TMI (Too much information)! Effects of given information on organic chemistry students’ approaches to solving mechanism tasks
Victoria
DeCocq
and
Gautam
Bhattacharyya
 *
*
Department of Chemistry, Missouri State University, 901 South National Avenue, Springfield, MO 65897, USA. E-mail: gautamb@missouristate.edu
First published on 8th October 2018
Abstract
We report our qualitative study of twenty-four students enrolled in the second-semester of a second-year undergraduate (sophomore-level) organic chemistry course, Organic Two. We asked the research participants to propose the product and electron-pushing mechanism of elementary mechanistic steps in the absence and presence of the corresponding overall transformation. We also asked the students about their preferences of representational systems when working on tasks common to Organic Two to ascertain the extent to which an external representation, rather than a task, might evoke a problem-solving strategy. In addition to familiarity to instructional materials, the main reason for which the students preferred line-angle formulas for nearly all of the task types is that the representational system allowed them most readily extract relevant, or otherwise useful, information without distracting them. However, line-angle formulas did not seem to cue students to the three-dimensional attributes of molecules; only dash-and-wedge structures and Newman and chair conformers did so. For the electron-pushing tasks, the research participants’ reasoning processes included at least some chemical characteristics of the species involved in the transformation when they were not given the product of reaction. When provided with the overall transformation, however, the students changed their focus to getting to the product. Consequently, they replaced correct answers with incorrect ones when given the reaction products. These results raise the possibility that traditional mechanism tasks may mask students’ mechanistic reasoning ability.
Introduction
Using the electron-pushing formalism (EPF) to teach reactions has dominated instruction in organic chemistry since Morrison and Boyd (1959) introduced their textbook over half a century ago (Ferguson and Bodner, 2008; Bhattacharyya, 2014; Flynn and Ogilvie, 2015). One of the expected virtues of this mechanistic approach was that it would provide a logical method for teaching and learning reactions, thereby reducing the need for memorization (Straumanis and Ruder, 2009). Yet, rote memorization continues to be the predominant strategy for “learning” organic chemistry (Grove and Bretz, 2012), and pervades the manner in which students solve electron-pushing tasks (Bhattacharyya and Bodner, 2005; Grove et al., 2012a). Consider, for example, Bhattacharyya and Bodner's (2005) study of graduate students taking an advanced organic chemistry course. Instead of relying on various theoretical constructs to inform their problem solving, the research participants were almost single-mindedly guided by atomic and bonding changes between the structural representations of the reactant(s) and the product(s). Thus, their most frequent justification for the steps of their proposed mechanisms was, “It gets me to the product” (p. 1405).Comparing and contrasting the reactant and product structures – or, mapping – has been subsequently observed in several other studies and, as such, seems to be a general procedure used by students attempting to solve electron-pushing tasks (Ferguson and Bodner, 2008; Anderson, 2009; Grove et al., 2012a; Bhattacharyya, 2014; Galloway et al., 2017; Caspari et al., 2018). Mapping appears to further instantiate means-ends-analysis, which is a general problem-solving strategy characterized by Newell and Simon (1972). In means-end-analysis, problem solvers use the differences between initial and goal states of the problem to take steps that, in the least appear to, reduce the gap between those states. “It gets me to the product”, therefore, is a quintessential example of means-end-analysis. Contrast this approach to retrosynthetic analysis in which individuals exclusively work backwards from the final state, the target molecule.
Broadly generalizing in the context of electron-pushing tasks, students seem to apply means-ends analysis in one of two ways. In one set of approaches, which emphasizes the reactant(s) and the pathway forward, students use the differences between the reactants(s) and product(s) to identify canonical mechanisms, such as SN1 or SN2, or specific functional group transformations (Bhattacharyya, 2014). The other set of approaches, conversely, focuses more on the product and is backward-chaining (Galloway, et al., 2017; Caspari et al., 2018). Caspari et al. have proposed that the often attendant “backward-oriented reasoning” is teleological in nature. Talanquer (2007) described teleology as a commonly-used, domain-general heuristic for explaining causation as, proverbially, the ends justifying the means. Regardless of the direction in which the emphasis is placed, the prior research suggests that students’ electron-pushing strategies are dominated by thoughts of how to obtain the product. It is possible, therefore, that their preoccupation with “getting to the product” may impede the consideration and/or use of other strategies.
External representations in problem solving
The effects of external representations (ERs) on problem solving strategy and success have been previously noted in cognitive science and educational psychology (Cheng et al., 2001; McKendree et al., 2002). Zhang (1997), coined the term “representational determinism” to propose that, “…the format of a representation can determine what information can be perceived, what processes can be activated, and what structures can be discovered from the specific representation” (p. 213). By mediating individuals’ internal processes, ERs play dual roles of facilitating and constraining cognitive activity.Consider, for example, the following task from Bodner and Domin (2000) in which more than 200 students were asked to predict all of the major products of the free-radical bromination of methylcyclopentane (Fig. 1A). The authors reported that the vast majority of students predicted three isomeric monobromination products (Fig. 1B). These students overlooked a fourth product; ironically, the highest-yielding one. All of the students who provided the fourth product first converted the line-angle structure of methylcyclopentane into one in which all of the hydrogen atoms were explicitly shown. None of the students who supplied only three products made this transformation.
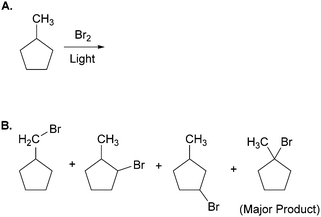 | ||
| Fig. 1 (A) Task given to more than 200 students by Bodner and Domin (2000). (B) The four primary products of the reaction. Note that the vast majority of students did not provide the fourth product. | ||
In another example – from Bhattacharyya and Bodner (2005) – the research participants were asked to explain the differential product distribution of the reaction shown in Fig. 2A. All of the students correctly accounted for the trans-dibromination products. However, only the students who recognized the possibility of the resonance delocalization shown in Fig. 2B were able to propose an acceptable electron-pushing mechanism for the cis-dibromination product of dihydropyran.
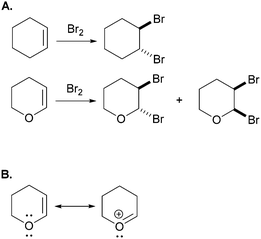 | ||
| Fig. 2 (A) Task from Bhattacharyya and Bodner (2005), in which students were asked to use electron-pushing to explain the observed difference in product distribution. (B) Resonance structures recognized by all the participants who successfully solved this task. | ||
The preceding two examples show how ERs can facilitate successful problem solving. Ferguson (2003), alternatively, demonstrated how ERs can limit students’ thought processes. In his study of decision-making during electron-pushing, Ferguson found that in one of the tasks students chose not to propose an intramolecular mechanistic pathway, which happened to be the correct one, because the two parts that would need to react looked too far away on the structure as drawn by the researcher. He termed this phenomenon as “Argument by ChemDraw” (p. 207). Consistent with Zhang's framework of representational determinism, these examples suggest that working with the “appropriate” ERs is a necessity for eventual success on problem-solving tasks in organic chemistry.
Present study
Previous findings on how students approach electron-pushing tasks suggest that their strategies are dominated by “getting to the product”, without substantial consideration of the kinetic or thermodynamic viability of their proposed mechanisms. Since research in cognition (Cheng et al., 2001; McKendree et al., 2002) indicates that the ERs in which tasks are presented affect students’ strategies, the goal of the present study was to determine whether students enrolled in the second-semester of a second-year undergraduate (sophomore-level) organic chemistry course, Organic Two, would change their strategy for solving an electron-pushing task in absence, or presence, of the overall product of the transformation.Methods
Theoretical framework
First proposed by Kenneth Craik (1943) as a way to describe peoples’ internal, cognitive representations, mental model theory is considered foundational to cognitive science (Nersessian, 2002). The concept of mental models has been widely applied by researchers from so many different disciplines that a consensus definition or set of characteristics has yet to emerge (Nersessian, 2002; Jones et al., 2011). We use, as such, Jones and colleagues’ concise generalization: “Mental models are cognitive representations of external reality” (Jones et al., 2011, p. 46). This description is purposefully broad so it can accommodate as many of the individual theories of mental models as possible. Among those theories, two of the more noteworthy were established by Gentner and Gentner (1983) and Johnson-Laird (1983). Though often conflated and confounded, these frameworks refer to significantly different constructs.Gentner and Gentner's (1983) version of mental models deals with knowledge structures in long-term memory; whereas Johnson-Laird's (1983) is more appropriate for cognitive processes associated with short-term memory. Since the present research is about problem-solving strategy and reasoning, we used Johnson-Laird's framework, which is based on three main assumptions (Johnson-Laird, 2010). First, people build mental models of what they believe to be true. This so-called “principle of truth” (p. 18244) can lead to logical fallacies since the “truth” is not absolute; it is based on the modelers’ beliefs and perceptions. Second, we construct unique mental models for each set of circumstances. Accordingly, our minds use a series of mental models to represent dynamic systems, much like cels are used to show the progression of motion in animations. Third, mental models are thought to be iconic in that, “the structure of a representation corresponds to the structure of what it represents” (p. 18244). Borrowed from the semiotician Charles Saunders Peirce, the key attribute of this final assumption is that correspondence between the representation and the represented is structural, implying that mental models should not be equated to mental images.
At first glance, Johnson-Laird's mental models framework may seem an inappropriate choice for research which is based on students’ interpretations of diagrammatic ERs since Holland et al. (1986) contend that knowledge in Johnson-Laird's theory is represented in working memory as propositional phrases. However, Nersessian (2002) suggests that there is no commitment to the format of the internal representation. Using terminology from Barsalou (1999), Nersessian explains that the internal representations are “amodal iconic symbols” (p. 142) in that the mental models do not possess perceptual characteristics of the “real systems”. Prior research on the learning organic chemistry suggests that students consider the ERs in electron-pushing diagrams as amodal iconic symbols. Ferguson's (2003) “Argument-by-ChemDraw”, for example, indicates that students treat the ERs as iconic symbols. Additionally, the fact that organic chemistry students do not attribute any physical reality to the substances and processes represented in electron-pushing diagrams as amodal (Bodner and Domin, 2000; Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008).
Participants and setting
Participants were recruited at a publically-funded, comprehensive university in the midwestern United States. After receiving approval from the Institutional Review Board (IRB), we solicited voluntary participation of Organic Two students during the eighth week of the semester, by which time the course instructor anticipated covering the content in the research tasks. In addition to explaining information associated with study participation we mentioned that each student would receive a $25 VISA gift card upon study completion.Twenty-four students, fourteen of whom were women, volunteered for the study. The study sample contained a larger proportion of women than the Organic Two course. Furthermore, at the time of the interviews, which were conducted between weeks ten and thirteen of the semester, none of the participants anticipated getting a grade lower than a C. As such, the sample anticipated performing better than the students in the course.
Interview protocol
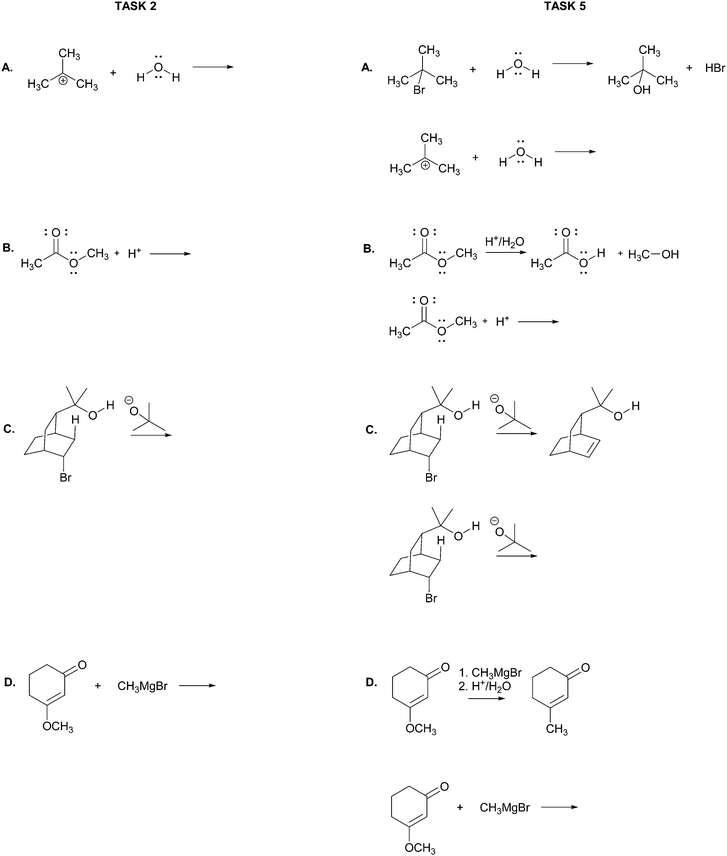
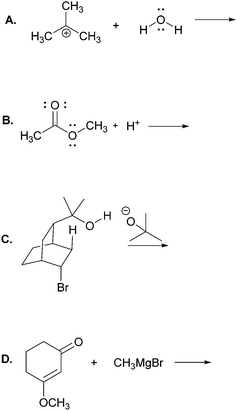
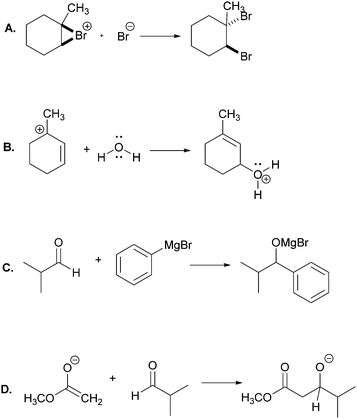
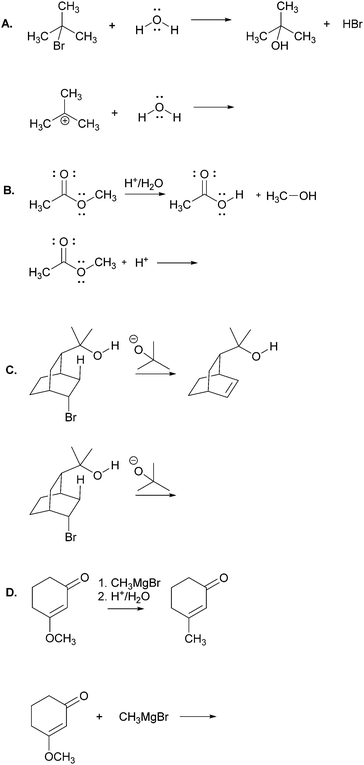
The main objective of the present research was to elicit students’ strategies for solving electron-pushing tasks whether given, or not, the product of the overall transformation. We would not be able to use, therefore, typical electron-pushing assessment items in which students are given an overall reaction – i.e. starting material(s), reagent(s), and product(s) – and asked to propose their mechanisms. We also precluded tasks previously used in Kraft et al. (2010), or Grove et al. (2012b), because the eventual goal of those tasks was to provide reaction products. As such, the research participants were implicitly required to turn their attention in that direction.In addition to excluding reaction products, another major consideration for this research was to develop tasks that would help maintain the focus of the study on problem-solving strategy without confounding it with electron-pushing ability or success. Based on these factors, we decided to give students the reactants of an elementary reaction step and ask them to use electron-pushing to provide the product of that step (Fig. 3). To avoid potential mental fixation on the product of the overall reaction, none of the elementary steps were meant to be the last of a multi-step mechanism. Thus, we first gave the research participants the reactants of an elementary mechanism step and asked them to provide the product of that step along with the electron-pushing. We later gave them the same task, but this time with the overall reaction, as shown in Fig. 3. We verified with the instructor of the course from which the participants were recruited that the content in these tasks had been covered in class by the time of the interviews.
Though the participants would certainly recognize the reappearance of the elementary mechanism steps when given to them the second time, we purposely did not give them different steps for the tasks with and without the overall reaction product because then it would leave the possibility that a change in strategy was caused by a change in task. However, to at least momentarily divert the students’ attention we gave them two other electron-pushing tasks, which are shown with the entire interview protocol in Appendix A, but are not further discussed in this manuscript. Solutions to Tasks 2 and 5 are shown in Appendix B.
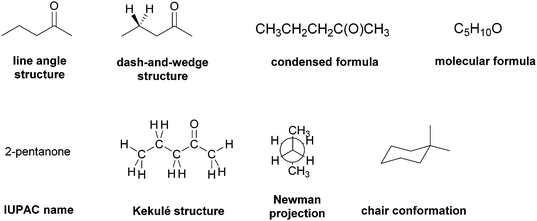
Readers will notice from Appendix A that prior to giving them the electron-pushing tasks we asked the research participants several questions about some of the commonly-used representational systems used to express structures in organic chemistry. We attempted to elicit the research participants’ mental models and the attendant expectations of these ERs as a way to ascertain the extent to which an ER, rather than a task, might evoke a problem-solving strategy. Thus, we chose eight representational systems – line-angle structures, dash-and-wedge structures, condensed formulas, molecular formulas, IUPAC names, Kekulé structures, Newman projections, and chair conformations – that were routinely and repeatedly used by the Organic Two professor in lectures and exams. Though we did not want to lead the students to think about these ERs in specific ways, we were concerned that if the interview prompts were too open-ended, they may not adequately elicit the participants’ mental models. Based on our prior experience (Strickland et al., 2010; DeFever et al., 2015), we took two steps to address these potential issues. First, we asked students about their potential use of the representational systems in the context of specific task types. These types had been previously identified by Stieff (2005) and Raker and Towns (2010) as common to sophomore-level organic chemistry courses and had been used by the instructor in course assessments. Second, we added an element of redundancy to the interview protocol, asking for the same type of information on multiple occasions.
Data collection and analysis
Using the research protocol described in the previous section, each participant was interviewed once between the tenth and thirteenth weeks of the semester. The interviews were audiotaped and videotaped and lasted between 35 to 45 minutes. To protect the participants’ anonymity, only their hands and drawings appeared on the video clips. For the questions involving students’ conceptions of the ERs, the research participants were given a sheet containing the eight representational systems and then verbally asked the respective questions by the interviewer. For the remaining tasks, the participants were given each task on its own sheet of paper on which the participants were asked to draw their responses and verbalize, to the greatest extent possible, their thoughts. To reduce stress associated with incorrectly completing the tasks, the interviewer reminded the participants that the researchers were not interested in their ability to correctly propose electron-pushing mechanisms.All of the audio recordings were transcribed verbatim. The videos were then used to annotate the transcripts. For example, in instances where participants pointed to structures using indefinite terms such as, “it” or “that”, the transcripts were accordingly modified to indicate the species identified by the participants. In accordance with the University's human subjects protocol, all of the students were given pseudonyms and their drawings were converted to ChemDraw files to protect their anonymity.
Though mental models are inherently an individual's construct, we used the group as the unit of analysis to examine the data of the students’ conceptions of the ERs. This approach, which we have used in our previous research (Bhattacharyya, 2006; Strickland et al., 2010), allowed us to look for commonalities across the sample instead of focusing on the differences. Accordingly, each item of this section of the interview protocol was analyzed separately, i.e. all of the participants’ responses were collected and then divided by preferred and not-preferred representational systems along with the spectrum of reasons for each category. Consider, for example, the students’ ER preferences when predicting the product of a reaction (Table 1). The numbers of respondents in each category are provided to give the reader a sense of its prevalence. Note, however, that a student may have had more than one choice which is why the totals exceed twenty-four (which is the total number of participants). Also, ERs that were chosen by three or fewer students are excluded.
| Predicting product(s) of reactions given the starting material(s) and reagent(s) | |||
|---|---|---|---|
| Representational system | Total number of students | Reason | |
| Preferred | Line-angle structure | 13 | Familiarity from course materials |
| Contains “relevant” information without too much “Irrelevant” | |||
| Dash-and-wedge | 11 | Familiarity from course materials | |
| Not-preferred | Dash-and-wedge | 5 | Distraction if task does not involve stereochemistry |
| IUPAC name | 9 | Need to recall more information because it adds an extra step | |
| Newman projection | 8 | Adds an extra step because need to convert to line-angle structures | |
| Chair conformer | 6 | Need to think in 3-D | |
Unlike the data on the ERs, the unit of analysis for the electron-pushing tasks was the individual because the objective was to determine whether the presence of the overall reaction affected the participants’ problem-solving reasoning. As such, the data were separated into the four different elementary steps and each participant's drawing and utterances without and with the overall reaction were compiled (an example of which is shown in Fig. 4). The twenty-four pairs of responses were further analyzed for changes in drawing and changes in reasoning.
 | ||
| Fig. 4 Patrice's responses responses to one of the electron-pushing tasks. Note that her written answer did not change, but her reasoning changed; becoming product-focused in the second case. | ||
Results and discussion
We support our claims in this section with direct quotes from the participants – all of whom have been given pseudonyms. Annotations to quotes are presented in square brackets and the letter, “I”, is used to indicate utterances made by the interviewer.Structural representations
There were three main factors that dictated students’ preferences for representational systems for each task type. Predictably, perhaps, one of them was familiarity from lectures and other course materials such as exams. Consider, for example, the following quote from Olivia explaining her choice for ERs when predicting reaction products:“The line formula. That is what we use; generally anyways. So that's the easiest.”
Like Olivia at least half the participants preferred to see and, ostensibly, use line-angle structures for reaction-based tasks, i.e. predicting products, supplying reagents, and/or proposing electron-pushing mechanisms. Landon, however, expressed a potential disadvantage of this sense of familiarity
“Line angle is most commonly used so it doesn’t signal anything for me.”
Unlike some of the representational systems (which are described below), line-angle structures did not cue students to any specific characteristic about the task.
The second factor that influenced the participants’ preferences was the perceived suitability of the representational systems for task types. Consider, for example, Hollis’ description of the information he derived from line-angle structures
“The line-angle to me is just the most simple form. It's easy to read and I can easily figure out hybridization and possible stereochemistry or hindrance and bond angles.”
In another interview, Devin elaborated that line-angle structures allowed him to notice,
“Heteroatoms, double and triple bonds, positive and negative charges.”
Similarly, Wendy explained why she would favor line-angle structures when asked to fill in reagents,
“Probably line structure because you can see it better and focus on the arrows and where it goes and what comes out. So if I have to fill in what's in the middle, I can figure it out. I can see it better.”
These quotes suggest that Hollis, Devin, and Wendy preferred to use line-angle structures when working on these tasks because the representational system allowed them to “see” what they felt was necessary.
Contrast these comments with those of Cynthia and Sophia about Kekule structures:
Cynthia: “The Kekule; I notice the hydrogens because it clutters it more.”
Sophia: “The Kekule is more obvious with the hydrogens but I don’t prefer it because it's busy.”
In the case of most tasks, the participants considered the explicit addition of the hydrogen atoms in Kekule structures distracting. There were, however, three task types for which students felt that Kekule structures would be advantageous. One of them was identifying acidic sites of compounds since all the hydrogen atoms are explicitly shown. Another was predicting a molecule's polarity, as explained by Bryson
“Uhm still leaning towards the same, the basic Kekule structure. It is nice being able to see; I relate the electronegativity towards seeing the letters. At this point I know an O; if I see an O that means electronegative.”
A third task type, for which about a quarter of the participants noted that they would prefer Kekule structures to line-angle ones, was for proposing electron-pushing mechanisms,
Landon: “Kekule, because when you do mechanisms a lot have to do with proton transfers so it helps to write out the hydrogens so you can do arrows easier. But I don’t usually use those because you usually don’t need that much information.”
Though he appreciated the potential benefits, like Cynthia and Sophia, Landon noted that Kekule structures can lead to information overload.
Similar to their limited preferences for Kekule structures, the participants indicated that they liked dash-and-wedge structures only when stereochemistry was a relevant aspect of the task. Consider, for example, the following quotes from Hayden and Madison:
Hayden: “There are times with stereochemistry I’d want dash-and-wedge but it can convolute noticing the functional groups or heteroatoms.”
Madison: “Usually if someone were to present it [pointing to a structure] to me in the dash-and-wedge that would tip me off to look for it [referring to stereochemistry].”
As Madison explained, dash-and-wedge structures cued participants to consider stereochemical aspects of tasks. According to Hayden, however, the representational system could otherwise distract him from other aspects of the structure.
In addition to the dash-and-wedge formalism only chair conformations of cyclohexane derivatives and Newman projections cued students to the three-dimensional characteristics of chemical structures. Consider, for example, Preston's comments about Newman projections:
“Also the Newman projection I could see being a little difficult [referring to using them to predict products of reactions] because sometimes it is hard to envision flat; how it would react with, how you would do like arrow-pushing and other things like that. …Just that like the Newman projection is kind of like you are looking at it straight on instead of flat like a line-angle structure.”
Like Preston, several other participants saw the “flat”-ness of line-angle structures as an advantage. This result is noteworthy because it implies that (one of) the most commonly-used representational systems in organic chemistry instruction does not cue three-dimensionality in the research participants’ minds. Furthermore, unless specifically cued, most students do not consider three-dimensionality relevant to most tasks in organic chemistry.
The third, and final, factor that affected students’ preferences for representational systems was the ease with which they could reason imagistically with it. Consider the following comments from Hollis and Olivia regarding their choices for predicting reaction products:
Hollis: “Probably between the line-angle and the dash-and-wedge because I visualize them better in my head.”
Olivia: And I never liked chair conformations because they flip and it is hard to visualize.”
Stieff (2011) has previously shown that students reason imagistically when asked to compare or translate representations of compounds. These quotes indicate that such reasoning may extend to several other task types.
In addition to expressing their preferences for representational systems they would want to use, the participants also spoke of types of ERs they would want to avoid. Chief among them were the IUPAC system of nomenclature, although several participants mentioned condensed, Newman, and chair structures as well. Bryson and Devin explained, for example, why they would not want tasks expressed in IUPAC names or condensed formulas
Bryson: “I don’t want to see the IUPAC name. Again, I just have to break it into what it actually looks like. I can do it but I kind of feel like it's a waste of time to demonstrate that.”
Devin: “I would be able to do it with the other ones, but the one I’m most comfortable with is line. Especially with the condensed and IUPAC I would have to convert it to a line angle structure anyways.”
As the quotes indicate, the main reason for almost all of the students was that getting a task in one of those systems would add an extra step to the problem-solving process. Penelope, however, offered a different reason for specifically avoiding IUPAC names
“IUPAC, because there is a lot more to memorize and if you want to show something simple you have to think more deeply about what it shows.”
Though expressed only by her, this comment is significant because we have seen a similar tendency to avoid nomenclature in our previous work (Bhattacharyya and Harris, 2018).
Electron-pushing tasks
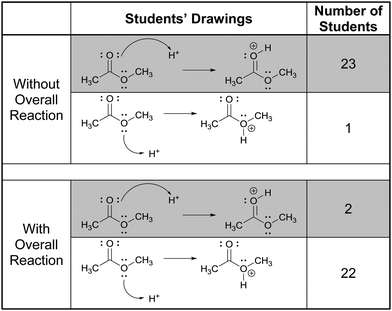
In this part of the interviews we asked the students to draw the product and accompanying electron-pushing of a single elementary step of a multi-step mechanism. The research participants were first given these tasks without any indication of the corresponding overall reaction (Fig. 3). After two more tasks, whose results are not discussed, the participants were again asked to draw the product and accompanying electron-pushing of the same four elementary steps but this time the overall reaction was provided. The students’ drawn responses and the frequencies of their answers to Tasks 2A and 5A are shown in Table 2. Note that Harper, who drew the answer to 2A that was different from all the others, drew a different answer to 5A. Her answer to 2A is reminiscent of what Grove et al. (2012a) described as the “cyclic approach” in which all of the EPF arrows for the entire mechanism are shown in a single step.| Correct answer(s) is/are shaded in grey.a The same student drew these two responses. |
|---|
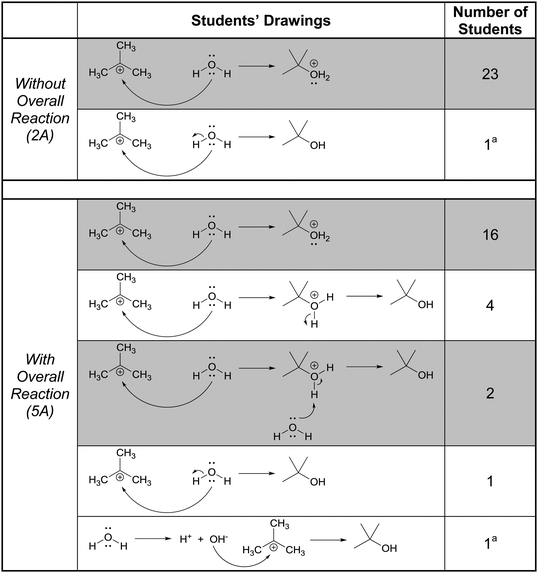
|
Rather than focus on the correctness of the students’ answers, however, the discussion here will concentrate on their reasoning. Consider, for example, Patrice's explanation for her drawing,
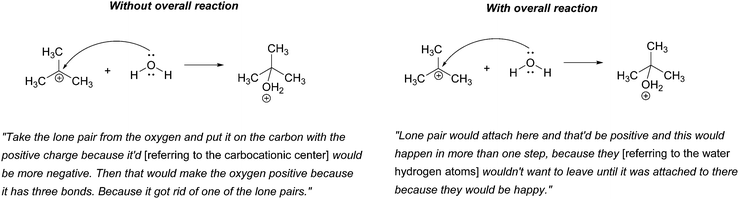
“Take the lone pair from the oxygen and put it on the carbon with the positive charge. Then that would make the oxygen positive because it has three bonds. Because it got rid of one of the lone pairs.”
In all, 21 of the participants recognized that the electron-rich region of one of the reactants – one of the non-bonding electron pairs on oxygen in this case – would attack the electron-poor region of the other – the carbocationic center. (The other three did not explain their answers.) Thus, their reasoning was forward-chaining since it was based on the reactive tendencies of the two reactants involved in the step. As such, it may be inferred that the students’ mental models of this task were reactant-based.
When explaining their drawn responses to 5A, however, 11 of the participants’ comments suggested that they switched their mental models to reflect a focus on the formation of the overall reaction product. Patrice, whose drawn response did not change, stated
“Lone pair would attach here and that’d be positive and this would happen in more than one step, because they [referring to the water hydrogen atoms] wouldn’t want to leave until it was attached to there because they would be happy.”
In this attempt Patrice used the subsequent deprotonation of the protonated alcohol to justify the attack of water to the carbocation; an example of one of the types of product-oriented, backward-chaining reasoning described by Caspari et al. (2018). Interestingly, six participants provided drawn answers that also showed the mechanism of the deprotonation step forming the overall reaction product, even though they were asked to show only the addition step.
Compared to those from Tasks 2A and 5A, the results from Tasks 2B and 5B, shown in Table 3, are far more dramatic. Without knowledge of the overall transformation, all but one of the participants proposed that protonation would occur at the carbonyl oxygen, which is the accepted answer for this step (Anslyn and Dougherty, 2006). Only about a third of those, however, recalled the correct reason for protonation at that site. Kalli, for example, noted
“It’ll be able to have resonance stabilization between it and the carbon meaning that it would be more stable with the charge by spreading out the charge between atoms in the molecule.”
Another third of the participants believed, as Hayden explained below, that the carbonyl oxygen would be protonated because it was more sterically available than the ester oxygen
“So I see a proton and it's going to protonate something that has electrons. And I see those two oxygens and between the two I guess I’m considering; because it; I’m thinking about sterics. This oxygen of the ester is sandwiched between two carbons so it's a little less accessible.”
The remaining students could not recall a specific reason for protonation at the carbonyl oxygen but, nonetheless, proposed it because it seemed familiar, as Madison explained in the following quote:
“I’m seeing the lone pairs on the oxygen with the double bond and I’m seeing the lone pairs on the oxygen that is single bonded so I know probably one of those oxygens with their lone pairs will go and attach to the proton that is free in solution. I can’t think of anything that would make it go to one; favor one oxygen over the other expect maybe that; well no because either way I add it it’ll put a positive charge on the oxygen. I guess what I’ve seen more often is that you protonate the oxygen of the double bond so I’m drawing an arrow from the lone pairs to the hydrogen.”
Provided with the overall reaction, however, nearly all of the participants reversed their earlier choice and predicted protonation at the ester oxygen. Almost all of the students who decided to make this change did so because they became preoccupied with making the –OCH3 into a good leaving group. Consider, for example, the following quote from Hollis:
“So in this one I’m deciding what happens in the entire reaction so I can figure out the steps. And this was a hydrogen that replaces what was a methyl group so the methyl group has to leave. And in order for that to happen this oxygen needs to bond to the hydrogen so that it’ll push out. That’ll give the oxygen a positive charge and therefore push out the methyl group.”
During her interview, Kalli explained the distracting effect the presence of the product in 5B had on her thought process in the following exchange:
I: “This is different from the answer you gave before. What made you change your mind?”
Kalli: “The way the products were drawn out.”
I: “Before when you didn’t have the overall reaction what were you looking at?”
Kalli: “The most stable thing that could be formed.”
I: “What are you focusing on now?”
Kalli: “How to get from that reactant to that product shown.”
This vignette suggests that the “traditional” presentation of electron-pushing tasks, i.e. in which starting material(s), reagent(s), and product(s) are given, may mask students’ ability to reason mechanistically.
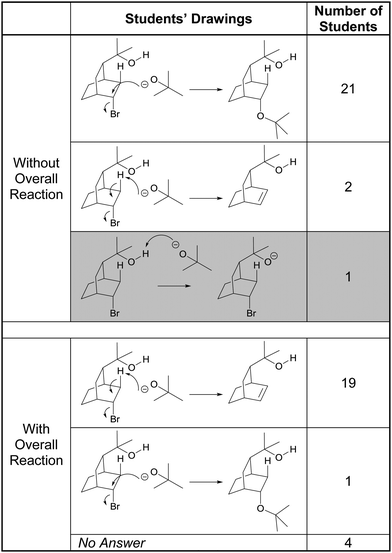
About a quarter of the participants seemed to have a visceral reaction to the bicyclic structures in Tasks 2C and 5C. Devin, for example, immediately stated, “Overwhelming from the chair conformation.”, before proceeding to analyze the structure. Without seeing the overall reaction, almost all of the students proposed the “SN2-like” displacement of bromide by tert-butoxide, as shown in Table 4. Consider the following comment by Preston:
“So this would be your nucleophile and I instantly recognize bromine as your leaving group and because of that I know that typically this is where the oxygen would attack which would cause the bromine to branch off. And then all of this up there and then B-r minus.”
We referred to the students’ proposed mechanisms as “SN2-like” because there is a retention of stereochemical configuration at the carbon center. All of those who drew the substitution product noticed the bromine atom and recognized bromide ion would be a good leaving group. Additionally, they identified tert-butoxide as a nucleophile but did not mention its basic properties. Even Emma, who proposed the deprotonation of the alcohol by the alkoxide – which is the accepted first step of this mechanism (Bhattacharyya and Bodner, 2005) – did so because of the representation of the starting material structure:
“I think maybe because there is an explicit hydrogen drawn out there as opposed to a normal alcohol; the hydrogens attached aren’t drawn out.”
The only participant who recognized the basic properties of the alkoxide was Campbell, who, along with Landon, proposed the elimination product. In fact, it was the basicity of tert-butoxide that cued Campbell to the elimination possibility, as she explained in the following quote:
“I recognized this structure [referring to the tert-butoxide] and everything we’ve done with this structure has been an elimination and this is a base, specifically bulky, and it's wanting a hydrogen so it’ll take away from there.”
Failure to recognize the dual reactivity of substances is a common oversight made by organic chemistry students (Bhattacharyya, 2014; Cruz-Ramírez de Arellano and Towns, 2014; Anzovino and Bretz, 2015).
When given the overall reaction, the formation of the carbon–carbon double bond along with the loss of bromine in the starting material led most of the participants to change their answers to show an E2 elimination mechanism. In the following exchange, Sophia explained the students’ propensity to favor substitution over elimination (in absence of the overall reaction):
Sophia: “Well bromine is just a really good leaving group and when it leaves it’ll leave behind a positive charge attracting a negative charge. But when I looked at the product I realized that couldn’t be it because these two are not reacting and combining it's creating an alkene but my initial thought was the negative charge to attack the slightly positive area.”
I: “Do you know why you initially thought substitution before elimination?”
Sophia: “I think substitution occurs more often so unless you expect an elimination reaction to take place I immediately think about substitution. I should have seen this is a bulky reagent and there is too much hindrance, if I would have looked at the product I would have seen the double bond but I didn’t look at the product until after I had drawn the substitution reaction.”
Sophia's comments suggest that her inclination towards the substitution product was due to seeing the corresponding reactions more often and is another example of the teleological, backwards reasoning recently described by Caspari et al. (2018).
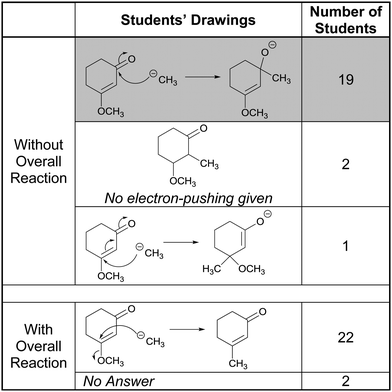
Lastly, the results from Tasks 2D and 5D are shown in Table 5. All of the participants who proposed attack of the Grignard reagent at the carbonyl carbon seemed to apply a form of rule-based reasoning. Consider, for example, the following comment from Wendy:
“Okay D. This is a Grignard reagent therefore direct and really the same thing as this [redrew reagent as CH3−]. It's a Grignard reagent which I think favors direct addition instead of conjugate and that's why it’d go to this first carbon instead of this one up here.”
Since they had an exam that included 1,2- versus 1,4-addition to α,β-unsaturated enones, all of the students recalled the “rule” for the reaction of Grignard reagents with those systems. Once again, the presence of the overall reaction steered the participants’ thoughts processing towards “getting to the product”, as Aidan described in the following exchange:
Aidan: “So I know this [referring to the Grignard reagent] is C-H-3-minus and it looks like based on the product that this takes place of that so I would say that the lone pair has to attack here [referring to the β-carbon of the enone system] and this [referring to –OCH3] has to leave as a leaving group.”
I: “Quick question when you first did these set of problems you didn’t have the overall reaction so what was the focus, or criteria used to determine the end product?”
Aidan: “I guess just looking at the reactant and what normally happens to them like carbonyls breaking apart and bromine groups leaving.”
I: “How about this time around when you had that [referring to the overall reaction]?”
Aidan: “Well this time around I can see what it is supposed to be going to. Then I can fill in the steps. I was trying to make the reactants look like products.”
Like Aidan, all of the participants changed their answers from the correct one to an implausible SN2-like displacement of methoxide at a sp2-carbon.
Conclusions
The primary goal of our study was to investigate whether organic chemistry students would change their mental models for a mechanism task when given the product of the overall reaction. The data offer compelling evidence that without the extra information, the research participants’ reasoning processes included at least some chemical characteristics of the species involved in the transformation. Though that reasoning may have been mistaken or included misconceptions, at least the students attempted to apply their knowledge of organic chemistry. When provided with the overall transformation, however, the students seemed to switch their reasoning strategy to a domain-general one namely, means-ends analysis. As such, the “take-home” message from this research is not about the nature of the students’ reasoning; rather it should be that there was a dramatic change in the reasoning. These results, therefore, raise the possibility that traditional mechanism tasks – i.e. those in which students are given the starting material(s), reagent(s), and product(s) – may not be the most effective at assessing mechanistic reasoning.The students’ choices of ERs also provide potential insight into their approaches to electron-pushing tasks. In addition to familiarity from instructional materials, the main reason for the students’ preference for line-angle formulas for nearly all of the task types is that the representational system allowed students to maximize germane cognitive load while minimizing extraneous cognitive load. Paas et al. (2003) explained that ERs support germane cognitive load when they allow individuals to readily extract relevant, or otherwise useful, information to complete tasks. Conversely, ERs which distract individuals from perceiving important information are said to induce extraneous cognitive load. As Devin's quote indicated, line-angle formulas allowed him to readily perceive heteroatoms, π-bonds, and charges; all of which are common loci of chemical reactivity. Furthermore, the participants found that in most cases the added hydrogen atoms in Kekulé structures were distracting. Thus, line-angle formulas seemed to best facilitate the formation of “productive” mental model, i.e. those that would lead to the successful completion of the tasks. The main concern, however, was that line-angle representational system did not seem to cue students to the three-dimensional attributes of molecules. In fact, only dash-and-wedge structures and Newman and chair conformers cued students to that possibility.
Implications for instruction
We see two main implications of our research. First, instructors should continue to encourage their students to write down intermediate solutions. Consider, for example, that the students could only solve Tasks 2A and 5A based solely on identifying electron-rich and electron-poor regions of chemical species. The participants who proposed acceptable solutions to the remaining tasks had to recognize the potential for resonance in only one of the protonation products (2B & 5B) or recall the rule for type of addition (2D & 5D). (2C and 5C had at least three potential options and was, therefore, the most complex of all of the electron-pushing tasks.) These additional considerations were necessary because the reactants for these three parts could have interacted at multiple positions. Since mental model theory suggests that individuals build separate mental models for each situation, perceiving all of the relevant features of the given structures and examining the possible reaction pathways would cause a working memory overload, especially in light of the facts our tasks covered only one elementary mechanistic step and half of them did not have the overall reaction (to further distract the participants)! It is for these reasons it is important to continue to emphasize to students the cognitive-load-reducing advantages of drawing partial or intermediate solutions. In addition to demonstrating this practice during lectures or tutorials, this behavior can be scaffolded on assessments as well. Bodé and Flynn (2016) reported, for example, that the students in their research on synthetic problem solving were required to show their “brainstorming” and “analysis” for marks during course exams. In the context of electron-pushing professors could allot part of the marks for identifying all of the nucleophilic, electrophilic, acidic, and basic sites of the reactants, showing the reactions between them and then evaluating which of those options would be most and/or least likely to happen. The value of helping students adopt this new problem-solving habit cannot be underestimated. In their study on combined spectral problem solving, Cartrette and Bodner (2010) observed that all of the more successful participants drew fragments of molecular structures as they attempted to solve the tasks.The second main implication of our research is that we need to reimagine the items for we use to assess mechanistic reasoning using the EPF. The vignettes with Aidan and Kalli indicate that the traditional way of presenting EPF tasks may mask students’ mechanistic reasoning ability. In fact, students’ means-ends-analysis approach to traditional mechanism tasks may even be a subconscious heuristic response. In addition to excluding the overall reaction, our data on the participants’ preferences of ERs suggests that using representational systems such as dash-and-wedge may also distract students from mechanistic reasoning.
Limitations
There are two main limitations to this study. First, there was only one type of task we used with the participants to examine the effects of the overall reaction product. As such, it is possible that the effects we saw may be confined to this instance. Additionally, we could have further detracted attention from products by changing the directions to, “Using the electron-pushing formalism, please indicate how these two substances would interact.” Alternatively, we could have given students the starting material(s) and reagent(s) and asked them, “How would this reaction start?” We leave these options to future research.The second limitation of our study is that, the students were “atypical” in that none of them got lower than a C in the course from which they were recruited. Furthermore, they seemed to spontaneously, engage in mechanistic reasoning practices, in that they drew the curved EPF arrows before drawing out the products. This is a significant departure from previous research in which graduate students “connected-the-dots” (Bhattacharyya and Bodner, 2005) or undergraduate students “decorated with arrows” (Grove et al., 2012a).
Conflicts of interest
There are no conflicts to declare.Appendix A: complete interview protocol
Task 1
• Which of the 8 representational systems described in the figure below would you find most useful when solving the following task types? Why?∘ Predicting the product(s) of a reaction given the starting material(s) and reagent(s)
∘ Choosing the appropriate reagents for a reaction given the starting material(s) and product(s)
∘ Proposing a reaction mechanism given the starting material(s), reagent(s), and product(s)
• Which of the representations are most useful for determining the following characteristics of a substance?
∘ Polarity
∘ Intermolecular forces
∘ Solubility in water
∘ Boiling point at ambient pressure
∘ Most acidic site(s)
∘ (Potentially) basic site(s)
∘ Nucleophilic site(s)
∘ Electrophilic site(s)
• Referring only to line-angle, dash-and-wedge, Kekule, and chair cyclohexane structures
∘ What type of information do you normally derive from each of these representations?
∘ What expectations, if any, do you have of task when you see it presented in one of these representations?
Task 2
Using curved arrows of the electron-pushing formalism please draw the mechanism and product of each of the following elementary steps.Task 3
For each elementary mechanistic step, please draw in the missing curved arrows.Task 4
Please choose the more likely of the two alternatives in each of the sets of mechanisms below. Note: the overall reaction is shown above each set of choices.Task 5
Using curved arrows of the electron-pushing formalism please draw the mechanism and product of each of the following elementary steps. Note: the corresponding overall reaction is shown above each elementary step.Appendix B: solutions to Tasks 2 & 5
Acknowledgements
We would like to thank all of the research participants for their time and efforts and Professor Matthew Siebert for allowing us to recruit students from his course. Financial support from the Department of Chemistry, College of Natural and Applied Sciences, and the Office of the Provost of Missouri State University is also gratefully acknowledged. We would also like to thank the referees for their helpful comments and suggestions.References
- Anderson, J., (2009), Learning the language of organic chemistry: how do students develop reaction mechanism problem-solving skills? Doctor of Philosophy, West Lafayette, Indiana: Purdue University.
- Anslyn, E. and Dougherty, D., (2006), Modern physical organic chemistry, Sausalito, CA: University Science Books.
- Anzovino M. E. and Bretz S. L., (2015), Organic chemistry students’ ideas about nucleophiles and electrophiles: The role of charges and mechanisms, Chem. Educ. Res. Pract., 16, 797–810.
- Barsalou, L., (1999), Perceptual symbol systems, J. Behav. Brain Sci., 22, 577–609.
- Bhattacharyya, G., (2006), Practitioner development in organic chemistry: How graduate students conceptualize organic acids, Chem. Educ. Res. Pract., 7, 240–247.
- Bhattacharyya, G., (2014), Trials and tribulations: Student approaches and difficulties with proposing mechanisms using the electron-pushing formalism, Chem. Educ. Res. Pract., 15, 594–609.
- Bhattacharyya G. and Bodner G. M., (2005), “It gets me to the product”: How students propose organic mechanisms, J. Chem. Educ., 82, 1402–1407.
- Bhattacharyya, G. and Harris, M. S., (2018), Compromised structures: Verbal descriptions of mechanism diagrams, J. Chem. Educ., 95, 366–375.
- Bodé, N. E. and Flynn, A. B., (2016), Strategies of successful synthesis solutions: Mapping, mechanisms, and more, J. Chem. Educ., 93, 593–604.
- Bodner, G. M. and Domin, D. S., (2000), Mental models: The role of representations in problem solving in chemistry, Univ. Chem. Educ., 4, 24–30.
- Cartrette, D. P. and Bodner, G. M., (2010), Non-mathematical problem solving in chemistry, J. Res. Sci. Teach., 47, 643–660.
- Caspari, I., Weinrich, M. L., Sevian, H. and Graulich, N., (2018), This mechanistic step is “productive”: Organic chemistry students’ backward-oriented reasoning, Chem. Educ. Res. Pract., 19, 42–59.
- Cheng, P. C., Lowe, R. K. and Scaife, M., (2001), Cognitive science approaches to understanding diagrammatic representations, Artif. Intell. Rev., 15, 79–94.
- Craik, K., (1943), The nature of explanation, Cambridge, England: Cambridge University Press.
- Cruz-Ramírez de Arellano, D. and Towns, M. H., (2014), Students’ understanding of alkyl halide reactions in undergraduate organic chemistry, Chem. Educ. Res. Pract., 15, 501–515.
- DeFever, R. S., Bruce, H., and Bhattacharyya, G., (2015), Mental rolodexing: Senior chemistry majors’ understanding of chemical and physical properties, J. Chem. Educ., 92, 415–426.
- Ferguson, R. L. (2003), Investigating chemistry students’ understanding of the arrow-pushing formalism, Doctor of Philosophy, West Lafayette, Indiana: Purdue University.
- Ferguson R. and Bodner G. M., (2008), Making sense of the arrow-pushing formalism among chemistry majors enrolled in organic chemistry, Chem. Educ. Res. Pract., 9, 102–113.
- Flynn A. B. and Ogilvie W. W., (2015), Mechanisms before reactions: A mechanistic approach to the organic chemistry curriculum based on patterns of electron flow, J. Chem. Educ., 92, 803–810.
- Galloway K. R., Stoyanovich C. and Flynn A. B., (2017), Students’ understanding of mechanistic language prior to learning organic reactions, Chem. Educ. Res. Pract., 18, 353–374.
- Gentner, D. and Gentner, D. R., (1983), Flowing waters or teeming crowds: Mental models of electricity, in Mental models, ed. D. Gentner and A. Stevens, Hillsdale, NJ: Lawrence Earlbaum Associates.
- Grove N. P. and Bretz S. L., (2012), A continuum of learning: From rote memorization to meaningful learning in organic chemistry, Chem. Educ. Res. Pract., 13, 201–208.
- Grove N. P., Cooper M. M. and Rush K. M., (2012a), Decorating with arrows: Toward the development of representational competence in organic chemistry, J. Chem. Educ., 89, 844–849.
- Grove N. P., Cooper M. M. and Cox E. L., (2012b), Does mechanistic thinking improve student success in organic chemistry? J. Chem. Educ., 89, 850–853.
- Holland, J., Holyoak, K., Nisbett, R. and Thagard, P., (1986), Induction: Processes of inference, learning, and discovery, Cambridge, MA: MIT Press.
- Johnson-Laird, P. N., (1983), Mental models: Towards a cognitive science of language, inference, and consciousness, Cambridge, MA: Harvard University Press.
- Johnson-Laird, P. N., (2010), Mental models and human reasoning, Proc. Nat. Acad. Sci. U. S. A., 107, 18243–18250.
- Jones, N. A., Ross, H., Lynam, T., Perez, P. and Leitch, A., (2011), Mental models: An interdisciplinary synthesis of theory and methods, Ecol. Soc., 16, 46.
- Kraft A., Strickland A. M. and Bhattacharyya G., (2010), Reasonable reasoning: Multi-variate problem-solving in organic chemistry, Chem. Educ. Res. Pract., 11, 281–292.
- McKendree, J., Small, C., Stenning, K. and Conlon, T., (2002), The role of representation in teaching and learning critical thinking, Educ. Rev., 54, 57–67.
- Morrison, R. T. and Boyd, R. N., (1959), Organic chemistry, Boston: Allyn and Bacon.
- Nersessian, N. J., (2002), The cognitive basis of model-based reasoning in science, in The cognitive basis of science, ed. P. Carruthers, S. Stich, and M. Siegal, New York: Cambridge University Press.
- Newell, A. and Simon, H. A., (1972), Human problem solving, Englewood Cliffs, NJ: Prentice-Hall.
- Paas, F., Renkl, A., and Sweller, J., (2003), Cognitive load theory and instructional design: Recent developments, Educ. Psychol., 38, 1–4.
- Raker, J. R. and Towns, M. H., (2010), Benchmarking problems used in second year level organic chemistry instruction, Chem. Educ. Res. Pract., 11, 25–32.
- Stieff, M., (2005), Dichotomous use of external representations in science learning, Paper presented at the annual meeting of the American Educational Research Association, Montreal, Canada.
- Stieff, M., (2011), When is a molecule three dimensional? A task-specific role for imagistic reasoning in advanced chemistry, Sci. Educ., 95, 310–336.
- Straumanis, A. R. and Ruder, S. M., (2009), New bouncing curved arrow technique for the depiction of organic mechanisms, J. Chem. Educ., 86, 1389–1391.
- Strickland, A. M., Kraft, A., and Bhattacharyya, G., (2010), What happens when representations fail to represent? Graduate students’ mental models of organic chemistry diagrams, Chem. Educ. Res. Pract., 11, 293–301.
- Talanquer, V., (2007), Explanations and teleology in chemistry education, Int. J. Sci. Educ., 29, 853–870.
- Zhang, J., (1997), The nature of representations in problem solving, Cogn. Sci., 21, 179–217.
| This journal is © The Royal Society of Chemistry 2019 |