A phenomenographic study of 10th grade students’ understanding of electrolytes
Shanshan
Lu
 a,
Hualin
Bi
a,
Hualin
Bi
 *a and
Xiufeng
Liu
*a and
Xiufeng
Liu
 b
b
aCollege of Chemistry, Engineering and Materials Science, Shandong Normal University, Jinan, Shandong, China. E-mail: bihualin@sdnu.edu.cn
bLearning and Instruction Graduate School of Education, State University of New York at Buffalo, Buffalo, NY, USA
First published on 4th October 2018
Abstract
Students have various conceptions of electrolytes in learning chemistry. The aim of this study is to identify 10th grade students’ understanding of the electrolyte concept by using a phenomenographic method. Eight students, whose abilities were at different levels, were selected and interviewed. The findings show that four distinctive categories of students’ conceptions of electrolytes are demonstrated, and a hierarchy in terms of the logical progression among them can be developed. Finally, teaching implications are given.
Introduction
An electrolyte has a property of conductivity in solutions. Learning the concept of an electrolyte can benefit the understanding of chemistry disciplinary core ideas of the structure and properties of matter. The concept of an electrolyte is typically learned in the context of aqueous solutions; specifically, students should know the characteristics of solutions of ionic compounds in water and the electrical neutrality condition in an electrolyte solution (College Board, 2014, pp. 19–38).Some studies on diagnosing students’ misconception of an electrolyte have shown that students have difficulties in understanding the conductivity of the electrolyte solution (Çalik, 2005; Devetak et al., 2009a, 2009b). A common difficulty is in recognizing what kind of particles in the electrolyte solution make the electric current. For instance, Çalik (2005) studied 8th grade students’ understanding of the conductivity of the solution and found that students believed that all solutions could conduct electricity. Devetak et al. (2009a, 2009b) studied 408 secondary school students’ microscopic understanding about electrolytes and found that 46% of students had an incomplete understanding of the concept of electrolyte dissociation. Even though the concept of electrical conductivity is directly related to microscopic particles of separated ions for electrolytes and molecules for nonelectrolytes, students believed that particles of an ionic compound are molecules in aqueous solutions. The same finding was found in Adadan and Savasci (2012)’s study. They assessed 756 students about the understanding of electrical conductivity in solutions at the 11th grade and found that many students encountered problems with identifying ionic and molecular substances and their dissociation in water.
The conductivity of an electrolyte solution is an important foundation for the understanding of electrochemistry. Some studies about students’ understanding of electrochemistry have shown another general difficulty that students are unclear about how the electric current occurs (Garnett and Treagust, 1992a, 1992b; Sanger and Greenbowe, 1997; Schmidt et al., 2007; Rahayu et al., 2011). Garnett and Treagust surveyed 12th grade students by using a semi-structured interview protocol after they had completed an electrochemistry course. The results showed that students had conceptual difficulties in learning electric circuits and the electrolytic cell. Students in the sample confused the nature of electric current in metallic conductors with that in electrolyte solutions. Sanger and Greenbowe (1997) replicated Garnett and Treagust's study by interviewing introductory college chemistry students after electrochemistry instruction. Students thought that electrons caused electric current in an electrolyte solution and moved through a solution by being attracted from one ion to another. Schmidt et al. (2007) investigated grade 11–13 students by using multiple-choice electrochemistry test items, identifying some students’ misconceptions, such as “electric current produces ions” and “electrons migrate through the solution from one electrode to the other”. Rahayu et al. (2011) investigated 244 Indonesian and 189 Japanese public senior high-school students and their understanding of the movement of ions in electrolyte solutions, and found that students from both countries had the same difficulties and misconceptions at the sub-microscopic level.
Students’ learning of the concept of electrolyte shows variations. Also, although the concept in the textbook taught by teachers is hopefully in accordance with the standpoint of modern science, the conception is considered as only one of several reasonable ways of understanding the phenomenon. Students’ conceptions can be considered as alternative ways of understanding the phenomenon.
Bussey et al. (2013) reported a fact that two students who were sitting in the same class and access the same materials could come to understand a particular chemistry concept differently, which constantly baffled the teachers. If teachers only focus on the difficulty in students’ learning, without attending to students’ variations in the understanding, they could fail their teaching goal, which has been demonstrated by some studies. For example, Supasorn (2015) implemented small-scale experiments in conjunction with a model kit to improve 12th grade students’ conceptual understanding of the mechanism of conductivity of electrolyte solutions. The findings showed that students’ conceptual understandings in respect of macro and symbolic levels were improved, but new misconceptions about the number of charged ions appeared at the sub-microscopic level. Another study was conducted by Rogers et al. (2000), in which they designed a teaching model to correct students’ misconceptions about current flow, and the results showed that the model did not address misconception related to electrolytes. Şen et al. (2015) implemented a teaching method called POGIL (Process Oriented Guided Inquiry Learning) in an 11th grade class. In light of the findings, he concluded that using computer animations or teaching pedagogies to enhance students’ full understanding of electric current was still a challenge. Schmidt et al. (2007) measured grade 11–13 students in upper secondary schools and identified the nature of conductivity of electrolyte solutions to be an inevitable problem in learning electrochemistry at an introductory chemistry level. Researchers agreed that students attempt to make sense of the conductivity of an electrolyte solution with the previous knowledge they have already developed (Garnett and Treagust, 1992a, 1992b; Schmidt et al., 2007). Marton and Pang (2006) argue that an affordance for learning is to create a pattern of variation and invariance that the student must experience to learn. Therefore, knowing the variations in students’ understanding of the concept of electrolyte or in the experiences of conductivity of solutions may benefit teachers in designing effective classroom teaching.
The concept of electrolyte is taught at different grades in different countries. In China, the concept of electrolyte is taught at 10th grade, which is the first year of high school. Students have had a year of general chemistry course at 9th grade and have learned about aqueous solutions. The 10th grade students have experience about the conductivity of metals. They have learned that the conductivity of metals is due to the electrons in the wires in the physics course. Their experience formed in daily life about conductivity phenomena and prior knowledge learned in other disciplinary courses can affect their understanding of conductivity of electrolyte solutions. The content of electrolytes in the 10th grade chemistry course includes electrolytes and non-electrolytes, electrolyte ionization in aqueous solution, conductivity of solutions, and ionic reactions.
The purpose of this study is to identify 10th grade students’ variations in their conceptions of electrolytes. Phenomenography is both a theory and a method to deal with students’ experience in learning, which uses descriptive categories to represent students’ different degrees of understanding of the whole phenomenon. The main question in this study is: what are the descriptive categories of 10th grade students’ conceptions of electrolytes?
Methodological framework
Phenomenography is an approach developed by Ference Marton (1981) and his colleagues, in which “phenomenon” refers to significant aspects of the reality or world, and “graphy” means describing, analyzing, and understanding people's experiences of various aspects of the world. For this study, the conductivity of a solution is considered as one phenomenon, and students’ conceptions of electrolytes are considered as different ways of experiencing the phenomenon. The aim of a phenomenographic study is to identify the qualitatively different ways in which students experience and understand a particular phenomenon (Marton and Booth, 1997).In the perspective of phenomenography, learning is viewed as changes in the learners’ conceptions relating to a phenomenon (Marton and Pang, 2006). That is, qualitatively different conceptions correspond to different levels of complexity derived from different ways of experiencing a phenomenon. Variations in conceptions are due to the different aspects of the phenomenon that learners are able to discern. By creating variations, phenomenographic research leads to a hierarchical set of increasingly more complex categories of conceptions. The differences in conceptions are not only between different individuals but also in different contexts within a person.
There are four underlying assumptions in phenomenographic research: (a) individuals conceptualize in a few identifiable qualitatively different ways when they reflect upon a phenomenon, (b) differences in conceptions may exist between individuals on the same phenomenon or within an individual in different contexts, (c) variations in students’ conceptions can be described by a few categories, and (d) categories of students’ conceptions form a hierarchical structure or “outcome space” (Marton and Booth, 1997; Liu and Ebenezer, 2002).
Methods
Participants
After obtaining permission from the school administration and the teacher for conducting our interview, the researcher (first author) explained the purpose of the research to the students and invited them to participate in the interview. Students were assured that their responses to interview questions will be kept confidential and will not be shared with their teachers. Students were also told that their performance in the interview would not affect their course grade. A purposeful sample was chosen to probe students’ conceptions of electrolytes. That is, among those who volunteered, eight students were chosen for the interview and signed consent forms. These students were at four different achievement levels, with one male and one female at each level. Students’ labels and characteristics are listed in Table 1.We interviewed these students face-to-face to probe their conceptions of the electrolyte concept after they finished electrolyte instruction. Research has shown that preconceptions are resistant to change (Rogers et al., 2000; Duit andTreagust, 2003; Vosniadou, 2009; Şen et al., 2015; Supasorn, 2015), thus students’ conceptions after instruction would reflect both students’ preconception and learned conceptions as the result of instruction, resulting in maximal variation in conceptions. We acknowledge that interviewing students only after instruction would not reveal changes in students’ conceptions due to instruction.
Interview design
A structured interview was designed to elicit students’ conceptions of electrolytes. A set of questions and tasks were carefully designed by following the protocol of “interview about instances” and “prediction interview”, which were used by Mintzes et al. (2005). As to the interview about instances, students are typically asked to recognize some examples of a concept and then explain their answers. As to the prediction interview, students are required to anticipate an outcome of a situation and explain or justify the prediction (Mintzes et al., 2005, pp. 74–77). These questions or tasks are designed to probe students’ understanding of three aspects of electrolyte solutions: the electrolyte, the conductivity of an electrolyte solution and its change, and particles and interactions in solutions.Questions are shown in Fig. 1. Q1, Q2, and Q3 pertain to electrolytes. Using these questions, we asked students to list examples of electrolytes, explain why he/she thought it is an electrolyte, and define electrolyte in their own words.
Q4, Q6, and Q7 are designed to probe the conductivity of an electrolyte solution and its change. Specifically, we asked students in Q4 to predict whether the electrolyte that he/she answered in Q1 could be conductive or not, then explain the prediction he/she had just made. We asked students in Q6 to predict the change of conductivity when adding another electrolyte into the existing solution, while in Q7 to predict the change of conductivity when adding a non-electrolyte into the existing solution. Q5, Q6 and Q7 were also designed to elicit students’ understanding of the particles and interactions in the solution. In light of our focus on the ions and molecules, not the ions’ reaction, the electrolyte referred to in Q6 won’t react with the existing electrolyte.
Procedures
Students were interviewed by one of the authors (labeled Researcher in the following excerpt) who also designed the structured interview protocol. The interviews were finished in two days after the electrolyte instruction. Each student's interview lasted 10–20 minutes. In order to elicit students’ understanding with maximal variation, before conducting the interview, the interviewer spent some time to develop a degree of comfort with the interviewee by talking informally about learning in the school.During the interview, the interviewer carefully listened to how students responded to the questions and focused on the insight about students’ understanding. Only after the intent and meaning of the student's response was clearly understood could the interviewer ask a new question to the student. Follow-up questions focused on students’ interpretations of the meaning of words they had just said, rather than an evaluation of students’ responses.
Data analysis
All interviews were audiotaped and transcribed verbatim into one Microsoft Word document and printed out before analysis. The interviews were coded in several steps by following Åkerlind's (2005) stages of phenomenographic research, including considering variations in each transcript, corroborating on variations, managing the data in ways of variation, and constructing the structure.Firstly, the first author read interview transcripts repeatedly to underline and identify meaningful words or sentences. Those meaningful words and sentences formed initial codes, then these codes were organized as interview topics for each participant (i.e., electrolytes, the conductivity of electrolyte solution and its change, and particles and interactions in solutions). The second author examined the transcripts with the developed codes to validate the initial codes. The percentage of these initial codes agreed by the second author was 95%.
The initial codes were then compared and analyzed for managing the data in ways of variation. Another new label was used to represent several related codes, which had a similar meaning of the concept. The above two steps were reiterated until the more obvious boundary variations were found in students’ understanding, and all the initial codes could be included. In other words, this analysis stage involved a summarized interpretation of the data, which attempted to organize the variation or patterns and their broader meanings. Next, a sentence or a proposition for each variation was created and labeled a “descriptive category”, followed by giving the most characteristic example for each descriptive category.
Finally, we analyzed a series of categories and descriptions and ordered the categories into a hierarchy based on the complexity degree to a logical progression.
Results
In light of the assumptions of phenomenographic research described in the Methodological Framework, categories of students’ conceptions of electrolytes were generated from students’ responses to interview questions. Different from the findings of students’ alternative conceptions research that focus on the types and prevalence of students’ misconceptions, the categories of student conceptions presented below reflect student conceptions that are both consistent with and different from scientific conceptions; they together demonstrate variation and hierarchy among student conceptions. Table 2 presents the categories of student conceptions.| Category A | Examples: |
| An electrolyte is a matter that can conduct electricity in solution. | • An electrolyte could conduct electric current in solution. |
| • An electrolyte is a compound. | |
| • The more electrolytes there are in a solution, the stronger the conductivity gets. | |
| Category B | Examples: |
| An electrolyte is a compound that produces ions in water to conduct electricity. | • Conducting electricity is due to ions in a solution. |
| • An electrolyte could produce ions in water. | |
| • The water molecule has no function in the ionization. | |
| Category C | Examples: |
| An electrolyte is a compound made of a cation and an anion and ionizes in water by interacting with water molecules to conduct electricity. | • Ions were produced by interaction between the electrolyte and water. |
| • The strength of conductivity relates to the amount of ions. | |
| • Unknown particles are in the non-electrolyte solution. | |
| Category D | Examples: |
| An electrolyte is a compound made of ions with electrical charges and can conduct electricity when it ionizes in the water. | • Ions are particles with positive or negative electrical charges. |
| • Free ions can conduct electricity when voltage is given. |
Descriptive categories
Researcher: please give an example of an electrolyte.
DF: ethanol, oh no, although ethanol is a compound, it is not an electrolyte. Eh, NaOH.
Researcher: why do you think NaOH is an electrolyte and ethanol is not an electrolyte?
DF: eh … (1 to 2 seconds pause), the NaOH solution can conduct electric current, but ethanol can’t conduct electric current.
The other five students (AM, BM, BF, CF, and DM) gave a compound solution as an example of an electrolyte. Taking CF's interview as an example:
Researcher: please give an example of an electrolyte.
CF: NaCl solution.
Researcher: why do you think NaCl solution is an electrolyte?
CF: eh…, NaCl solution can conduct electric current and NaCl is a compound.
Researcher: can you define electrolyte in your own words?
CF: electrolyte is a compound that can produce current in water.
With regard to descriptive category A, another understanding of electrolyte is that the more electrolytes there are in a solution, the stronger the conductivity gets. All the students gave the same answer to Q6, stating that a solution would become stronger after adding another electrolyte. Taking AM's interview as an example:
Researcher: after adding KOH solid into the NaCl solution, how does the conductive strength of the solution change? Becomes stronger, weaker, or no change?
AM: becomes stronger.
Researcher: why do you think it will become stronger?
AM: because the KOH is also an electrolyte, the concentration of the electrolyte will increase when it dissolves in water. Therefore, the effect of conductivity will become stronger.
Researcher: what about adding a sugar cube into the NaCl solution, how do you think the conductive strength of the solution will change?
AM: it becomes weaker.
Researcher: why do you think so?
AM: because sugar is a non-electrolyte, it won’t conduct electric current when it dissolves in water. However, eh … it will prevent the conductivity of the solution.
Researcher: after adding a KOH solid into the NaCl solution, how does the conductive strength of the solution change? Becomes stronger, weaker, or no change?
AF: will the KOH react with the NaCl?
Researcher: can you predict whether they react or not by using what you have learned?
AF: let me see … they won’t react. Therefore, the conductive strength will increase.
Researcher: why do you think so?
AF: because new ions are added to the existing solution (here is the NaCl solution), and they don’t react, that is no neutralization.
Researcher: what kind of “new ions” you just said? How were the new ions produced?
AF: oh, they are K + and OH − , they combined together and formed the KOH molecules. Water molecules separate the KOH molecules to release K + and OH − .
Although student AF mentioned that water molecules had an effect on electrolyte ionization, she didn’t show an understanding about the function of water molecules in the ionization. The following is the continuation of student AF's interview:
Researcher: could you give me more description of how water molecules separated the KOH molecules?
AF: as I said, K + and OH − are combined together. The water molecules separated them, changing molecules into ions in the solution.
When the eight students were asked to answer what functions water molecules had when the electrolyte dissolved in the water in response to Q5, half of the students (BM, CM, DM, and DF) responded that water molecules had no function. Taking BM's interview as an example:
BM: when KOH dissolves in water, it will produce K + .
Researcher: how did KOH produce ions?
BM: when it dissolved in the water, it becomes ions.
Researcher: what is the function of the water molecules?
BM: I think water molecules have no function.
Researcher: please give an example of an electrolyte.
CM: sodium chloride (NaCl).
Researcher: why do you think it is an electrolyte?
CM: I feel that … it has ions. When it is dissolved, it will be active.
Researcher: what do you mean by “active”?
CM: that is, I feel the sodium chloride is made of two kinds of small balls, they separate into two parts, and they are free.
Researcher: can you define electrolyte in your own words?
CM: an electrolyte is matter that can separate into ions, and then produce electric current as learned in the physics course.
Researcher: what did you learn about the electric current in the physics course?
CM: I forgot it. I guess it has a cation and an anion: one is positive and the other is negative. They will have the transfer effect.
Researcher: what do you mean by “transfer effect”?
CM: that is the conductive transfer. Specifically, the electricity brings ions back and forth.
Moreover, the students showed different understanding of the function of the water molecules in this category. Such as, students of AM and CF thought electrolyte ionization was due to water molecules’ movement; student BF responded that water molecules went into the inner space of the electrolyte and separate it into ions. Three students’ responses are shown as follows.
AM: when KOH dissolved in water, because of water molecules’ irregular movement all the time, they can separate KOH molecules into K + and OH − .
CF: eh… because water molecules automatically move all the time, so, after adding NaCl solid into the water, the movement of water molecules makes the NaCl move together, and then NaCl becomes loose and produces Na + and Cl − .
BF: Due to the molecule diffusion, water molecules are inside of, no, they enter the interior of the KOH, eh… then water molecules come out. The KOH is broken, with the ions produced.
Researcher: please give an example of an electrolyte.
AF: One? NaCl.
Researcher: why do you think it is an electrolyte?
AF: if NaCl dissolves in water, the NaCl molecules will be separated by water into ions. These ions can conduct electric current due to their electrical charges.
Researcher: can you define electrolyte in your own words?
AF: an electrolyte is a compound that can conduct electric current in the solution or in the molten state.
Researcher: why do you think the NaCl solution can conduct electric current?
AF: because NaCl produces Na + and Cl − in solution. Both Na + and Cl − have an electric charge and they can conduct electric current in water, which is different from electrons.
Hierarchy of categories of descriptions
The above four qualitative categories of description can be ordered in a hierarchy in terms of the logical progression among them. As illustrated in Fig. 2, students’ understanding of electrolytes is expanding from category A to category D. Category A is subsumed by category B, category B is subsumed by category C, and category D subsumes all categories of understanding about the phenomenon of conductivity of electrolyte solutions. Thus, categories A–D represent different degrees of understanding of the whole. This hierarchy does not mean that all categories are completely correct or wrong; it only shows qualitative differences in learning outcomes that are to be found when students learn the concept of electrolyte.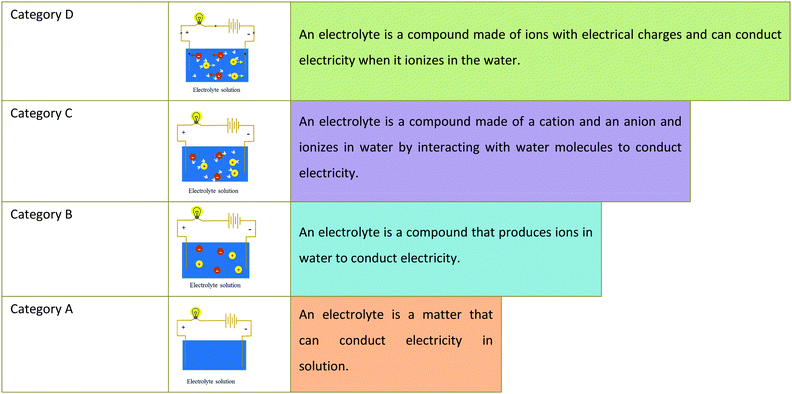 | ||
| Fig. 2 The relationship among descriptive categories of electrolytes. Note: the figure is drawn by the authors, and the authours have the copyright of this figure. | ||
Specifically, for category A, students can discern electrolytes with the property of conducting electricity. Students knew the difference in properties between electrolytes and non-electrolytes. In this study, none of the students thought that a non-electrolyte could conduct electricity. In their responses to Q7, six of the eight students thought a non-electrolyte wouldn’t change the strength of the existing electrolyte solution. Two of the eight students thought that a non-electrolyte would weaken the strength of electrolyte's conductivity.
As for category B, students can discern ions in the electrolyte solution with a partial understanding of electrolyte ionization. Students knew the particles that made up the electrolyte and the electrolyte solution.
As for category C, students can recognize the structure of the electrolyte, which is composed of ions and interactions between the particles. Students knew the structure of matter and the interaction between the particles.
As for category D, students knew more about the characteristics of ions than in category C. Students thought that ions have an electrical charge, and the nature of conductivity of ions is different from the nature of conductivity of electrons.
The phenomenographic method focuses on variations, and the aim of this method is to reveal those variations by capturing qualitatively distinct categories. Based on this hierarchy of categories, possible pathways of students experiencing learning about electrolytes could be anticipated across a group or within an individual at different times or in different contexts (Marton and Booth, 1997, p. 124). Fig. 3 presents the possible pathways of conceptual progression.
Discussion
Four distinct categories of descriptions created for 10th grade students demonstrate variations in students’ learning the electrolyte concept. Using phenomenography, more insight about students’ understandings of electrolytes was found compared to previous studies. As found in a previous study, students had difficulty in understanding the conductivity of an electrolyte solution; they were unclear about the kinds of particles in electrolyte solutions (Çalik, 2005). In the present study, we found that students believed that the particles made of ionic compounds are molecules. For example, in category B, as AF responded, “K+ and OH− combined together and formed the KOH molecule.” “Water molecules separate the KOH molecules to release K+ and OH−.” Thus, student AF thought that “KOH molecules” exist in the KOH solution before they are ionized. This result is similar to what Devetak et al. (2009a, 2009b) and Adadan and Savasci's (2012) found, where students thought that there were molecules in some ionic compounds’ solution, such as NaCl. In this study, we also found students’ other difficulties in understanding the electrolyte concept, such as: they didn’t know the characteristics of the ions in the solution, they didn’t know how ions were produced in water by using the interaction between ions and water molecules, they didn’t know why ions could form electric current, and they didn’t know what kind of particles existed in the nonelectrolyte solution, etc.Understanding of electrolytes is an important factor to get a clear understanding of electrochemistry. Studies found that higher-grade students, even introductory-college-chemistry students, were unclear about the mechanism of how electric current occurred in the learning of electric circuit and electrolytic cells (Garnett and Treagust, 1992a, 1992b). In this study, we found students had some particular ideas that would affect their understanding of electrochemistry. One is that although students know that ions are produced in electrolyte solutions and can conduct electricity, which is similar to Rahayu et al.'s (2011) result, they still have difficulties in understanding how these ions make electric current, as one student said, “the electric current brings ions back and forth”. Another is that students are unclear about the characteristics of ions. Sanger and Greenbowe's (1997) study found that students thought electrons made the electric current in an electrolyte solution, and Schmidtet al.'s study found that students confused the nature of conductivity of a solution and of a metal (2007). As to why students confused the conductivity of ions and electrons, we argue that they lack a deep understanding of the characteristics of ions, such as ions have electrical charges, ions are free moving in the solution, the relationship of ions and electrons from a sub-micro perspective, and ions would move regularly upon adding voltage.
The conductivity of a solution is an important property of an electrolyte. In categories A and B, students mainly focus on the macro traits of the matter or properties. However, in categories C and D, we found that students predict matter's properties by their structure. A proper knowledge of structure would help students understand the properties of the electrolyte.
Conclusions and implications
There are four distinctive descriptive categories of electrolytes for 10th grade students; they are: an electrolyte is a compound conducting electricity; an electrolyte is a compound producing ions in water; an electrolyte is matter made of ions and ionizes in water by interacting with water molecules; and an electrolyte is a compound made of ions with electrical charges and can conduct electric current when it ionizes in water. Furthermore, a hierarchical structure is constructed among the categories. The hierarchical structure can inform teachers to plan and implement instruction to support students to develop an in-depth conceptual understanding of electrolytes because the hierarchy among the categories suggests possible pathways for students to change conceptions. The hierarchy may also inform research on developing learning progressions on electrolytes in particular and on electrochemistry in general.To improve students’ deeper understanding of electrolytes, prior research has suggested using models and computer animations at the sub-microscopic level. In this study, we found that students had difficulties in understanding the conductivity of electrolyte solutions. The four descriptive categories and the hierarchical structure created in this study will benefit the design of models and computer animations. For example, in Rogers's (2000) study, a teaching model was designed to correct the misconception in electrochemistry. Even though the model led to a significant improvement in students’ understanding of what was occurring at the microscopic level in an electrochemical cell and addressed one main misconception of students about “electrons moving in the solution”, other misconceptions were not addressed. Another was “a negative electrode implying that the electrode is negatively charged”. This misconception can be interpreted in the perspective of categories C and D, wherein students lack the understanding of substructures of atoms and ions. Future teaching pedagogy using models may focus on the following aspects: the mechanism of conductivity of an electrolyte solution, the characteristics of ions, the function of water molecules in ionization, the particles present in a nonelectrolyte solution, and how the ions form electric current.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank all research participants in this study for providing their time and responses. The authors also thank the instructors for their support.References
- Åkerlind G. S., (2005), Variation and commonality in phenomenographic research methods, High. Educ. Res. Dev., 24(4), 321–334.
- Adadan E. and Savasci F., (2012), An Analysis of 16–17-Year-Old Students' Understanding of Solution Chemistry Concepts Using a Two-Tier Diagnostic Instrument, Int. J. Sci. Educ., 34(4), 513–544.
- Bussey T. J., Orgill M. K. and Crippen K. J., (2013), Variation theory: a theory of learning and a useful theoretical framework for chemical education research, Chem. Educ. Res. Pract., 14(1), 9–22.
- Çalik M., (2005), A Cross-Age Study of Different Perspectives in Solution Chemistry from Junior to Senior High School, Int. J. Sci. Math. Educ., 3(4), 671–696.
- College Board, (2014), AP Chemistry: Course and Exam Description, http://media.collegeboard.com/digitalServices/pdf/ap/ap-chemistry-course-and-exam-description.pdf, accessed Feb. 2018.
- Devetak I., Vogrinc J. and Glažar S., (2009a), Assessing 16-year-old Students’ Understanding of Aqueous Solution at Submicroscopic Level, Res. Sci. Educ., 39(2), 157–179.
- Devetak I., Lorber E. D., Jurisevic M. and Glazar S. A., (2009b), Comparing Slovenian year 8 and year 9 elementary school pupils' knowledge of electrolyte chemistry and their intrinsic motivation, Chem. Educ. Res. Pract., 10(4), 281–290.
- Duit R. and Treagust D. F., (2003), Conceptual change: a powerful framework for improving science teaching and learning, Int. J. Sci. Educ., 25(6), 671–688.
- Garnett P. J. and Treagust D. F., (1992a), Conceptual Difficulties Experienced by Senior High School Students of Electrochemistry: Electric Circuits and Oxidation-Reduction Equations, J. Res. Sci. Teach., 29(2), 121–142.
- Garnett P. J. and Treagust D. F., (1992b), Conceptual Difficulties Experienced by Senior High School Students of Electrochemistry: Electrochemical (Galvanic) and Electrolytic Cells, J. Res. Sci. Teach., 29(10), 1079–1099.
- Liu X. and Ebenezer J., (2002), Descriptive categories and structural characteristics of students' conceptions: an exploration of the relationship, Res. Sci. Technol. Educ., 20(1), 111–132.
- Marton F., (1981), Phenomenography—Describing Conceptions of the World around Us, Instruct. Sci., 10(2), 177–200.
- Marton F. and Booth S., (1997), Learning and awareness, Mahwah, New Jersey: Lawrence Earlbaum Associates Publishers, pp. 110–136.
- Marton F. and Pang M. F., (2006), On Some Necessary Conditions of Learning, J. Learn. Sci., 15(2), 193–220.
- Mintzes J. J., Wandersee J. H. and Novak J. D., (2005), Assessing science understanding: a human constructivist view, San Diego, California, USA: Academic Press, pp. 74–77.
- Rahayu S., Treagust D. F., Chandrasegaran A. L., Kita M. and Ibnu S., (2011), Assessment of Electrochemical Concepts: A Comparative Study Involving Senior High-School Students in Indonesia and Japan, Res. Sci. Technol. Educ., 29(2), 169–188.
- Rogers F., Huddle P. A. and White M. D., (2000), Using a Teaching Model to Correct Known Misconceptions in Electrochemistry, J. Chem. Educ., 77(1), 104.
- Sanger M. J. and Greenbowe T. J., (1997), Common student misconceptions in electrochemistry: galvanic, electrolytic, and concentration cells, J. Res. Sci. Teach., 34(4), 377–398.
- Schmidt H. J., Marohn A. and Harrison A. G., (2007), Factors that prevent learning in electrochemistry, J. Res. Sci. Teach., 44(2), 258–283.
- Şen Ş., Yılmaz A. and Geban Ö., (2015), The effects of process oriented-guided inquiry learning environment on students' self-regulated learning skills, Problems of Education in the 21st Century, 66, 54–66.
- Supasorn S., (2015), Grade 12 students' Conceptual Understanding and Mental Models of Galvanic Cells Before and after Learning by Using Small-Scale Experiments in Conjunction with a Model Kit, Chem. Educ. Res. Pract., 16(2), 393–407.
- Vosniadou S., (ed.), (2009), International handbook of research on conceptual change, New York and London: Routledge.
| This journal is © The Royal Society of Chemistry 2019 |


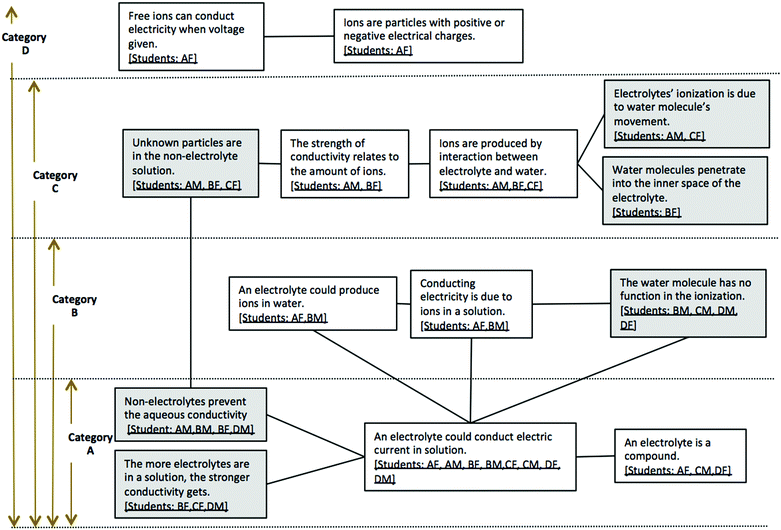
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) The frames represent examples in every category; the white frames are the ones consistant with scientific conceptions, and the gray frames are the ones different from scientific conceptions. The connections between the frames are labeled as lines, which are made when the same person is in two different frames.
The frames represent examples in every category; the white frames are the ones consistant with scientific conceptions, and the gray frames are the ones different from scientific conceptions. The connections between the frames are labeled as lines, which are made when the same person is in two different frames.