Open Access Article
Open Access ArticleNon-natural 2H-azirine-2-carboxylic acids: an expedient synthesis and antimicrobial activity†
Pavel A. Sakharov a,
Alexander N. Koronatov
a,
Alexander N. Koronatov a,
Alexander F. Khlebnikov
a,
Alexander F. Khlebnikov a,
Mikhail S. Novikova,
Artem G. Glukhareva,
Elizaveta V. Rogachevaab,
Liudmila A. Kraevab,
Vladimir V. Sharoykoa,
Tatiana B. Tennikova
a,
Mikhail S. Novikova,
Artem G. Glukhareva,
Elizaveta V. Rogachevaab,
Liudmila A. Kraevab,
Vladimir V. Sharoykoa,
Tatiana B. Tennikova a and
Nikolai V. Rostovskii
a and
Nikolai V. Rostovskii *a
*a
aInstitute of Chemistry, Saint Petersburg State University, 7/9 Universitetskaya nab., Saint Petersburg, 199034, Russia. E-mail: n.rostovskiy@spbu.ru
bPasteur Institute of Epidemiology and Microbiology, 14 Mira Street, Saint Petersburg, 197101, Russia
First published on 21st November 2019
Abstract
Non-natural 2H-azirine-2-carboxylic acids were obtained in high yields by FeCl2-catalyzed isomerization of 5-chloroisoxazoles to azirine-2-carbonyl chlorides followed by their hydrolysis. The 3-aryl- and 3-heteroaryl-substituted acids are stable during prolonged storage, exhibit antibacterial activity against ESKAPE pathogens and show a low level of cytotoxicity.
Introduction
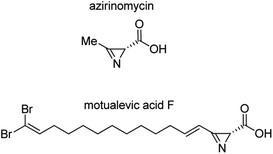
Infectious diseases still remain among the five main causes of death in the World and cause 13 million deaths per year.1 At the same time, the effectiveness of existing antibacterial drugs has been increasingly reduced during recent decades due to the constant growth in the number of microorganisms that are resistant to the drugs.2 Resistant microorganisms are becoming clinically widespread and they pose a serious threat to world health. Therefore, development of new compounds with antibacterial activity is one of the most significant challenges in modern medicinal and pharmaceutical chemistry.3In 1971, azirinomycin (3-methyl-2H-azirine-2-carboxylic acid), the first example of an azirine-2-carboxylic acid, was isolated from the soil bacterial strain Streptomyces aureus (Fig. 1).4 It was found that azirinomycin exhibited a wide spectrum of antibacterial activity in vitro against both Gram-positive and Gram-negative bacteria.4a In vivo studies in mice have shown rather high toxicity of azirinomycin of 50% purity isolated from natural raw materials.4a As far as azirinomycin was very unstable compound, it was not definitely established whether the observed toxicity was related to azirinomycin, its decomposition products or impurities present in the sample.
Motualevic acid F, isolated from the marine sponge Siliquariaspongia (Fig. 1), was the first reported example of long chain 2H-azirine-2-carboxylic acids.5 Motualevic acid F showed inhibitory activity against Staphylococcus aureus and methicillin-resistant Staphylococcus aureus at low acid loadings.5 According to observations, the presence of a carboxyl group in the structure of azirine derivative was important for displaying antimicrobial activity that was confirmed by the fact that esters of azirinomycin4a and motualevic acid F ((4E)-(R)-antazirine)5 showed much lower antimicrobial activity.
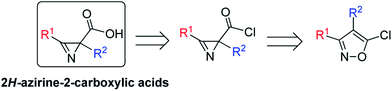
This data shows that azirine-2-carboxylic acid derivatives are perspective candidates in antibiotics development. Although the compounds with a free carboxyl group showed high antibacterial activity, further studies in this direction slowed down. This was primarily due to the lack of methods for the synthesis of azirine-2-carboxylic acids. The hydrolysis of motualevic acid F methyl ester has long been the only example of the synthesis of this class of carboxylic acids.6 Very recently another synthetic acid, 3-phenyl-2H-azirine-2-carboxylic acid,7 was prepared by the hydrolysis of its methyl ester. The poor availability of some azirine-2-carboxylic esters and the strongly basic conditions of the hydrolysis motivate the search for new approaches to azirine-2-carboxylic acids as potential antibiotics.
In this work, a new effective method for the synthesis of azirine-2-carboxylic acids has been developed involving FeCl2-catalyzed isomerization of 5-chloroisoxazoles to azirine-2-carbonyl chlorides followed by their hydrolysis (Scheme 1).
Results and discussion
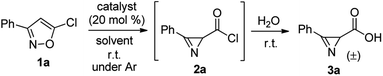
The catalytic isomerization of isoxazoles, bearing a heteroatom substituent at the C5 position, to 2H-azirine-2-carboxylic acid derivatives has been intensively studied and widely used in recent years.8,9 In particular, it has been demonstrated that 5-chloroisoxazoles can be transformed to 2H-azirine-2-carbonyl chlorides under treatment with anhydrous FeCl2 in acetonitrile.10Keeping this fact in mind we started searching for a catalyst suitable for the implementation of the two step reaction sequence “isoxazole isomerization – azirinecarbonyl chloride hydrolysis” in one synthetic operation. At first we tested FeCl2·4H2O in acetonitrile using isoxazole 1a as a model compound (Scheme 2, Table 1, entry 1). However, only traces of azirinecarboxylic acid 3a were detected even after addition of water. The use of anhydrous FeCl2 in acetonitrile for isomerization of 1a and addition of water after full conversion of the isoxazole into chloride 2a allowed to obtain azirine-2-carboxylic acid 3a in almost quantitative yield (entry 2). FeCl2 catalysis of 2a isomerization in other solvents (acetone, tetrahydrofuran, toluene) turned out to be less effective (entries 3–5). We also tested several other salts as catalysts for isomerization of chloroisoxazoles. Among Fe(NTf2)2,9d,i CuI and Rh2(Piv)4 (ref. 9a) only the latter allowed to synthesize acid 3a in moderate yield (entry 8).
| Entry | Solvent | Catalyst (20 mol%) | Time, h | Yield of 3ab (%) |
|---|---|---|---|---|
| a NTf2 = bis(trifluoromethylsulfonyl)imide, Piv = trimethylacetate.b Isolated yields.c Isomerization was carried out at 110 °C with 5 mol% of Rh2(Piv)4. | ||||
| 1 | MeCN | FeCl2·4H2O | 100 | Trace |
| 2 | MeCN | FeCl2 | 2 | 98 |
| 3 | Acetone | FeCl2 | 24 | 60 |
| 4 | THF | FeCl2 | 48 | 35 |
| 5 | PhMe | FeCl2 | 100 | 10 |
| 6 | MeCN | Fe(NTf2)2 | 100 | 0 |
| 7 | MeCN | CuI | 100 | Trace |
| 8 | PhMe | Rh2(Piv)4 | 2 | 51c |
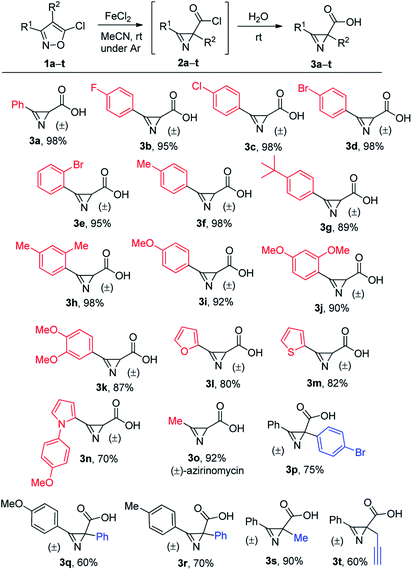
Next, we examined the scope of acids that could be prepared by the method using anhydrous FeCl2 (Scheme 3). The most starting materials, 5-chloroisoxazoles, were obtained from the corresponding isoxazol-5-ones and POCl3 (see ESI†). Azirinecarboxylic acids 3 bearing halogen, alkyl, and methoxy groups in the aryl ring were synthesized under these conditions in good to excellent yields (compounds 3a–k). Moreover, azirinecarboxylic acids containing heterocyclic substituent (furan, thiophene, pyrrole) at the C3 atom were also obtained but in slightly lower yields (compounds 3l–n). This method was applied to the synthesis of racemic form of natural azirinomycin (compound 3o). It was synthesized in high yield under the standard reaction conditions. Finally, 4-aryl- and 4-alkyl-substituted isoxazoles gave acids with quaternary azirine C2 atom in good yields (compounds 3p–t). It is important that all reactions were carried out at room temperature and in most cases there was no need to perform chromatographic purification in order to obtain pure products.
The structures of acids were established on the basis of 1H, 13C NMR and IR spectroscopy as well as the data of HRMS. The strong band of azirine C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond in IR spectra lies in 1763–1781 cm−1 range which is characteristic of C3-substituted azirines.11
N bond in IR spectra lies in 1763–1781 cm−1 range which is characteristic of C3-substituted azirines.11
rac-Azirinomycin was found to be unstable in pure form without solvent: it underwent spontaneous explosive decomposition. On the contrary, other synthesized acids containing an aryl or heteroaryl substituent at the C3 atom are quite stable and can be stored at −20 °C for months without notable decomposition. It was noticed that the solid acids melted with vigorous decomposition. We studied this process in detail for acid 3a using thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). According to the TGA data, weight loss of the sample of acid 3a under heating is 27.1% that corresponds to the loss of CO2 (27.3%) (ESI†). The DSC analysis showed that the acid melted first (a small endothermic region) and afterwards the exothermic decomposition of the sample occurred (ESI†).
2H-Azirine-2-carboxylic acids 3a–t are soluble in water and common organic solvents except aliphatic hydrocarbons. The measured solubility of acid 3a in water is ca. 1.5 mg mL−1. The acidity of compound 3a (pKa ≈ 3.2) was also estimated using the potentiometric method (ESI†).
Acids 3 can be easily converted to the corresponding potassium salts 4 in high yield (95% for compound 3a) using potassium tert-butoxide in methanol. The salt is much more soluble in water (ca. 35 mg mL−1) and more thermally stable than the acid. Thus, the preparation of the salts is especially useful as a solution to the problem of long-term storage of thermally unstable azirine-2-carboxylic acids.
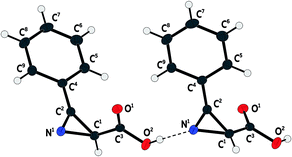
The structure of acid 3a was also studied by X-ray diffraction analysis (Fig. 2). In crystal phase, the acid 3a does not exist in form of zwitter-ion unlike amino acids: according to the carbon–oxygen bond distances (1.21 and 1.33 Å), there is a carboxylic group in the structure. However, chains which are formed by intermolecular hydrogen bonding between azirine nitrogen atom of one molecule and the hydrogen atom of the carboxylic group of another azirine molecule are observed in the crystal structure.
We also obtained primary data on antibacterial activity of the non-natural 2H-azirine-2-carboxylic acids. A screening of the antibacterial activity of synthesized acids against of the so-called ESKAPE pathogens (i.e. the ones most prone to developing resistance to drugs)12 was carried out initially by the disk diffusion method (DDM; see ESI† for details). Subsequently, for the lead compounds 3a,b,d,e,m antibacterial activities were evaluated by determining the minimum inhibitory concentration (MIC) and compared to Sulfamethoxazole used as a positive control (Table 2). In many cases, MICs of 2H-azirine-2-carboxylic acids toward ESKAPE pathogens were similar to that of Sulfamethoxazole. Moreover, compounds 3a,b,d,e inhibited growth of Staphylococcus aureus at concentrations even lower than Sulfamethoxazole. Notably, methyl ester of acid 3a (compound 3a′) displayed activity only against Klebsiella pneumoniae (DDM; see ESI† for details). This fact indicates that a free carboxylic group is essential for displaying antibacterial activity of azirine-2-carboxylic acids.
| Acid | E. faecium | S. aureus | K. pneumoniae | A. baumannii | P. aeruginosa | E. aerogenes |
|---|---|---|---|---|---|---|
| a MIC values are reported as μM. | ||||||
| 3a | 56 | 56 | 225 | 450 | 56 | 56 |
| 3b | 50 | 50 | 200 | 600 | 600 | 600 |
| 3d | 304 | 19 | 76 | 608 | 608 | 304 |
| 3e | 152 | 38 | 76 | 608 | 608 | 608 |
| 3m | >864 | 864 | 864 | 216 | 54 | >864 |
| Sulfamethoxazole | 16 | 64 | 64 | 128 | 32 | 32 |
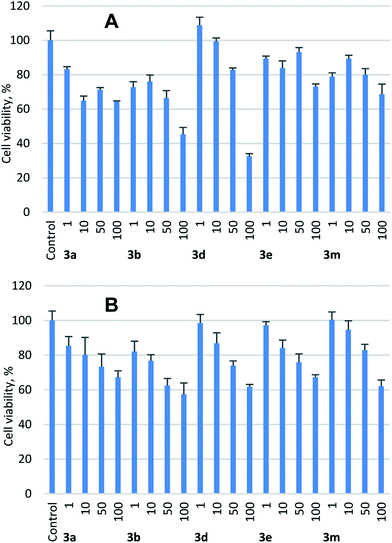
Considering initially documented toxicity of azirinomycin,4a the in vitro data on cytotoxicity of synthetic azirine-2-carboxylic acids 3 is of obvious interest. Compounds 3a,b,d,e,m were tested at 1–100 μM concentrations for their ability to affect the cell culture viability of non-cancerous human retinal pigment epithelial cell line ARPE-19 (ref. 13) and human epithelial kidney cell line HEK293.14 As followed from the MTT assay data (Fig. 3; see ESI† for details), the studied compounds displayed no appreciable cytotoxicity within the concentration range tested on both the cell lines. The pattern of cell viability in the presence of tested compounds was practically the similar in ARPE-19 and HEK293 cell lines (Fig. 3). Overall HEK293 cell line was less sensitive to cytotoxic effect of the tested compounds compared to ARPE-19 cell line. The lowest cell viability was detected for compounds 3b,d at 100 μM concentrations in ARPE-19 cell line.
Conclusions
In conclusion, a high-yield synthesis of 3-mono- and 2,3-disubstituted 2H-azirine-2-carboxylic acids from readily accessible 5-chloroisoxazoles has been developed. The method is based on FeCl2-catalyzed isomerization of 5-chloroisoxazoles followed by hydrolysis of formed azirine-2-carbonyl chlorides. 3-Aryl-2H-azirine-2-carboxylic acids exist as OH form in crystal, are stable during prolonged storage at −20 °C, but undergo decarboxylation after melting. These compounds exhibit antibacterial activity against ESKAPE pathogens comparable to that of Sulfamethoxazole and show low-level cytotoxicity.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support of the Scientific Council of the President of the Russian Federation (MK-2698.2019.3). This research used resources of the Magnetic Resonance Research Centre, Chemical Analysis and Materials Research Centre, Centre for X-ray Diffraction Studies, Thermogravimetric and Calorimetric Research Centre and Chemistry Educational Centre of the Research Park of St. Petersburg State University.Notes and references
- C. Nathan, Nature, 2004, 431, 899–902 CrossRef CAS.
- (a) J. Davies and D. Davies, Microbiol. Mol. Biol. Rev., 2010, 74, 417–433 CrossRef CAS PubMed; (b) U. Theuretzbacher, Curr. Opin. Pharmacol., 2011, 11, 433–438 CrossRef CAS PubMed; (c) R. J. Fair and Y. Tor, Perspect. Med. Chem., 2014, 6, 25–64 Search PubMed.
- (a) R. A. Fisher, B. Gollan and S. Helaine, Nat. Rev. Microbiol., 2010, 8, 501–510 CrossRef PubMed; (b) L. Freire-Moran, B. Aronsson, C. Manz, I. C. Gyssens, A. D. So, D. L. Monnet, O. Cars and E.-E. W. Group, Drug Resist. Updates, 2011, 14, 118–124 CrossRef; (c) J. Rai, G. K. Randhawa and M. Kaur, Int. J. Appl. Basic Med. Res., 2013, 3, 3–10 CrossRef PubMed.
- (a) E. O. Stapley, D. Hendlin, M. Jackson, A. K. Miller, S. Hernandez and J. M. Mata, J. Antibiot., 1971, 24, 42–47 CrossRef CAS PubMed; (b) T. W. Miller, E. W. Tristram and F. J. Wolf, J. Antibiot., 1971, 24, 48–50 CrossRef CAS PubMed.
- J. L. Keffer, A. Plaza and C. A. Bewley, Org. Lett., 2009, 11, 1087–1090 CrossRef CAS PubMed.
- V. D. Kadam and G. Sudhakar, Tetrahedron, 2015, 71, 1058–1067 CrossRef CAS.
- N. V. Rostovskii, I. A. Smetanin, A. V. Agafonova, P. A. Sakharov, J. O. Ruvinskaya, A. F. Khlebnikov and M. S. Novikov, Org. Biomol. Chem., 2018, 16, 3248–3257 RSC.
- For review see: (a) E. E. Galenko, A. F. Khlebnikov and M. S. Novikov, Chem. Heterocycl. Compd., 2016, 52, 637–650 CrossRef CAS; (b) A. F. Khlebnikov, M. S. Novikov and N. V. Rostovskii, Tetrahedron, 2019, 75, 2555–2624 CrossRef CAS.
- For recent examples see: (a) N. V. Rostovskii, A. V. Agafonova, I. A. Smetanin, M. S. Novikov, A. F. Khlebnikov, J. O. Ruvinskaya and G. L. Starova, Synthesis, 2017, 49, 4478–4488 CAS; (b) A. V. Agafonova, I. A. Smetanin, N. V. Rostovskii, A. F. Khlebnikov and M. S. Novikov, Chem. Heterocycl. Compd., 2017, 53, 1068–1071 CrossRef CAS; (c) E. E. Galenko, V. A. Bodunov, A. V. Galenko, M. S. Novikov and A. F. Khlebnikov, J. Org. Chem., 2017, 82, 8568–8579 CrossRef CAS PubMed; (d) A. V. Galenko, E. E. Galenko, F. M. Shakirova, M. S. Novikov and A. F. Khlebnikov, J. Org. Chem., 2017, 82, 5367–5379 CrossRef CAS PubMed; (e) K. Okamoto, A. Nanya, A. Eguchi and K. Ohe, Angew. Chem., Int. Ed., 2018, 56, 1039–1043 CrossRef PubMed; (f) Y. Ge, W. Sun, B. Pei, J. Ding, Y. Jiang and T.-P. Loh, Org. Lett., 2018, 20, 2774–2777 CrossRef CAS PubMed; (g) V. A. Bodunov, E. E. Galenko, A. V. Galenko, M. S. Novikov and A. F. Khlebnikov, Synthesis, 2018, 50, 2784–2798 CrossRef CAS; (h) L. D. Funt, O. A. Tomashenko, M. S. Novikov and A. F. Khlebnikov, Synthesis, 2018, 50, 4809–4822 CrossRef CAS; (i) E. E. Galenko, M. S. Novikov, F. M. Shakirova, J. R. Shakirova, I. V. Kornyakov, V. A. Bodunov and A. F. Khlebnikov, J. Org. Chem., 2019, 84, 3524–3536 CrossRef CAS PubMed.
- (a) K. I. Mikhailov, E. E. Galenko, A. V. Galenko, M. S. Novikov, A. Yu. Ivanov, G. L. Starova and A. F. Khlebnikov, J. Org. Chem., 2018, 83, 3177–3187 CrossRef CAS PubMed; (b) P. A. Sakharov, M. S. Novikov and A. F. Khlebnikov, J. Org. Chem., 2018, 83, 8304–8314 CrossRef CAS PubMed; (c) V. A. Bodunov, E. E. Galenko, P. A. Sakharov, M. S. Novikov and A. F. Khlebnikov, J. Org. Chem., 2019, 84, 10388–10401 CrossRef CAS PubMed.
- F. Palacios, A. M. Ochoa de Retana, E. Martínez de Marigorta and J. M. de los Santos, Org. Prep. Proced. Int., 2002, 34, 219–269 CrossRef CAS.
- J. A. Karlowsky, D. J. Hoban, M. A. Hackel, S. H. Lob and D. F. Sahm, J. Med. Microbiol., 2017, 66, 61–69 CrossRef PubMed.
- K. C. Dunn, A. E. Aotaki-Keen, F. R. Putkey and L. M. Hjelmeland, Exp. Eye Res., 1996, 62, 155–169 CrossRef CAS PubMed.
- F. L. Graham, J. Smiley, W. C. Russell and R. Nairn, J. Gen. Virol., 1977, 36, 59–74 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure for the preparation of new compounds and the biological studies, spectroscopic data for all new compounds, TGA and DSC data. CCDC 1956437 (3a). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ra09345a |
| This journal is © The Royal Society of Chemistry 2019 |