations of atomic structure, similar to the findings of McKagan et al. (2008) where students appeared to mix characteristics from multiple models of the atom.
Previous research has not only investigated how students illustrate the atom, but also documented that students invoke quantum mechanics vocabulary and concepts to describe Bohr-like models. In a study conducted by Cervellati and Perugini (1981), students were asked to provide written descriptions of an orbital. Students’ definitions were divided into five categories, with approximately 30% of the students having defined the orbital as a trajectory or an energy level. These findings are consistent with other reports in the literature where students confused or defined orbitals as shells, subshells, paths, spheres or as a region in which there is a high probability for an electron to be found (Tsaparlis, 1997; Taber, 2002, 2005; Wright, 2003; Park and Light, 2009; Stefani and Tsaparlis, 2009). A central theme of all these studies is that students often fail to distinguish between concepts related to quantum mechanics and their pre-existing ideas about classical mechanics. According to Park and Light (2009), for students to gain a conceptual understanding of the electronic structure of the atom using quantum mechanics, students must first develop an understanding of both energy quantization and probability and how these ideas are connected to one another (Park and Light, 2009).
Most studies that have explored students’ ideas of the atom were carried out at the secondary level (Harrison and Treagust, 1996, 2000; Budde et al., 2002b; Cokelez and Dumon, 2005; Adbo and Taber, 2009; Akaygun, 2016; Papageorgiou et al., 2016; Zarkadis et al., 2017) or in physics (Budde et al., 2002a, 2002b; McKagan et al., 2008; Ünlü, 2010). There is limited research regarding first year university chemistry students’ (Park and Light, 2009) or physical chemistry students’ ideas about the electronic structure of the atom (Muniz et al., 2018). Furthermore, there is no research regarding how these ideas evolve from first year university chemistry to physical chemistry, despite the expectation for more nuanced disciplinary understandings among advanced undergraduate students (Holme and Murphy, 2015; Holme et al., 2018). In particular, there is no research on how university chemistry students in both the first and third year interpret multiple representations of the atom. The research described below investigated first year university chemistry, physical chemistry and biophysical chemistry students’ interpretations of multiple representations of the atom and the representations that best match the students’ mental models of electronic structure.
Methods
Research questions
The findings reported in this manuscript are one part of a research study that investigates students’ thinking about the electronic structure of the atom with regards to both probability and energy quantization. The specific research questions addressed and discussed herein are1. Which representations of the electronic structure of an atom best align with students’ ideas?
2. How do students interpret the features of multiple representations of the electronic structure of an atom?
Research design
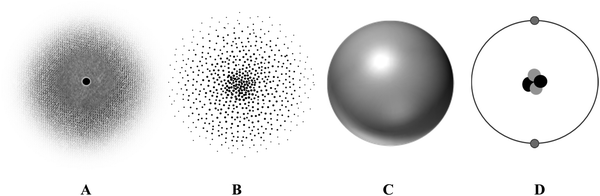
Semi-structured, think aloud (Bowen, 1994) interviews were conducted to elicit students’ ideas about the electronic structure of the atom. The interview-guide was designed using Novak's meaningful learning theory (Novak, 1977, 1993; Novak and Gowin, 1984; Bretz, 2001) and Johnstone's multiple levels of representations (Johnstone, 1991, 2006, 2010). Novak's meaningful learning theory states that for a student to form substantive connections among concepts, three conditions must be met. First, the individual student must possess relevant prior knowledge information about the concept being presented. Second, the concept to be learned must be meaningfully connected to its prior knowledge. Lastly, the student must decide to integrate the new material to previously learned, relevant information (Novak, 1977, 1993; Novak and Gowin, 1984; Bretz, 2001). Accordingly, the interview guide was designed to elicit students’ prior knowledge about the electronic structure of the atom before asking the students to discuss external representations of the atom that were provided in the interview. Johnstone's multiple levels of representations were used to guide decisions as to the type of representations used in the interview. According to Johnstone, chemistry is understood at three different levels: the symbolic level (equations or diagrams), the particulate level (invisible molecular level), and the macroscopic level (tangible and visible). As students gain knowledge in chemistry, they are expected to form connections among these three domains (Johnstone, 1991; Johnstone, 1997; Johnstone, 2010). However, students find that making connections among these domains is challenging (Johnstone, 1991). Therefore, the interview guide explored the extent to which students could articulate an understanding of not only the electronic structure of the atom in both the particulate domain and the symbolic domain, but also their understanding of the nature of the connections between these two domains.The semi-structured interviews consisted of four phases. Phase 1 was designed to elicit students’ prior knowledge about the electronic structure of the atom, along with their understandings of the concepts of probability and energy quantization. Students were asked to draw an atom as they pictured it in their mind. Students were also asked to draw any representations that they were familiar with that could be used to represent the energy of an atom. During Phase 2, students were shown an energy level diagram for the hydrogen atom (symbolic representation) and asked to describe the features of the diagram. The third phase of the interview focused on particulate representations and consisted of two tasks. In the first task of Phase 3, students were shown four representations of the atom (Fig. 1), and they were asked to describe the main features of each of these four representations. Students were then asked to rank the representations from one through four with “one” being the representation that they found to best match their mental model of the atom and “four” being the representation that least matched their mental model of the atom. As students ranked the representations, they were prompted to provide verbal descriptions of the features of the representations that led them to discriminate among them and to rank the representations in a particular order. In the second task of Phase 3, students were asked to draw the carbon atom as they picture it in their minds and to explain their drawing. Students were then provided with two different sets of representations for the 1s, 2s, and 2p orbitals and asked to choose which representation(s) they would use to depict the structure of the carbon atom and provide their reasoning. In Phase 4 of the interview, students were asked to consider once again each of the representations that they had created or been provided during the interview and, in doing so, to describe any connection (or lack thereof) between the energy level diagram of Phase 2 and the orbitals. This manuscript reports findings from the first task of Phase 3, where students were asked to rank atomic representations and to describe their salient features.
Representations
The research began with conducting a pilot study to examine the efficacy of both the interview guide questions and the representations in eliciting student thinking. During the pilot study, students were shown hand-drawn representations of the atom and were told that other chemistry students had sketched the drawings. From the pilot study, many students mentioned having difficulty interpreting the drawings because they were not sure if the representations matched what the students intended to draw or whether a limitation of the drawings was the students’ ability to physically depict their ideas of the atom. During the pilot study, many students decided either to modify the drawings or to draw their own depiction of the atom. Because the goal was to understand how students interpret the different features in the multiple representations of the atom, it was decided to forego using hand-drawn images of the atom and instead use computer-generated figures (created by the authors) similar to those commonly found in textbooks.Due to the variety of atomic representations chemistry students are exposed to, and the distinct historical models of the atom reported in the literature (Justi and Gilbert, 2000), four representations typically found in first year university chemistry textbooks were chosen (Fig. 1) in order to elicit students’ understandings. Fig. 1A was chosen because this representation depicts an electron cloud which is typically used to introduce students to the idea that the ‘surface’ of the atom is somewhat undefined. This representation also afforded an opportunity to investigate students’ interpretations of the shade gradient. Fig. 1A has been recommended as a representation suitable to introduce quantum mechanics rather than Fig. 1B (Budde et al., 2002a, 2002b; Wright, 2003). Fig. 1B was chosen because this representation, which is typically used to depict electron probability density, has been associated with learning difficulties for both secondary chemistry students and undergraduate physics students (Harrison and Treagust, 2000; Budde et al., 2002b). This representation afforded the opportunity to investigate students’ interpretations of the dots in the figure. Fig. 1C was chosen because it depicts the spherical shape of the atom and is commonly used in ball-and-stick or space-filling models of molecules (Griffiths and Preston, 1992; Harrison and Treagust, 1996; Wright, 2003; Cokelez and Dumon, 2005; Chittleborough and Treagust, 2007). This representation afforded the opportunity to investigate students’ interpretations of the boundary surface depicted in the figure. Lastly, Fig. 1D was chosen because this representation depicts the planetary orbit model, better known as the Bohr model. This model is commonly introduced during introductory science courses, and it is the model students tend to retain in their minds (Harrison and Treagust, 1996; Justi and Gilbert, 2000; Budde et al., 2002b; Wright, 2003; McKagan et al., 2008; Adbo and Taber, 2009; Park and Light, 2009). Although the Bohr model for the helium atom is not a scientifically accurate depiction of the electronic structure of the atom, we wanted to understand how students’ ideas of the Bohr model might influence the interpretations of Fig. 1A–C. All four representations were printed in black and white on a single sheet of paper, with ample space for students to draw or write on the representations if they so desired.
Sample
This research was conducted at a primarily undergraduate institution in the midwestern United States. Participants were recruited from three courses: first-semester, first-year university chemistry (FYC), second-semester physical chemistry (PC), and second-semester biophysical chemistry (BPC). Students were sampled from these courses because the quantum model of the atom is taught in all three courses. Students in the FYC are mostly first-year students majoring in chemistry, biochemistry, engineering or another science discipline. FYC students are introduced to the ideas of energy quantization and taught the historical development of how chemists came to understand the electronic structure of the atom.PC and BPC students are taught a more detailed explanation of the mathematical foundation for the quantum model. At the institution where this research was conducted, chemistry and biochemistry students have the option to enroll in either physical chemistry or biophysical chemistry, both of which are two-semester sequences offered in the Department of Chemistry & Biochemistry. In both sequences, students are taught the fundamentals of physical chemistry including kinetics, thermodynamics, quantum chemistry, and spectroscopy. However, students who choose to enroll in the BPC sequence are taught these concepts in the context of biomacromolecules.
Each course was taught by a different instructor. Students from all three courses were purposefully sampled from a list of students who volunteered to participate in the research based upon their grades in previous chemistry courses, major, college level, race/ethnicity and gender (see Table 1). The purposeful sampling ensured that the sample was representative of the students enrolled in the courses. A total of 34 students (20 of whom identified as female, 14 of whom identified as male) were interviewed, with twenty-six students who were enrolled in FYC and eight students who were enrolled in either PC or BPC. Thirteen students were either chemistry or biochemistry majors, and the remaining 21 students were majors in either engineering or another science discipline.
| Students | Gender | College level | Race/ethnicity | Major |
|---|---|---|---|---|
| FYC (n = 26) | Females n = 13 | Freshmen n = 22 | White n = 21 | Science discipline n = 21 |
| Males n = 13 | Sophomores n = 4 | Asian n = 2 | Chemistry n = 4 | |
| Hispanic n = 2 | Biochemistry n = 1 | |||
| Am. Indian/Alaska n = 1 | ||||
| PC/BPC (n = 8) | Females n = 7 | Juniors n = 3 | White n = 5 | Biochemistry n = 5 |
| Males n = 1 | Seniors n = 5 | Asian n = 2 | Chemistry n = 3 | |
| African Am. n = 1 | ||||
Data collection and analysis
The first author conducted the semi-structured interviews during fall 2016 and spring of 2017. All interviews were conducted in English. All students were interviewed after they had been taught the quantum model of the atom and within 1–2 weeks after being tested upon this content. The Institutional Review Board approved the research protocol. All students provided informed consent to participate in the study and to permit both audio and video recording of their interviews. The interviews typically lasted for 45 to 60 minutes. Each participant received a nominal compensation of a $20.00 gift card for their time. All students were assigned pseudonyms and are referred to by these pseudonyms in this manuscript.All the interviews were transcribed verbatim, and data was managed using NVivo 11 for Windows (QSR International Pty Ltd, 2015). The video was used to clarify transcripts when students used the words “this” or “that,” and to annotate the transcripts with gestures made by the students to augment their explanations. A Live-scribe smartpen was used to capture any drawings or marks that were made during the interview upon the representations themselves (Linenberger and Bretz, 2012). The interview transcripts and students’ drawings were analyzed using constant comparative analysis (Strauss and Corbin, 1998; Fram, 2013) to describe and classify students’ ideas about the electronic structure of the atom and their interpretations of the multiple representations of the atom. Each student's interpretations of the multiple representations were compared to one another and examined for both similarities and contradictions within one student. Students were also compared to each another for their interpretations of a representation. Lastly, all students’ interpretations were compared to findings in the literature. To ensure the trustworthiness of the data analysis, the authors met weekly to discuss and revise the codebook (Lincoln and Guba, 1985). In addition, the authors met periodically with other chemistry education researchers who were not involved in the data collection and analysis in order to ensure the confirmability and credibility of the findings (Bretz, 2008).
Results and discussion
The primary goal of Phase 3 of the semi-structured interviews was to gain insights into how students think about the electronic structure of the atom and how they interpret the features of multiple representations of the atom. Students were told that each of the four representations in Fig. 1 depicted a helium atom. The FYC and PC/BPC students’ rankings (Fig. 2) and interpretations of the representations (Table 2) are described and discussed below.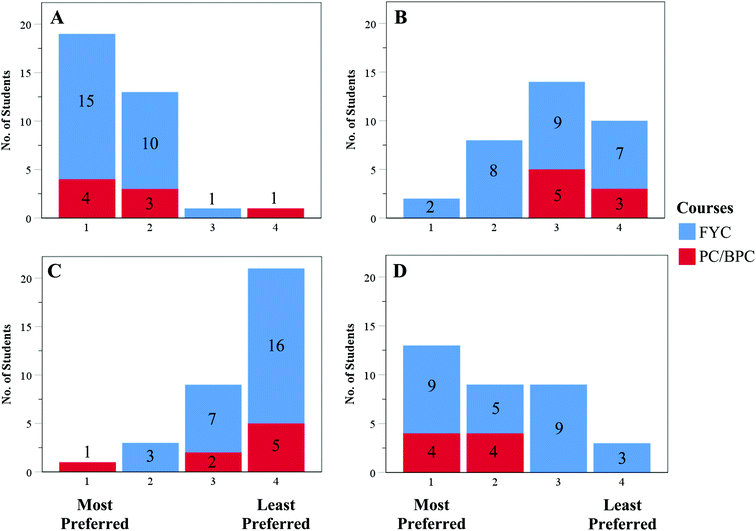 | ||
| Fig. 2 Results on students’ selection of atomic models as their mental model of electronic structure of the atom. | ||
| Representation | Students’ interpretation | Total | FYC | PC/BPC | |
|---|---|---|---|---|---|
| A | A.1 | Probability or likeness of finding electrons | 20 | 14 | 6 |
| A.2 | Location and/or movement of electrons | 5 | 4 | 1 | |
| A.3 | Number of electrons present in the atom | 4 | 4 | — | |
| A.4 | Connection (or lack of) to energy quantization | 3 | 2 | 1 | |
| A.5 | Presence of matter | 3 | 3 | — | |
| A.6 | Energy (or force of attraction) within the atom | 3 | 3 | — | |
| A.7 | Charges within the atom | 1 | — | 1 | |
| B | B.1 | Probability or likeliness of finding electrons | 16 | 13 | 3 |
| B.2 | Multiple particles | 7 | 5 | 2 | |
| B.3 | Location of electrons over time | 6 | 4 | 2 | |
| B.4 | Location of electrons | 1 | 1 | — | |
| B.5 | Mass of the nucleus | 1 | 1 | — | |
| B.6 | Forces with the atom | 1 | 1 | — | |
| C | C.1 | Size/shape of an atom | 11 | 7 | 4 |
| C.2 | Not representative of an atom | 9 | 6 | 3 | |
| C.3 | Location of electrons | 8 | 8 | — | |
| C.4 | Even probability/likelihood of finding electrons | 6 | 3 | 3 | |
| D | D.1 | Movement of electrons | 15 | 10 | 5 |
| D.2 | Orbital where electrons are (most likely to be) | 12 | 10 | 2 | |
| D.3 | Energy level (shell) within orbital | 6 | 3 | 3 | |
| D.4 | Distance between the electrons and nucleus | 2 | 2 | — | |
Students’ rankings of multiple representations of the atom
The most preferred representation (Fig. 2A) was Fig. 1A with 19 of 34 students (15 FYC, 4 PC/BPC) ranking this as first, i.e., the representation which most matched their mental model of the atom. Nearly two-thirds of these 19 students mentioned that one essential feature of the representation was the nucleus. Students offered a variety of reasons for citing the nucleus as an important feature that “needed” to be present in a representation of an atom, including that the nucleus provides a reference point for where the electrons are most likely to be in the atom, the nucleus holds the atom together, and the nucleus could be used to determine the identity of the element.Of the 19 students who ranked Fig. 1A as their most preferred representation, 9 (5 FYC, 4 PC/BPC) of them also ranked Fig. 1D as their second most preferred representation (Fig. 2D), once again mentioning the importance of the clearly depicted nucleus and also being able to easily count the number of electrons. These students preferred Fig. 1D over Fig. 1B and C because Fig. 1D was a “familiar” representation that they had seen in their textbooks, had been shown by a high school teacher, or would be the one they would draw or imagine when asked to think about the atom. These findings echo previously published results with secondary students, university physics students, and pre-service teachers who preferred to either draw or select a representation that resembles the Bohr model when they were asked to represent or select a representation of the atom (Harrison and Treagust, 1996, 2000; McKagan et al., 2008; Ünlü, 2010; Akaygun, 2016; Papageorgiou et al., 2016; Zarkadis et al., 2017; Muniz et al., 2018).
Students ranked Fig. 1B and C as their least preferred representations of the atom (Fig. 2B and C). Nearly one-third of our sample (7 FYC, 3 PC/BPC) ranked Fig. 1B as their least preferred representation. Although Fig. 1B is intended to be similar to Fig. 1A by depicting electron probability, students found this representation to be confusing and unfamiliar. Both FYC and PC/BPC students found it difficult to interpret the number of dots “clustered” towards the center of the figure and were troubled by the absence of a clearly depicted nucleus. To many students, this representation was problematic because they interpreted the dots to be electrons at the center of the atom, where they would have expected to see a nucleus and the likelihood of finding electrons would be zero. Although in many textbooks this representation is shown as a cross-section of an orbital where the electron probability is dependent on volume, the students in this study seemed to think of Fig. 1B as a two-dimensional representation of the atom and therefore “confusing.”
A total of 21 students (16 FYC, 5 PC) ranked Fig. 1C as their least preferred representation of the atom (Fig. 2C). Sixteen of the 21 students interpreted this representation as a solid sphere that provided no details about the nucleus or electrons in the atom, making it a poor representation of the atom and not one they would use to depict an atom. These results were unexpected given that the use of representations similar to Fig. 1C are ubiquitous in chemistry textbooks in ball-and-stick or space-filled models of molecules. These results diverge from findings in studies where students drew atoms as spheres (Griffiths and Preston, 1992; Harrison and Treagust, 1996; Cokelez and Dumon, 2005).
Both FYC and PC/BPC students in our sample preferred the representation of the atom in Fig. 1A which is aligned with the quantum model of the atom while the second most preferred representation was Fig. 1D which is aligned with the classical model of the atom. These findings echo those previously published by McKagan et al. (2008) with students enrolled in a modern physics course who had a strong preference for both quantum-like models and the Bohr model, often mixing features of the two distinct models. In our sample, the two most preferred representations showed “clear” depiction of the nucleus (Fig. 1A and D) and the electrons (Fig. 1D), suggesting that students prefer to think about the atom using concrete features that they can use to identify the “different components” of the atom, just like they would in an everyday life object (Harrison and Treagust, 1996, 2000; Sewell, 2002; Wright, 2003).
Most research regarding students’ ideas of the atom has been conducted with secondary students. The findings reported above suggest that even when students have been formally taught and tested upon the quantum model of the atom (including in multiple courses as is the case with the PC/BPC students), many still prefer to think of the atom using the concrete Bohr model. Thirteen students who selected Fig. 1A as their most preferred representation mentioned the “cloud of electrons.” However, half the students who mentioned the “electron cloud” or “cloud of electrons” had difficulty explaining what that phrase meant. The remaining students described the electron cloud as synonymous with an orbital where electrons were most likely to be found, where the electrons moved, or as energy levels. Similar challenges with differentiating among orbitals, electron probability, and energy levels have been reported with secondary students (Cervellati and Perugini, 1981; Taber, 2002; Stefani and Tsaparlis, 2009).
Although students were asked to rank the representations of the atom from the one that most matched their idea of the atom to the one that least matched, three students (1 FYC, 2 PC/BPC) chose to make two different sets of rankings. They initially ranked the representations to reflect how they thought about the atom, and then they re-ranked the representations from “most accurate” to “least accurate.” Each of these students thought it was important to draw a distinction between what they had been taught as “accurate” versus how they thought about the atom. The 2 PC/BPC students discussed the idea of electron probability and how Fig. 1A and B seemed to depict this idea, but they wanted to emphasize that these were not the models that they actively used when thinking about the atom.
Students’ interpretations of multiple representations of the atom
After students ranked the four representations for the helium atom, they were asked to interpret the main features of each representation. Salient features included the shading in Fig. 1A, the multiple dots in Fig. 1B, the uniform surface of Fig. 1C, and the ring in Fig. 1D. A summary of the students’ interpretations can be found in Table 2. Note that the number of interpretations does not sum to 34 (the number of students in the sample) because some students offered multiple interpretations, while other students stated that they were unsure what the representation depicted and therefore did not provide an interpretation.“This [dark shade] is kind of a representation of the electron cloud… the nucleus is there [pointing at the center] and then the darker region represents like the most likely area for an electron to be found … it's kind of like a gradient as it fades [pointing at the edge ofFig. 1A] and [shades] kind of like represents how is less and less likely for the electron to be found.” (Diego, FYC)
Five students interpreted the change in the shading to indicate how likely the electron was to be at a given “region” or “area,” whereas 9 students attributed this likelihood to the attraction between the electron and the nucleus.
An alternative interpretation of the shading in Fig. 1A was the idea that the change in color represented different energy levels. For example, Santos thought that the darker shade was the first energy level or 1s orbital while the lighter shade depicted the second energy level or the 2s orbital (Table 2, A.4):
“…that is like the probability… you can kind of see like a line here [gestures a circle]… so, you can kind of think of that as being one of the energy levels, and it is a lower energy level. It's closer to the nucleus here, and most of the time, it's [electron] going to be in that lower non-excited state, and that's why it's so dense there. It's kind of hard to see it on the paper, but it starts getting lighter because an electron can be quantized and jump into that umm…other energy level…that's why it starts getting a lighter shading.” (Santos, FYC)
Elena associated the shading with shells, indicating that the lighter shade meant “no chance” of finding electrons because the helium atom only has two electrons and “they will be closer to the nucleus because it will fill that like…shell first.” (Elena, FYC).
Although previous studies have reported that students use the terms ‘orbital,’ ‘energy levels’, and ‘shells’ interchangeably (Cervellati and Perugini, 1981; Taber, 2002; Stefani and Tsaparlis, 2009), students in this research study were explicitly asked to distinguish among these terms when they used them interchangeably. However, students who used these terms to interpret Fig. 1A could not explain what these terms meant nor how they differed. Furthermore, some (n = 3) students’ ideas about quantized energy levels heavily influenced the way they interpreted the representations as was the case for Agustina who thought the shading of Fig. 1A was an inaccurate way to depict an atom because
“…[I]f you just have random electrons all over the place, like not in quantized energy levels and not circling the nucleus as well, it just wouldn’t work. I can’t remember what experiment they did to prove this wrong…” (Agustina, BPC)
Agustina's rejection of this representation suggests that she has conceptualized the atom in terms of the Bohr model, even though she has been taught and tested on the quantum model and is just months from graduation. She invoked the concept of quantized energy which has been identified by Park and Light (2009) as a pivotal concept to be mastered before learning about the electronic structure of the atom. Here, however, Agustina's ideas about quantized energy interfere with representations of electron probability.
Three students interpreted the shading of Fig. 1A to represent mass within the atom (Table 2, A.5). Two students thought that the darker shading represented the mass of the nucleus in comparison to the small mass of the electrons. Felipe (FYC) explained that the nucleus contained most of the mass and that is why Fig. 1A was darkest at the center and lightest on the edge where the electrons were. One student thought that the shading represented other matter between the nucleus and the electrons. This has previously been reported in the literature for young children who think the structure of matter is continuous and that matter occupies all space (Anderson, 1990). This same idea could influence how students interpret representations of the atom. Likewise, Joaquin (FYC) mentioned that while he remembered having to use a representation similar to Fig. 1D in his course that he was unfamiliar with Fig. 1A. These results suggest that even when students are shown a quantum model representation, they might invoke ideas they hold about the Bohr model to generate their own interpretations of representations like Fig. 1A.
“… places that it [electron] could be. Or looking at like points …I don’t really like that we are kind of looking at like a cloud that's like a point, because a point it's just kind of like I mean…too specific, that like I mean that's like Heisenberg would not approve this representation [Figure B]. Like you can't know the exact location of the electrons.” (Santos, FYC)
Alternatively, 6 students thought of these dots as the location of electrons over time (Table 2, B.3):
“I assumed that they [dots] are the location, but I feel like that's more over like a separate period of time where maybe they use data or something and they like, kind of like put it in a diagram… like they would measure it every time [and] that's where it would be. But like, I guess that kind of shows, like it's denser towards the center too…so, that kind of shows probability, [Fig. 1A] is more like drawing out [Fig. 1B], I guess over a certain amount of time. But I’ve never seen [Fig. 1B] before. I never really thought about it before now.” (Luis, FYC)
Just like Luis, many students thought of this representation as the results of an experiment, even when they mentioned that they were familiar with the figure or thought of it as “inaccurate” for showing dots in the middle of the figure. Budde and colleagues have cautioned that if students did not understand that “a quantum mechanical measurement changes the state of the electron” and students thought of “classical measurements,” this would lead students to think that each dot represented the chronological position of an electron (Budde et al., 2002b). When presented with an unfamiliar representation like Fig. 1B, students like Luis can and do use their prior knowledge to interpret features of a representation they do not understand.
The dots in Fig. 1B were interpreted by 7 students as multiple particles (Table 2, B.2), specifically multiple helium atoms:
“Well I think, since it's the little dots, I would assume it's either umm…what composes the inside of an atom or it's just different like helium atoms that are coming together to form like ah…so it would just be like a bunch of monomers forming a polymer together. That's what I would think it would be.” (Joaquin, FYC)
Or as the electrons, neutrons, and protons that make up the atom. Six students interpreted this representation in terms of the composition of the atom but did not consider the internal structure of the atom, leading them to provide an erroneous interpretation of Fig. 1B.
“… [I]t's kind of just a ball. If you look at any of the other three, it's completely different while the others show somewhere where the electrons could possibly be rather than a fixed circle, and … [ Fig. 1A ] and [ Fig. 1D ] show the nucleus as well. Umm… whereas this one is kind of like all clumped together, there is not a distinction between anything… it's not showing where the electrons would be, like more likely to be or less likely or where the nucleus is or any of that.” (Miguel, FYC)
These 9 students did not think Fig. 1C depicted the helium atom because it did not show the composition of the atom. One student thought it depicted only the nucleus of the helium atom but that no electrons were shown. This student's idea echoed the thinking in Harrison and Treagust's (2000) case study of a high school student who thought of the “ball model” as the nucleus of that atom.
Six other students interpreted the surface of Fig. 1C as depicting a uniform probability of finding electrons in the atom (Table 2, C.4). When asked to interpret the figure, Elena said:
“I think it's like saying… there is an equal probability of finding the electrons anywhere and I don’t think that's really accurate.” (Elena, FYC)
Students with this idea thought that the uniform color and defined shape of the representation suggested that electrons have an equal probability of being anywhere within the atom. In addition, three of these six students mentioned that this representation inadequately depicted Heisenberg's uncertainty principle. Although the idea of probability has been proposed as a threshold concept for students to develop an accurate mental model of the atom (Park and Light, 2009), students like Elena who have learned about probability now face interference from that concept when interpreting this representation. The purpose of Fig. 1C is to indicate the overall spherical nature atoms. However, students find it difficult to separate what features they think an “accurate” representation of an atom must include from a representation intended to simplify some characteristics of the internal structure of the atom. These findings add to previous reports of students’ difficulties differentiating between models of the sub-microscopic level and actual atoms (Treagust et al., 2003; Chittleborough and Treagust, 2007).
Fifteen students said that the ring around the nucleus of the atom represented the movement of electrons (Table 2, D.1) with 11 students specifically referring to the ring as a path: “…the path electrons are following…” (Rafael, FYC). Only two students referred to the ring as the “orbit” of electrons. Interestingly, seven of these fifteen students said that the movement of electrons depicted in this figure was “true” for this “old-school” model, but that the electrons would be moving “randomly” or “can be just anywhere” (Makson, FYC) rather than rotating around the nucleus. Students described the electrons as moving randomly for a variety of reasons, including that electrons move very rapidly, the particle and wave duality of the electrons, and their understanding of the Heisenberg Uncertainty Principle:
“So, random…you can’t tell the speed and location at the exact same time… I think you can say unpredictable…you know it's [the electron's] relative location, but you don’t necessarily know how it's [the electron's] moving. So, it [the electron] could be like all over the place.” (Luis, FYC)
Misinterpreting the Heisenberg uncertainty principle leads students to think that because it is not possible to know both the location and the momentum of the electron simultaneously, the electron must be moving randomly. These findings indicate that even when students invoke ideas from the quantum model of the atom, they can still be influenced by their ideas of classical theories and experiences in the macroscopic world.
Twelve students said that the ring around the nucleus represented the orbital where electrons are located or are most likely to be (Table 2, D.2). This finding echoes previously reported results with secondary students who adopted quantum terms to label their prior knowledge about the Bohr model (Cervellati and Perugini, 1981; Tsaparlis, 1997; Taber, 2002).
Conclusions
While many reports of student thinking about the structure of the atom exist in the literature, most of those studies investigated the thinking of young children or secondary students. In this study, university students enrolled in first year chemistry, physical chemistry, or biophysical chemistry were interviewed. The study explored their understandings of the electronic structure of the atom through their rankings and interpretations of multiple representations of the helium atom.Many students had difficulties interpreting the main features of the four representations investigated and offered conflicting interpretations across the representations. Both FYC and PC/BPC students were challenged to make connections across the representations of the electron cloud, the probability model and the boundary surface model. Students struggled to recognize the purpose and explanatory power of different atomic models, e.g., recognizing the boundary surface model as a representation of the overall shape of the atom.
The analyses of this investigation revealed that students at all levels were torn between the electron cloud and the Bohr model when asked to indicate which model best matched their mental model of the atom. We conclude that the co-existence of these two contradicting models that both align with their mental model of the atom led students to conflate ideas between the two models. For example, some students used quantum terms to describe their understanding of the atom by referring to the orbit in a Bohr model as an orbital. Also, their classical ideas such as thinking of the electron as a particle also influenced these students’ interpretations and their ability to distinguish between the Bohr model and the quantum model.
Park and Light (2009) have argued that both energy quantization and probability are threshold concepts that must be mastered before students can develop appropriate mental models of the electronic structure of the atom. The research presented herein showed how students who have learned about these two concepts found it challenging to integrate these concepts when interpreting representations of atomic models. Their poorly organized and integrated ideas about the quantum model hindered their ability to explain the different atomic models, potentially impeding students’ growth and understandings in other chemistry concepts where the understanding of the atom might be critical. Our data suggest that mastery of threshold concepts alone is necessary, but not sufficient, for understanding the quantum model of the atom. Students need additional opportunities to work with integrating these threshold concepts with one another.
Limitations
There are limitations to the research reported here. First, different representations may have generated different rankings and interpretations from students. Second, given the small number of PC/BPC students who were interviewed, we were not able to detect any differences between the FYC students and the PC/BPC students. A study with a larger sample of PC/BPC students would be warranted. Third, although we reported the students’ rankings and interpretations of multiple representations, some students did invoke quantum concepts such as energy quantization and probability. Additional analyses of student thinking about these important concepts and the connection between them were conducted using both qualitative and quantitative data, but these findings are beyond the scope of this manuscript.Implications for teaching and research
These findings have implications for both the chemistry classroom and for chemistry education research. Previous research has suggested that students’ understanding of atomic structure could affect their conceptual understanding of phase changes (Griffiths and Preston, 1992; Adbo and Taber, 2009), electronic transition and atomic emission (Taber, 2002; Bretz and Murata Mayo, 2018), and chemical bonding and reactions (Griffiths and Preston, 1992; Sewell, 2002; McKagan et al., 2008). More research is needed to investigate how students thinking with multiple representations of the atom changes as students progress from first year university courses to physical chemistry and perhaps even beyond to graduate school. Even though the number of PC/BPC students in this sample was small, the findings from this study provide important insights into these students’ thinking as students who are just months from completing their undergraduate degrees.The research protocol for this study asked students to rank and interpret representations independent of any specific context in order to examine how students think with these representations so as to not predispose students to think that any particular interpretation was desired by the researchers. The group of students who insisted on ranking the representations twice – both as they thought about them and as they were taught by faculty to think about them –suggests that how students invoke and reason with models depends on the nature of the task. Researchers need to give careful thought to the nature of tasks designed to elicit student thinking during interviews. Some students expressed conflicting ideas about the purpose of specific representations. Although some research has been done on task-dependent use of atomic representations (Papageorgiou et al., 2016; Zarkadis et al., 2017) with secondary students, future research should investigate university chemistry students’ ideas as to the intent of different representations and which representations students prefer to use for a given task.
In the classroom, teachers should assess students’ prior knowledge about the atom before introducing them to any representations. Rather than emphasize only which representation of the atom is considered to be most scientifically accurate, teachers should create opportunities for students to discuss both the strengths and the limitations of representations, as well as their purpose. Even so, the findings of this study reveal that students cannot adequately integrate quantum ideas into existing prior knowledge. Students need opportunities to explore how energy quantization and probability are depicted in the representations in Fig. 1. After learning about the quantum model, students may hold personal models of the atom (e.g., Bohr) and offer quantum ideas only in response to assessments. Teachers need to examine their classroom assessments to determine to what extent they can detect and measure the challenges identified in this research. Chemistry education research studies are needed to design assessment tools that capable of measuring thinking with representations of energy quantization and probability that teachers can use in their classrooms.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the students who volunteered to participate in this study. This material is based upon work supported by the National Science Foundation under Grant No. 1432466. Any opinions, findings, and conclusions or recommendations expressed in this manuscript are those of the authors and do not necessarily reflect the views of the National Science Foundations.References
- Adbo K. and Taber K. S., (2009), Learners’ mental models of the particle nature of matter: a study of 16-year-old swedish science students, Int. J. Sci. Educ., 31(6), 757–786.
- Akaygun S., (2016), Is the oxygen atom static or dynamic? The effect of generating animations on students’ mental models of atomic structure, Chem. Educ. Res. Pract., 17(4), 788–807.
- Anderson B., (1990), Pupils’ conceptions of matter and its transformation (age 12–16), Stud. Sci. Educ., 18, 53–85.
- Bowen C. W., (1994), Think-aloud methods in chemistry education: understanding student thinking, J. Chem. Educ., 71(3), 184–190.
- Bretz S. L., (2001), Novak's theory of education: human constructivism and meaningful learning, J. Chem. Educ., 78(8), 1107.
- Bretz S. L., (2008), Qualitative Research Designs in Chemistry Education Research, in Bunce D. M. and Cole R. S. (ed.), Nuts and Bolts of Chemical Education Research., pp. 79–99.
- Bretz S. L. and Murata Mayo A. V., (2018), Development of the flame test concept inventory: measuring student thinking about atomic emission, J. Chem. Educ., 95(1), 17–27.
- Budde M., Nidder H., Scott P. and Leach J., (2002a), The Quantum atom model “Electronium”: a successful teaching tool, Phys. Educ., 37(3), 204–210.
- Budde M., Niedderer H., Scott P. and Leach J., (2002b), “Electronium”: a quantum atomic teaching model, Phys. Educ., 37(3), 197–203.
- Cervellati R. and Perugini D., (1981), The understanding of the atomic orbital concept by Italian high school students, J. Chem. Educ., 58(7), 568–569.
- Chittleborough G. and Treagust D. F., (2007), The modelling ability of non-major chemistry students and their understanding of the sub-microscopic level, Chem. Educ. Res. Pract., 8(3), 274–292.
- Cokelez A. and Dumon A., (2005), Atom and molecule: upper secondary school French students’ representations in long-term memory, Chem. Educ. Res. Pract., 6(3), 119–135.
- Fram S. M., (2013), The constant comparative analysis method outside of grounded theory, Qual. Rep., 18, 1–25.
- Griffiths A. K. and Preston K. R., (1992), Grade-12 students’ misconceptions relating to fundamental characteristics of atoms and molecules, J. Res. Sci. Teach., 29(6), 611–628.
- Harrison A. G. and Treagust D. F., (1996), Secondary students’ mental models of atoms and molecules: implications for teaching chemistry, Sci. Educ., 80(5), 509–534.
- Harrison A. G. and Treagust D. F., (2000), Learning about atoms, molecules, and chemical bonds: a case study of multiple-model use in Grade 11 chemistry, Sci. Educ., 84(3), 352–381.
- Holme T. A. and Murphy K. L., (2015), The ACS Exams Institute undergraduate chemistry anchoring concepts content map I: general chemistry, J. Chem. Educ., 89(6), 721–723.
- Holme T. A., Reed J. J., Raker J. R. and Murphy K. L., (2018), The ACS Exams Institute undergraduate chemistry anchoring concepts content map IV: physical chemistry, J. Chem. Educ., 95(2), 238–241.
- Johnstone A. H., (1991), Why is science different to learn? Things are seldom what they seem, J. Comput. Assist. Learn., 7, 75–83.
- Johnstone A. H., (1997), Chemistry teaching - science or alchemy? J. Chem. Educ., 74(3), 262–268.
- Johnstone A. H., (2006), Chemical education research in Glasgow in perspective, Chem. Educ. Res. Pract., 7(2), 49–63.
- Johnstone A. H., (2010), You can’t get there from here, J. Chem. Educ., 87(1), 22–29.
- Justi R. and Gilbert J., (2000), History and philosophy of science through models: some challenges in the case of “the atom.”, Int. J. Sci. Educ., 22(9), 993–1009.
- Lincoln Y. W. and Guba E. G., (1985), Naturalistic Inquiry, Newbury Park: Sage Publications.
- Linenberger K. J. and Bretz S. L., (2012), A novel Technology to investigate students’ understandings of enzyme representations, J. Coll. Sci. Teach., 42(1), 45–49.
- McKagan S. B., Perkins K. K. and Wieman C. E., (2008), Why we should teach the Bohr model and how to teach it effectively, Phys. Rev. Spec. Top. Educ. Res., 4, 010103.
- Muniz M. N., Crickmore C., Kirsch J. and Beck J. P., (2018), Upper-Division Chemistry Students’ Navigation and Use of Quantum Chemical Models, Chem. Educ. Res. Pract., 19, 767–782.
- Novak J. D., (1977), A Theory of Education, Ithaca, NY: Cornell Univeristy Press.
- Novak J. D., (1993), Human constructivism: a unification of psychological and epistemological phenomena in meaning making, Int. J. Pers. Constr. Psychol., 6, 167–193.
- Novak J. D. and Gowin D. B., (1984), Learning How to Learn, London: Cambridge University Press.
- NVivo Qualitative Data Analysis Software, (2015).
- Papageorgiou G., Markos A. and Zarkadis N., (2016), Students’ representations of the atomic structure-the effect of some individual differences in particular task contexts, Chem. Educ. Res. Pract., 17, 209–219.
- Park E. J. and Light G., (2009), Identifying atomic structure as a threshold concept: student mental models and troublesomeness, Int. J. Sci. Educ., 31(2), 233–258.
- Sewell A., (2002), Cells and atoms – are they related? Aust. Sci. Teach. J., 48(2), 26–30.
- Stefani C. and Tsaparlis G., (2009), Students’ levels of explanations, models, and misconceptions in basic quantum chemistry: a phenomenographic study, J. Res. Sci. Teach., 46(5), 520–536.
- Strauss A. and Corbin J., (1998), Basics of Qualitative Research: Techniques and Proceudres for Developing Grounded Theory, Thousand Oaks, CA: Sage Publications.
- Taber K. S., (2002), Conceptualizing quanta: illuminating the ground state of student understanding of atomic orbitals, Chem. Educ. Res. Pract. Eur., 3(2), 145–158.
- Taber K. S., (2005), Learning quanta: barriers to stimulating transitions in student understanding of orbital ideas, Sci. Educ., 89(1), 94–116.
- Treagust D. F., Chittleborough G. and Mamiala T., (2003), The role of submicroscopic and symbolic representations in chemical explanations, Int. J. Sci. Educ., 25(11), 1353–1368.
- Tsaparlis G., (1997), Atomic orbitals, molecular orbitals, and related concepts: conceptual difficulties among chemistry students, Res. Sci. Educ., 27(2), 271–287.
- Ünlü P., (2010), Pre-service physics teachers’ ideas on size, visibility and structure of the atom, Eur. J. Phys., 31, 881–892.
- Wright T., (2003), Images of atoms, Aust. Sci. Teach. J., 49(1), 18–24.
- Zarkadis N., Papageorgiou G. and Stamovlasis D., (2017), Studying the consistency between and within the student mental models of atomic structure, Chem. Educ. Res. Pract., 18, 893–902.
| This journal is © The Royal Society of Chemistry 2019 |